The Optimal Cutoff Value of Tumor Markers for Prognosis Prediction in Ampullary Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Demographics
3.2. Tumor Marker Distribution
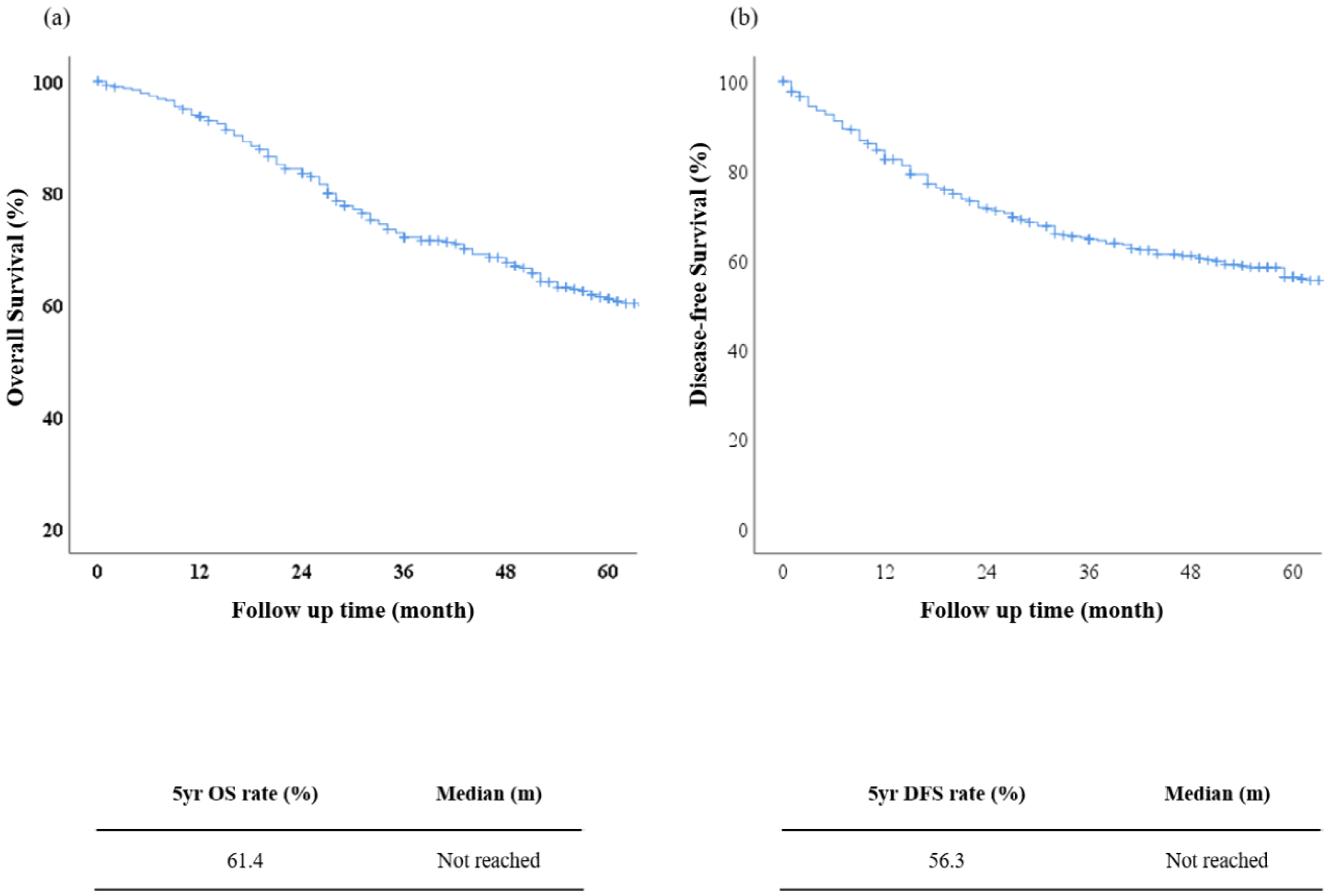
3.3. Survival Analysis
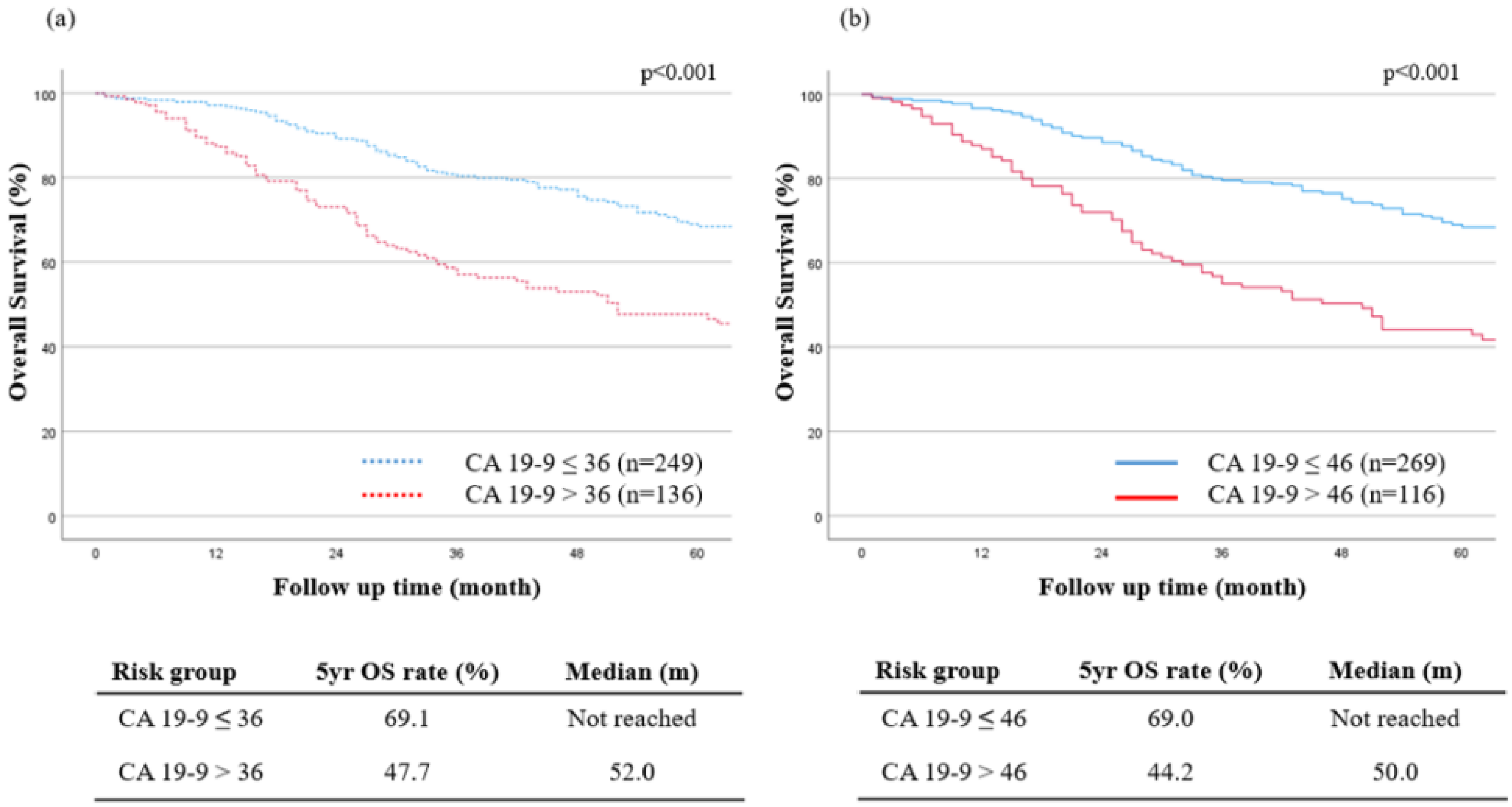
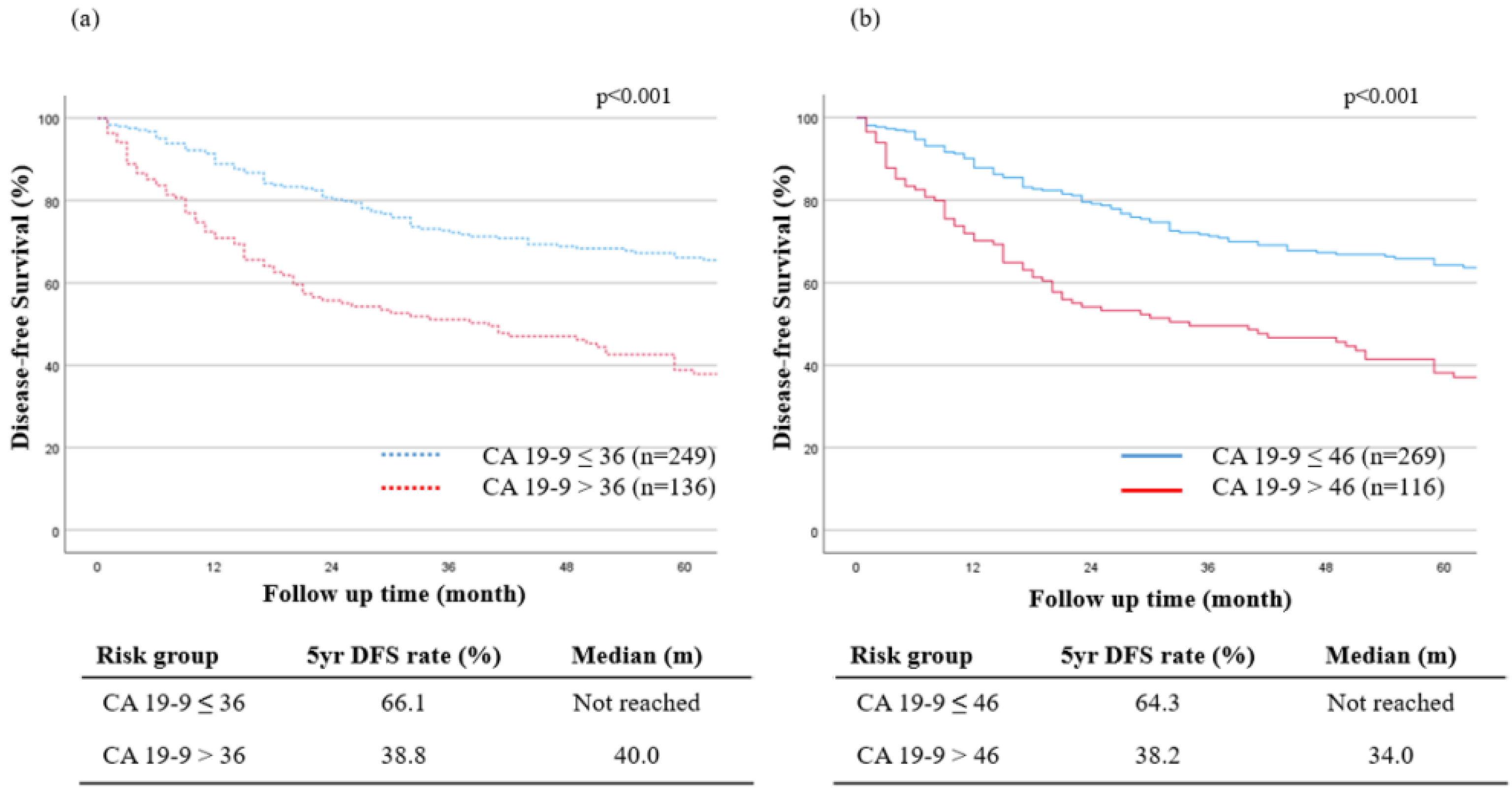
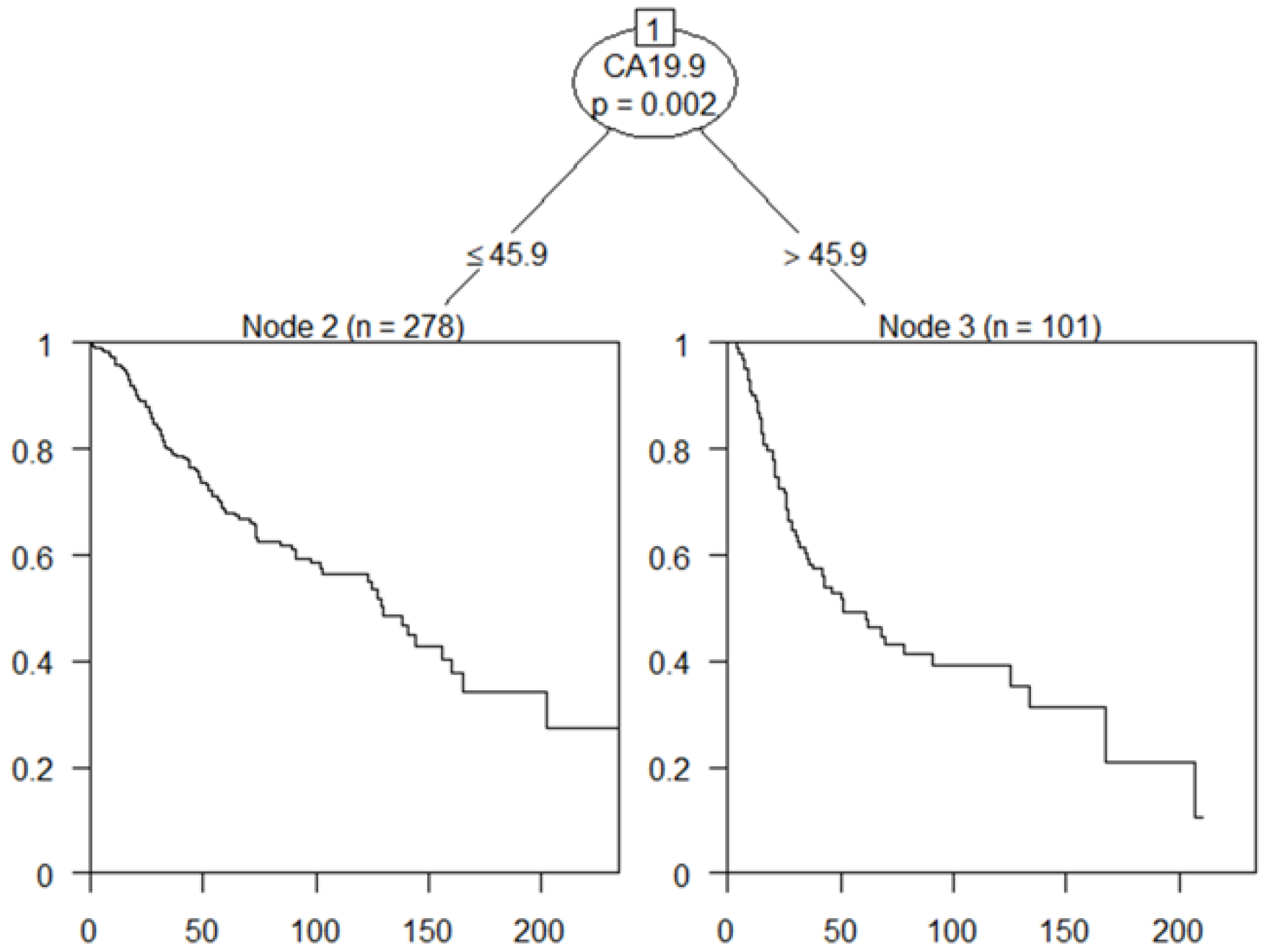
3.4. The New Optimal Cutoff Survival Value according to the Tumor Marker
3.5. Prognostic Factors for OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byun, Y.; Choi, Y.J.; Han, Y.; Kang, J.S.; Kim, H.; Kwon, W.; Jang, J.Y. Outcomes of 5000 pancreatectomies in Korean single referral center and literature reviews. J. Hepatobiliary Pancreat. Sci. 2021, 29, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kwon, W.; Kim, J.R.; Byun, Y.; Jang, J.Y.; Kim, S.W. Recurrence patterns after pancreaticoduodenectomy for ampullary cancer. J. Hepatobiliary Pancreat. Sci. 2019, 26, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Kim, S.W.; Park, S.J.; Lee, H.S.; Jang, J.Y.; Choi, M.G.; Kim, W.H.; Lee, K.U.; Park, Y.H. Clinicopathologic analysis of early ampullary cancers with a focus on the feasibility of ampullectomy. Ann. Surg. 2005, 242, 92. [Google Scholar] [CrossRef]
- Virji, M.A.; Mercer, D.W.; Herberman, R.B. Tumor markers in cancer diagnosis and prognosis. CA Cancer J. Clin. 1988, 38, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Clinical uses of tumor markers: A critical review. Crit. Rev. Clin. Lab. Sci. 2001, 38, 225–262. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, M.; Göçmen, E.; Tez, M.; Ertan, T.; Keskek, M.; Koç, M. Value of preoperative serum CA 19-9 levels in predicting resectability for pancreatic cancer. Can. J. Surg. 2006, 49, 241. [Google Scholar] [PubMed]
