Prospective Observational Study of Prevalence, Assessment and Treatment of Pancreatic Exocrine Insufficiency in Patients with Inoperable Pancreatic Malignancy (PANcreatic Cancer Dietary Assessment—PanDA)
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Statistical Analysis
2.2.1. Planned Sample Size
2.2.2. Data Analysis
2.2.3. Descriptive Analyses
2.2.4. Design of the Diagnostic Panel
2.2.5. Survival Analysis
3. Results
3.1. Prevalence of PEI and Patient Nutritional Status
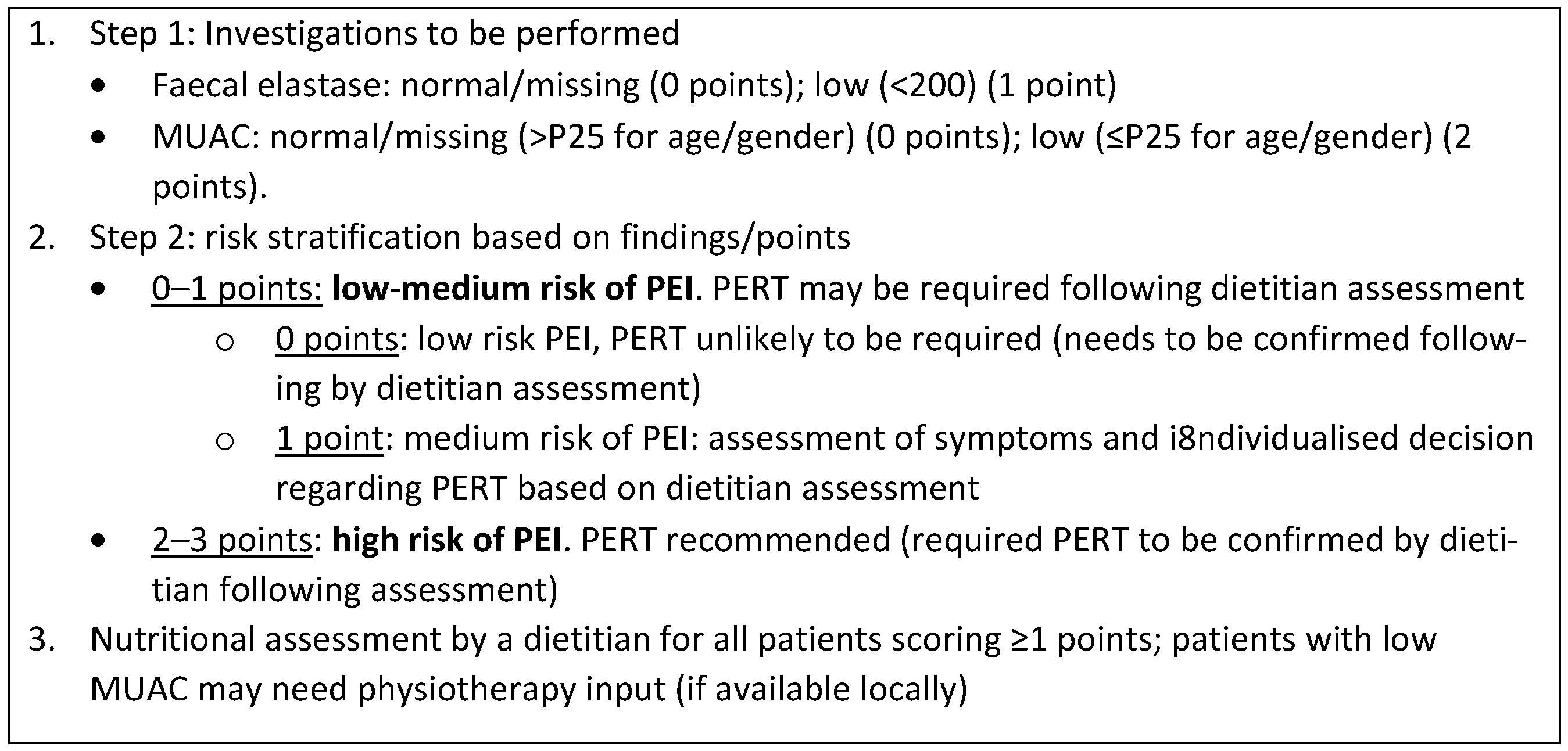
3.2. Design of PEI Screening Panel
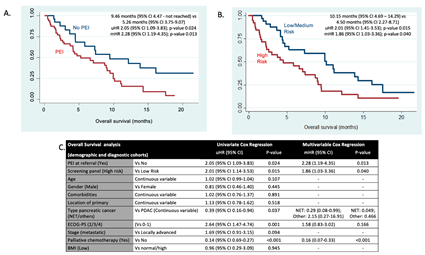
3.3. Survival Analysis: PEI and Screening Panel Results
3.4. Follow-Up Cohort
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malvezzi, M.; Bertuccio, P.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2014. Ann. Oncol. 2014, 25, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- McCallum, L.; Lamarca, A.; Valle, J. Prevalence of symptomatic pancreatic exocrine insufficiency in patients with pancreatic malignancy: Nutritional intervention may improve survival. Cancer Res. Front. 2016, 2, 352–367. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Akirov, A.; Larouche, V.; Alshehri, S.; Asa, S.L.; Ezzat, S. Treatment Options for Pancreatic Neuroendocrine Tumors. Cancers 2019, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.S.; Tesselaar, M.E.; van Leerdam, M.E.; Srirajaskanthan, R.; Ramage, J.K. Nutritional and vitamin status in patients with neuroendocrine neoplasms. World J. Gastroenterol. 2019, 25, 1171–1184. [Google Scholar] [CrossRef]
- Cade, J.E.; Hanison, J. The pancreas. Anaesth. Intensive Care Med. 2017, 18, 527–531. [Google Scholar] [CrossRef]
- Lindkvist, B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J. Gastroenterol. 2013, 19, 7258–7266. [Google Scholar] [CrossRef]
- Porta, M.; Fabregat, X.; Malats, N.; Guarner, L.; Carrato, A.; de Miguel, A.; Ruiz, L.; Jaroid, M.; Costafreda, S.; Coll, S.; et al. Exocrine pancreatic cancer: Symptoms at presentation and their relation to tumour site and stage. Clin. Transl. Oncol. 2005, 7, 189–197. [Google Scholar] [CrossRef]
- Halloran, C.M.; Cox, T.F.; Chauhan, S.; Raraty, M.G.T.; Sutton, R.; Neoptolemos, J.P.; Ghaneh, P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: A prospective study. Pancreatology 2011, 11, 535–545. [Google Scholar] [CrossRef]
- Gooden, H.M.; White, K.J. Pancreatic cancer and supportive care--pancreatic exocrine insufficiency negatively impacts on quality of life. Support. Care Cancer 2013, 21, 1835–1841. [Google Scholar] [CrossRef]