- Levy, C.; Lymp, J.; Angulo, P.; Gores, G.J.; Larusso, N.; Lindor, K.D. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2005, 50, 1734–1740. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.; Kim, H.; Han, Y.; Byun, Y.; Choi, Y.; Kang, J.; Kwon, W.; Jang, J.Y. Preoperative carbohydrate antigen 19-9 and standard uptake value of positron emission tomography-computed tomography as prognostic markers in patients with pancreatic ductal adenocarcinoma. J. Hepatobiliary Pancreat. Sci. 2020, 29, 1133–1141. [Google Scholar] [CrossRef]
- Yamashita, S.; Passot, G.; Aloia, T.A.; Chun, Y.S.; Javle, M.; Lee, J.E.; Vauthey, J.N.; Conrad, C. Prognostic value of carbohydrate antigen 19-9 in patients undergoing resection of biliary tract cancer. Br. J. Surg. 2017, 104, 267–277. [Google Scholar] [CrossRef]
- Scarà, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Pavai, S.; Yap, S.F. The clinical significance of elevated levels of serum CA 19-9. Med. J. Malays. 2003, 58, 667–672. [Google Scholar]
- Scroggie, D.L.; Mavroeidis, V.K. Surgical ampullectomy: A comprehensive review. World J. Gastrointest. Surg. 2021, 13, 1338. [Google Scholar] [CrossRef]
- Hong, S.S.; Han, S.S.; Kwon, W.; Jang, J.Y.; Kim, H.J.; Cho, C.K.; Ahn, K.S.; Yang, J.D.; Park, Y.; Min, S.K.; et al. Comparison of Oncologic Outcomes between Transduodenal Ampullectomy and Pancreatoduodenectomy in Ampulla of Vater Cancer: Korean Multicenter Study. Cancers 2021, 13, 2038. [Google Scholar] [CrossRef]