- Hendifar, A.E.; Petzel, M.Q.B.; Zimmers, T.A.; Denlinger, C.S.; Matrisan, L.M.; Picozzi, V.J.; Rahib, L.; Precision Promise Consortium. Pancreas Cancer-Associated Weight Loss. Oncologist 2019, 24, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.M.; Cahen, D.L.; de Wit, J.; Looman, C.W.N.; van Eijck, C.; Bruno, M.J. Prospective assessment of the influence of pancreatic cancer resection on exocrine pancreatic function. Br. J. Surg. 2014, 101, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.M.; Cahen, D.L.; de Wit, J.; Looman, C.W.N.; van Eijck, C.; Bruno, M.J. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J. Clin. Gastroenterol. 2014, 48, e43–e46. [Google Scholar] [CrossRef]
- Watson, L. Exocrine insufficiency and pancreatic enzyme replacement therapy in pancreatic cancer. Clin. Oncol. R. Coll. Radiol. 2010, 22, 391. [Google Scholar] [CrossRef] [PubMed]
- Dumasy, V.; Delhaye, M.; Cotton, F.; Deviere, J. Fat malabsorption screening in chronic pancreatitis. Am. J. Gastroenterol. 2004, 99, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Carnie, L.E.; Lamarca, A.; McNamara, M.G.; Bibby, N.; O’Reilly, D.A.; Valle, J.W. The assessment of pancreatic exocrine function in patients with inoperable pancreatic cancer: In need of a new gold-standard. Pancreatology 2020, 20, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.M.; Cahen, D.L.; Kuipers, E.J.; Bruno, M.J. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 337–347. [Google Scholar] [CrossRef]
- Benini, L.; Amodio, A.; Campagnola, P.; Agugiaro, F.; Cristofori, C.; Micciolo, R.; Magro, A.; Gabbrielli, A.; Cabrini, G.; Moser, L.; et al. Fecal elastase-1 is useful in the detection of steatorrhea in patients with pancreatic diseases but not after pancreatic resection. Pancreatology 2013, 13, 38–42. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.E.; Iglesias-García, J.; Vilariño-Insua, M.; Iglesias-Rey, M. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin. Gastroenterol. Hepatol. 2007, 5, 484–488. [Google Scholar] [CrossRef]
- Lindkvist, B.; Domínguez-Muñoz, J.E.; Luaces-Regueira, M.; Castiñeiras-Alvariño, M.; Nieto-Garcia, L.; Iglesias-Garcia, J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology 2012, 12, 305–310. [Google Scholar] [CrossRef]
- Pancreatric Section, British Society of Gastroenterology; Pancreatic Society of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland; Royal College of Pathologists; Special Interest Group for Gastro-Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 2005, 54 (Suppl. S5), v1–v16. [Google Scholar] [CrossRef] [PubMed]
- Sabater, L.; Ausania, F.; Bakker, O.J.; Boadas, J.; Dominguz-Munoz, J.E.; Falconi, M.; Fernandez-Cruz, L.; Frulloni, L.; Gonzalez-Sanchez, V.; Larino-Noia, J.; et al. Evidence-based Guidelines for the Management of Exocrine Pancreatic Insufficiency After Pancreatic Surgery. Ann. Surg. 2016, 264, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Toouli, J.; Biankin, A.V.; Oliver, M.R.; Pearce, C.B.; Wilson, J.S.; Wray, N.H.; Australasian Pancreatic Club. Management of pancreatic exocrine insufficiency: Australasian Pancreatic Club recommendations. Med. J. Aust. 2010, 193, 461–467. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.E. Chronic pancreatitis and persistent steatorrhea: What is the correct dose of enzymes? Clin. Gastroenterol. Hepatol. 2011, 9, 541–546. [Google Scholar] [CrossRef]
- Domínguez-Muñoz, J.E.; Iglesias-García, J.; Iglesias-Rey, M.; Vilariño-Insua, M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut 2006, 55, 1056–1057. [Google Scholar] [CrossRef] [PubMed]
- Carnie, L.E.; Farrell, K.; Barratt, N.; Abraham, M.; Gillespie, L.; Satyadas, T.; McNamara, M.G.; Hubner, R.A.; Geraghty, J.; Bibby, N.; et al. Pancreatic Enzyme Replacement Therapy for Patients Diagnosed With Pancreaticobiliary Cancer: Validation of an Algorithm for Dose Escalation and Management. Pancreas 2021, 50, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Ketterer, K.; Marsch, C.; Fechtner, K.; Krakowski-Roosen, H.; Buchler, M.W.; Friess, H.; Martignoni, M.E. Pancreatic cancer related cachexia: Influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer 2009, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Heiligensetzer, M.; Krakowski-Roosen, H.; Büchler, M.W.; Friess, H.; Martignoni, M.E. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J. Gastrointest. Surg. 2008, 12, 1193–1201. [Google Scholar] [CrossRef]
- Carnie, L.E.; Lamarca, A.; Vaughan, K.; Abedin Kapacee, Z.; McCallum, L.; Backen, A.; Barriuso, J.; McNamara, M.G.; Hubner, R.A.; Abraham, M.; et al. Prospective observational study of prevalence, assessment and treatment of pancreatic exocrine insufficiency in patients with inoperable pancreatic malignancy (PANcreatic cancer Dietary Assessment (PanDA): A study protocol. BMJ Open 2021, 11, e042067. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE). 196. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 6 April 2023).