- Liu, B.; Heckler, M.; Heger, U.; Roth, S.; Klaiber, U.; Büchler, M.W.; Strobel, O.; Michalski, C.W.; Hackert, T. Definition of an extended minimum level of lymphadenectomy in non-pancreatic periampullary cancer resections. HPB 2018, 20, 1028–1033. [Google Scholar] [CrossRef]
- Rizzo, A.; Dadduzio, V.; Lombardi, L.; Ricci, A.D.; Gadaleta-Caldarola, G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Curr. Oncol. 2021, 28, 3393–3402. [Google Scholar] [CrossRef]
- Bonet, M.; Rodrigo, A.; Vázquez, S.; Carrizo, V.; Vilardell, F.; Mira, M. Adjuvant therapy for true ampullary cancer: A systematic review. Clin. Transl. Oncol. 2020, 22, 1407–1413. [Google Scholar] [CrossRef]
- Skórzewska, M.; Kurzawa, P.; Ciszewski, T.; Pelc, Z.; Polkowski, W.P. Controversies in the diagnosis and treatment of periampullary tumours. Surg. Oncol. 2022, 44, 101853. [Google Scholar] [CrossRef]
- Ahn, D.H.; Bekaii-Saab, T. Ampullary cancer: An overview. Am. Soc. Clin. Oncol. Educ. Book 2014, 34, 112–115. [Google Scholar] [CrossRef]
- Lemke, J.; Schäfer, D.; Sander, S.; Henne-Bruns, D.; Kornmann, M. Survival and prognostic factors in pancreatic and ampullary cancer. Anticancer Res. 2014, 34, 3011–3020. [Google Scholar]
- Lee, H.S.; Jang, J.S.; Lee, S.; Yeon, M.H.; Kim, K.B.; Park, J.G.; Lee, J.Y.; Kim, M.J.; Han, J.H.; Sung, R.; et al. Diagnostic Accuracy of the Initial Endoscopy for Ampullary Tumors. Clin. Endosc. 2015, 48, 239–246. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Z.; Zhang, Y.; Wang, P.; Gao, H. Combination of CA19-9 and the Neutrophil-to-Lymphocyte Ratio for the Differential Diagnosis of Gallbladder Carcinoma. Cancer Manag. Res. 2020, 12, 4475. [Google Scholar] [CrossRef]
- Qin, X.L.; Wang, Z.R.; Shi, J.S.; Lu, M.; Wang, L.; He, Q.R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 2004, 10, 427. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Han, Y.; Sohn, H.; Kang, J.S.; Kwon, W.; Jang, J.Y. Prognostic Value of Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen 19-9 (CA 19-9) in Gallbladder Cancer; 65 IU/mL of CA 19-9 Is the New Cut-Off Value for Prognosis. Cancers 2021, 13, 1089. [Google Scholar] [CrossRef] [PubMed]
- Humphris, J.L.; Chang, D.K.; Johns, A.L.; Scarlett, C.J.; Pajic, M.; Jones, M.D.; Colvin, E.K.; Nagrial, A.; Chin, V.T.; Chantrill, L.A.; et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann. Oncol. 2012, 23, 1713–1722. [Google Scholar] [CrossRef]
- Kurihara, C.; Yoshimi, F.; Sasaki, K.; Iijima, T.; Kawasaki, H.; Nagai, H. Clinical value of serum CA19-9 as a prognostic factor for the ampulla of Vater carcinoma. Hepatogastroenterology 2013, 60, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Kau, S.Y.; Shyr, Y.M.; Su, C.H.; Wu, C.W.; Lui, W.Y. Diagnostic and prognostic values of CA 19-9 and CEA in periampullary cancers. J. Am. Coll. Surg. 1999, 188, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, W.; Zhang, N.; He, X. Preoperative platelet-lymphocyte ratio is an independent prognostic factor in ampullary carcinoma following pancreaticoduodenectomy. Oncol. Lett. 2018, 16, 4879–4888. [Google Scholar] [CrossRef]