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]

| Demographic Cohort (n = 50) | Diagnostic Cohort (n = 25) | Demographic vs. Diagnostic Cohort | Joint Demographic and Diagnostic Cohorts (n = 75) | Full Follow-Up Cohort (n = 37) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | p-Value | n | % | n | % | ||
| Baseline Characteristics | ||||||||||
| Age at study entry | Median (range) (95% CI) | 65.63 (25.56–87.53) (60–68) | 69.63 (50.98–84.68) (64–72) | 0.2259 | 65.87 (25.56–87.53) (62–68) | 70.57 (44–85.11) (65–72) | ||||
| Gender | Female | 27 | 54.0 | 11 | 44.0 | 0.414 | 38 | 50.67 | 17 | 45.95 |
| Male | 23 | 46.0 | 14 | 56.0 | 37 | 49.33 | 20 | 54.05 | ||
| Comorbidities | None | 20 | 40.0 | 17 | 68.0 | 0.116 | 37 | 49.33 | 13 | 35.14 |
| Mild | 19 | 38.0 | 4 | 16.0 | 23 | 30.67 | 18 | 48.65 | ||
| Moderate | 7 | 14.0 | 2 | 8.0 | 9 | 12.0 | 5 | 13.51 | ||
| Severe | 4 | 8.0 | 2 | 8.0 | 6 | 8.0 | 1 | 2.7 | ||
| Localisation primary pancreatic tumour | Head/neck | 25 | 50.0 | 13 | 52.0 | 0.713 | 38 | 50.67 | 20 | 54.05 |
| Body | 16 | 32.0 | 6 | 24.0 | 22 | 29.33 | 10 | 27.03 | ||
| Tail | 9 | 18.0 | 6 | 24.0 | 15 | 20.0 | 6 | 16.22 | ||
| Biopsy confirmed cancer | No | 1 | 2.0 | 0 | 0.0 | 1.000 | 1 | 1.33 | 0 | 0.0 |
| Yes | 49 | 98.0 | 25 | 100.0 | 74 | 98.67 | 37 | 100.0 | ||
| Type of pancreatic cancer | Adenocarcinoma | 44 | 88.0 | 20 | 80.0 | 0.064 | 64 | 85.33 | 33 | 89.19 |
| NET | 6 | 12.0 | 2 | 8.0 | 8 | 10.67 | 2 | 5.41 | ||
| Other | 0 | 0.0 | 3 | 12.0 | 3 | 4.0 | 2 | 5.41 | ||
| Differentiation (if NET) | Grade 1 | 1 | 2.0 | 0 | 0.0 | 1.000 | 1 | 1.33 | 1 | - |
| Grade 2 | 3 | 6.0 | 1 | 4.0 | 4 | 5.33 | 0 | - | ||
| Grade 3 | 2 | 4.0 | 1 | 4.0 | 3 | 4.0 | 1 | - | ||
| Not NET | 44 | 88.0 | 23 | 92.0 | 67 | 89.33 | 35 | - | ||
| Ki 67 (if NET) | Median (range) (ki67) | 10 (2–80) (0–56.45) | 17.5 (3–32) (0–100) | 0.7777 | 10 (2–80) (0.12–45.38) | 14 (1–27) (0–100) | ||||
| Functional (if NET) | Yes | 0 | 0.0 | 0 | 0.0 | n/a | 0 | 0.0 | 0 | - |
| No | 6 | 12.0 | 2 | 8.0 | 8 | 10.67 | 2 | - | ||
| Not NET | 44 | 88.0 | 23 | 92.0 | 67 | 89.33 | 35 | - | ||
| ECOG PS at study entry | 0 | 7 | 14.0 | 9 | 36.0 | 0.230 | 16 | 21.33 | 6 | 16.22 |
| 1 | 28 | 56.0 | 11 | 44.0 | 39 | 52.0 | 21 | 56.76 | ||
| 2 | 12 | 24.0 | 5 | 20.0 | 17 | 22.67 | 7 | 18.92 | ||
| 3 | 2 | 4.0 | 0 | 0.0 | 2 | 2.67 | 3 | 8.11 | ||
| 4 | 1 | 2.0 | 0 | 0.0 | 1 | 1.33 | 0 | 0.0 | ||
| Stage at study entry | Localised | 0 | - | 0 | - | 0 | - | 1 | 2.7 | |
| Locally advanced | 16 | 32.0 | 7 | 28.0 | 0.723 | 23 | 30.67 | 18 | 48.65 | |
| Metastatic | 34 | 68.0 | 18 | 72.0 | 52 | 69.33 | 18 | 48.65 | ||
| Treatment and outcomes | ||||||||||
| Treatment intention | Palliative | 50 | 100.0 | 25 | 100.0 | n/a | 75 | 100.0 | 37 | 100.0 |
| Did patient received systemic treatment | No | 13 | 26.0 | 4 | 16.0 | 0.386 | 17 | 22.67 | 11 | 29.73 |
| Yes | 37 | 74.0 | 21 | 84.0 | 58 | 77.34 | 26 | 70.27 | ||
| Line of treatment | First-line | 35 | 70.0 | 21 | 84.0 | 0.263 | 56 | 74.67 | 25 | 67.57 |
| Other line | 2 | 4.0 | 0 | 0.0 | 2 | 2.67 | 1 | 2.7 | ||
| None | 13 | 26.0 | 4 | 16.0 | 17 | 22.67 | 11 | 29.73 | ||
| Type of systemic treatment | Gemcitabine | 6 | 12.0 | 3 | 12.0 | 0.834 | 9 | 12.0 | 7 | 18.92 |
| FOLFIRINOX | 9 | 18.0 | 6 | 24.0 | 15 | 20.0 | 5 | 13.51 | ||
| GemCap | 5 | 10.0 | 4 | 16.0 | 9 | 12.0 | 7 | 18.92 | ||
| GemNabPaclitaxel | 6 | 12.0 | 4 | 16.0 | 10 | 13.33 | 5 | 13.51 | ||
| Sunitinib | 0 | 0.0 | 1 | 4.0 | 1 | 1.33 | 0 | 0.0 | ||
| SSA | 2 | 4.0 | 0 | 0.0 | 2 | 2.67 | 1 | 2.7 | ||
| TemCap | 3 | 6.0 | 0 | 0.0 | 3 | 4.0 | 0 | 0.0 | ||
| Carboplatin and Etoposide | 1 | 2.00 | 1 | 4.0 | 2 | 2.67 | 0 | 0.0 | ||
| Other * | 5 | 10.0 | 2 | 8.0 | 7 | 9.33 | 1 | 2.7 | ||
| None | 13 | 26.0 | 4 | 16.0 | 17 | 22.67 | 11 | 29.73 | ||
| * If Other (which) | NUC1031 | 2 | - | 0 | - | n/a | 2 | - | 0 | - |
| CisGem | 0 | - | 1 | - | 1 | - | 1 | - | ||
| FOLFOX + NabPaclitaxel | 3 | - | 1 | - | 4 | - | 0 | - | ||
| Chemotherapy dose intensity (%) | Median (range) (95% CI) | 68.5 (11.1–100) (58.96–77.37) | 80.6 (11.1–100) (55.06–84.28) | 0.8514 | 73.9 (11.1–100) (61.10–76.28); 54 observations | 52.6 (11.1–100) (40.88–58.84) | ||||
| Best radiological response | Progressive disease | 4 | 8.0 | 2 | 8.0 | 0.350 | 6 | 8.0 | 3 | 8.11 |
| Stable disease | 12 | 24.0 | 8 | 32.0 | 20 | 26.67 | 12 | 32.43 | ||
| Partial response | 14 | 28.0 | 3 | 12.0 | 17 | 22.67 | 4 | 10.81 | ||
| Not documented or no treatment received | 20 (13 never started treatment) | 40.0 | 12 | 48.0 | 32 (17 never started treatment) | 42.67 | 18 | 48.65 | ||
| Radiological progression documented at time of last data lock | Yes | 22 | 44.0 | 5 | 20.0 | 0.009 | 27 | 36.0 | 5 | 13.51 |
| Death documented at time of last data lock | Yes | 41 | 82.0 | 10 | 40.0 | <0.001 | 51 | 68.0 | 15 | 40.54 |
| Overall survival (estimated) | Median (95% CI) | 7.39 (1.14–9.95) | 5.85 (4.11-nr) | 0.79784 (log rank) | 7.29 (4.37–9.49) | 4.27 (2.17–nr) | ||||
| Follow-up | Median (range) (95% CI) | 7.23 (0.16–21.59) | 3.28 (0.46–9.03) | 0.014 | 4.50 (0.16–21.55) (5.39–8.01) | 2.49 (0–13.21) (1.75–3.27) | ||||
| Follow-Up Cohort (n = 37) | |||
|---|---|---|---|
| N | % | ||
| Dietitian feedback questionnaire completed (n = 37) | No | 14 | 37.84 |
| Yes | 23 | 62.16 | |
| Test completed (if questionnaire done) | No | 0 | 0.00 |
| Yes | 23 | 100.00 | |