- Alexakis, N.; Gomatos, I.P.; Sbarounis, S.; Toutouzas, K.; Katsaragakis, S.; Zografos, G.; Konstandoulakis, M.M. High serum CA 19-9 but not tumor size should select patients for staging laparoscopy in radiological resectable pancreas head and peri-ampullary cancer. Eur. J. Surg. Oncol. 2015, 41, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Suzuki, K.; Nishio, K.; Ishida, Y.; Okada, R.; Goto, Y.; Naito, M.; Wakai, K.; Ito, Y.; Hamajima, N. Smoking and serum CA19-9 levels according to Lewis and secretor genotypes. Int. J. Cancer 2008, 123, 2880–2884. [Google Scholar] [CrossRef] [PubMed]

| Variables N = 385 | |
|---|---|
| Age, years, mean ± SD | 62.45 ± 9.95 |
| Gender, n (%) | |
| Male | 205 (53.2) |
| Female | 180 (46.8) |
| BMI, kg/m2, mean ± SD | 23.59 ± 3.28 |
| ASA score, n (%) | |
| I | 125 (32.5) |
| II | 232 (60.3) |
| III | 28 (7.2) |
| Diabetes mellitus, n (%) | 73 (19.0) |
| CEA, ng/mL, median (IQR) | 1.6 (1.1–2.4) |
| CA 19-9, U/mL, median (IQR) | 18.6 (7.85–67.45) |
| Total bilirubin > 1.3 mg/dL, n (%) | 198 (50.1) |
| Operation time, min, mean ± SD | 315.99 ± 79.12 |
| Operation type, n (%) | |
| Whipple | 69 (17.9) |
| PPPD | 316 (82.1) |
| Operation method, n (%) | |
| Open | 375 (97.4) |
| Robotic | 10 (2.6) |
| EBL, cc, mean ± SD | 398.54 ± 386.95 |
| Differentiation, n (%) | |
| WD | 128 (33.2) |
| MD & PD | 244 (66.8) |
| Lymphatic invasion (+), n (%) | 142 (36.9) |
| Venous invasion (+), n (%) | 31 (8.1) |
| Perineural invasion (+), n (%) | 80 (20.8) |
| T stage, n (%) | |
| T1 | 128 (33.2) |
| T2 | 119 (30.9) |
| T3 | 128 (33.2) |
| T4 | 10 (2.7) |
| Lymph node (+), n (%) | 114 (29.6) |
| R0 resection, n (%) | 383 (99.5) |
| Hospital stay, day, mean ± SD | 20.72 ± 11.47 |
| POPF (+), n (%) | 201 (52.2) |
| Grade B-C POPF (+), n (%) | 57 (14.8) |