| Dietitian intervention was helpful (if questionnaire done) | 1 (≥) | 19 | 82.61 |
| 2 (significantly) | 4 | 17.39 | |
| 3 (not too much) | 0 | 0.00 | |
| 4 (not at all) | 0 | 0.00 | |
| Not answered | 0 | 0.00 | |
| Dietitian intervention improved my quality of life (if questionnaire done) | 1 (very much) | 7 | 30.43 |
| 2 (significantly) | 10 | 43.48 | |
| 3 (not too much) | 2 | 8.70 | |
| 4 (not at all) | 2 | 8.70 | |
| Not answered | 2 | 8.70 | |
| Dietitian intervention improved my symptoms (if questionnaire done) | 1 (very much) | 6 | 26.09 |
| 2 (significantly) | 7 | 30.43 | |
| 3 (not too much) | 6 | 26.09 | |
| 4 (not at all) | 2 | 8.70 | |
| Not answered | 2 | 8.70 | |
| I would suggest the dietitian input to be available for other patients in the future | Yes | 21 | 91.30 |
| No | 1 | 4.35 | |
| Not answered | 1 | 4.35 | |
| Free text comments |
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnie, L.E.; Shah, D.; Vaughan, K.; Kapacee, Z.A.; McCallum, L.; Abraham, M.; Backen, A.; McNamara, M.G.; Hubner, R.A.; Barriuso, J.; et al. Prospective Observational Study of Prevalence, Assessment and Treatment of Pancreatic Exocrine Insufficiency in Patients with Inoperable Pancreatic Malignancy (PANcreatic Cancer Dietary Assessment—PanDA). Cancers 2023, 15, 2277. https://doi.org/10.3390/cancers15082277
Carnie LE, Shah D, Vaughan K, Kapacee ZA, McCallum L, Abraham M, Backen A, McNamara MG, Hubner RA, Barriuso J, et al. Prospective Observational Study of Prevalence, Assessment and Treatment of Pancreatic Exocrine Insufficiency in Patients with Inoperable Pancreatic Malignancy (PANcreatic Cancer Dietary Assessment—PanDA). Cancers. 2023; 15(8):2277. https://doi.org/10.3390/cancers15082277
Chicago/Turabian StyleCarnie, Lindsay E., Dinakshi Shah, Kate Vaughan, Zainul Abedin Kapacee, Lynne McCallum, Marc Abraham, Alison Backen, Mairéad G. McNamara, Richard A. Hubner, Jorge Barriuso, and et al. 2023. "Prospective Observational Study of Prevalence, Assessment and Treatment of Pancreatic Exocrine Insufficiency in Patients with Inoperable Pancreatic Malignancy (PANcreatic Cancer Dietary Assessment—PanDA)" Cancers 15, no. 8: 2277. https://doi.org/10.3390/cancers15082277
APA StyleCarnie, L. E., Shah, D., Vaughan, K., Kapacee, Z. A., McCallum, L., Abraham, M., Backen, A., McNamara, M. G., Hubner, R. A., Barriuso, J., Gillespie, L., Lamarca, A., & Valle, J. W. (2023). Prospective Observational Study of Prevalence, Assessment and Treatment of Pancreatic Exocrine Insufficiency in Patients with Inoperable Pancreatic Malignancy (PANcreatic Cancer Dietary Assessment—PanDA). Cancers, 15(8), 2277. https://doi.org/10.3390/cancers15082277