| Complication (CD grade ≥3a), n (%) | 79 (20.5) |
| Adjuvant chemotherapy, n (%) | 182 (47.3) |
| Adjuvant radiation therapy, n (%) | 158 (41.0) |
| Recurrence, n (%) | 133 (34.5) |
| Variables N = 385 | CA 19-9 ≤ 36 n = 249 | CA 19-9 > 36 n = 136 | p-Value | CA 19-9 ≤ 46 n = 269 | CA 19-9 > 46 n = 116 | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Age (mean ± SD, yr) | 62.45 ± 9.95 | 62.19 ± 9.77 | 62.93 ± 10.32 | 0.484 | 62.29 ± 9.73 | 62.83 ± 10.50 | 0.628 | |
| Gender | Male | 205 (53.2%) | 135 (54.2%) | 70 (51.5%) | 0.606 | 143 (53.2%) | 62 (53.4%) | 0.958 |
| Female | 180 (46.8%) | 114 (45.8%) | 66 (48.5%) | 126 (46.8%) | 54 (46.6%) | |||
| Total bilirubin (mg/dL) | <2.0 | 215 (55.8%) | 162 (65.1%) | 53 (39.0%) | <0.001 | 173 (64.3%) | 42 (36.2%) | <0.001 |
| ≥2.0 | 170 (44.2%) | 87 (34.9%) | 83 (61.0%) | <0.001 | 96 (35.7%) | 74 (63.8%) | <0.001 | |
| Operation type | PPPD | 316 (82.1%) | 208 (83.5%) | 108 (79.4%) | 0.313 | 227 (84.4%) | 89 (76.7%) | 0.072 |
| Whipple | 69 (17.9%) | 41 (16.5%) | 28 (20.6%) | 42 (15.6%) | 27 (23.3%) | |||
| Differentiation | WD | 128 (33.2%) | 100 (40.2%) | 28 (20.6%) | <0.001 | 102 (37.9%) | 26 (22.4%) | 0.002 |
| MD | 210 (54.5%) | 129 (51.8%) | 81 (59.6%) | 142 (52.8%) | 68 (58.6%) | |||
| PD | 34 (8.8%) | 13 (5.2%) | 21 (15.4%) | 17 (6.3%) | 17 (14.7%) | |||
| T stage | T1 | 128 (33.2%) | 106 (42.6%) | 22 (16.2%) | <0.001 | 110 (40.9%) | 18 (15.5%) | <0.001 |
| T2 | 119 (30.9%) | 80 (32.1%) | 39 (28.7%) | 86 (32.0%) | 33 (28.4%) | |||
| T3 | 128 (33.2%) | 63 (25.3%) | 65 (47.8%) | 72 (26.8%) | 56 (48.3%) | |||
| T4 | 10 (2.6%) | 0 (0.0%) | 10 (7.8%) | 1 (0.4%) | 9 (7.8%) | |||
| N stage | Negative | 271 (70.4%) | 197 (79.1%) | 74 (54.4%) | <0.001 | 211 (78.4%) | 60 (51.4%) | <0.001 |
| Positive | 114 (29.6%) | 52 (20.9%) | 62 (45.6%) | 58 (21.6%) | 56 (48.3%) | |||
| Complication (CD grade ≥3a) | No | 306 (79.5%) | 200 (80.3%) | 106 (77.9%) | 0.580 | 217 (80.7%) | 89 (76.7%) | 0.379 |
| Yes | 79 (20.5%) | 49 (19.7%) | 30 (22.1%) | 52 (19.3%) | 27 (23.3%) | |||
| Chemotherapy | No | 203 (52.7%) | 148 (59.4%) | 55 (40.4%) | <0.001 | 158 (58.7%) | 45 (38.8%) | <0.001 |
| Yes | 182 (47.3%) | 101 (40.6%) | 81 (59.6%) | 111 (41.3%) | 71 (61.2%) | |||
| Radiotherapy | No | 227 (59.0%) | 163 (65.5%) | 64 (47.1%) | <0.001 | 173 (64.3%) | 54 (46.6%) | 0.001 |
| Yes | 158 (41.0%) | 86 (34.5%) | 72 (52.9%) | 96 (35.7%) | 62 (53.4%) | |||
| Recurrence | No | 252 (65.5%) | 180 (72.3%) | 72 (52.9%) | <0.001 | 192 (71.4%) | 60 (51.7%) | <0.001 |
| Yes | 133 (34.5%) | 69 (27.7%) | 64 (47.1%) | 77 (28.6%) | 56 (48.3%) | |||
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Patients (n = 385) | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Preoperative CEA, ≤5.0/>5.0 (ng/mL) | 362/23 | 1.80 (1.07–3.01) | 0.026 | 1.09 (0.64–1.85) | 0.630 | |
| Preoperative CA19-9, ≤46/>46 (U/mL) | 269/116 | 2.03 (1.50–2.75) | <0.001 | 1.37 (1.02–1.88) | 0.048 | |
| Total bilirubin, <2.0/≥2.0 (mg/dL) | 215/170 | 1.54 (1.14–2.09) | 0.005 | 1.33(0.98–1.82) | 0.071 | |
| Histologic grade | WD | 128 | Reference | - | Reference | - |
| MD | 210 | 2.29 (1.56–3.35) | <0.001 | 1.83 (1.24–2.70) | 0.002 | |
| PD | 34 | 5.77 (3.45–9.64) | <0.001 | 4.63 (2.74–7.83) | <0.001 | |
| T stage | T1 | 128 | Reference | - | Reference | - |
| T2 | 119 | 1.87 (1.21–2.90) | 0.005 | 0.99 (0.62–1.59) | 0.173 | |
| T3/4 | 138 | 3.14 (2.08–4.73) | <0.001 | 1.35 (0.85–2.15) | 0.057 | |
| N stage | N0 | 271 | Reference | - | Reference | - |
| N+ | 114 | 2.82 (2.09–3.79) | <0.001 | 1.65 (1.19–2.30) | 0.003 | |
| R status | R0 | 383 | Reference | - | Reference | - |
| R1 | 2 | 1.16 (0.16–8.30) | 0.881 | 0.67 (0.09–4.90) | 0.704 | |
| Adjuvant chemotherapy | No | 203 | Reference | - | Reference | - |
| Yes | 182 | 2.84 (2.07–3.90) | <0.001 | 2.12 (1.49–3.00) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Kim, H.; Sohn, H.; Lee, M.; Jung, H.; Jo, Y.; Han, Y.; Kwon, W.; Jang, J.-Y. The Optimal Cutoff Value of Tumor Markers for Prognosis Prediction in Ampullary Cancer. Cancers 2023, 15, 2281. https://doi.org/10.3390/cancers15082281
Lee S, Kim H, Sohn H, Lee M, Jung H, Jo Y, Han Y, Kwon W, Jang J-Y. The Optimal Cutoff Value of Tumor Markers for Prognosis Prediction in Ampullary Cancer. Cancers. 2023; 15(8):2281. https://doi.org/10.3390/cancers15082281
Chicago/Turabian StyleLee, Seungho, Hongbeom Kim, Heeju Sohn, Mirang Lee, Hyesol Jung, Youngjae Jo, Youngmin Han, Wooil Kwon, and Jin-Young Jang. 2023. "The Optimal Cutoff Value of Tumor Markers for Prognosis Prediction in Ampullary Cancer" Cancers 15, no. 8: 2281. https://doi.org/10.3390/cancers15082281
APA StyleLee, S., Kim, H., Sohn, H., Lee, M., Jung, H., Jo, Y., Han, Y., Kwon, W., & Jang, J.-Y. (2023). The Optimal Cutoff Value of Tumor Markers for Prognosis Prediction in Ampullary Cancer. Cancers, 15(8), 2281. https://doi.org/10.3390/cancers15082281






