Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications
Abstract
Simple Summary
Abstract
1. Introduction
2. Methodology of Literature Search and Selection
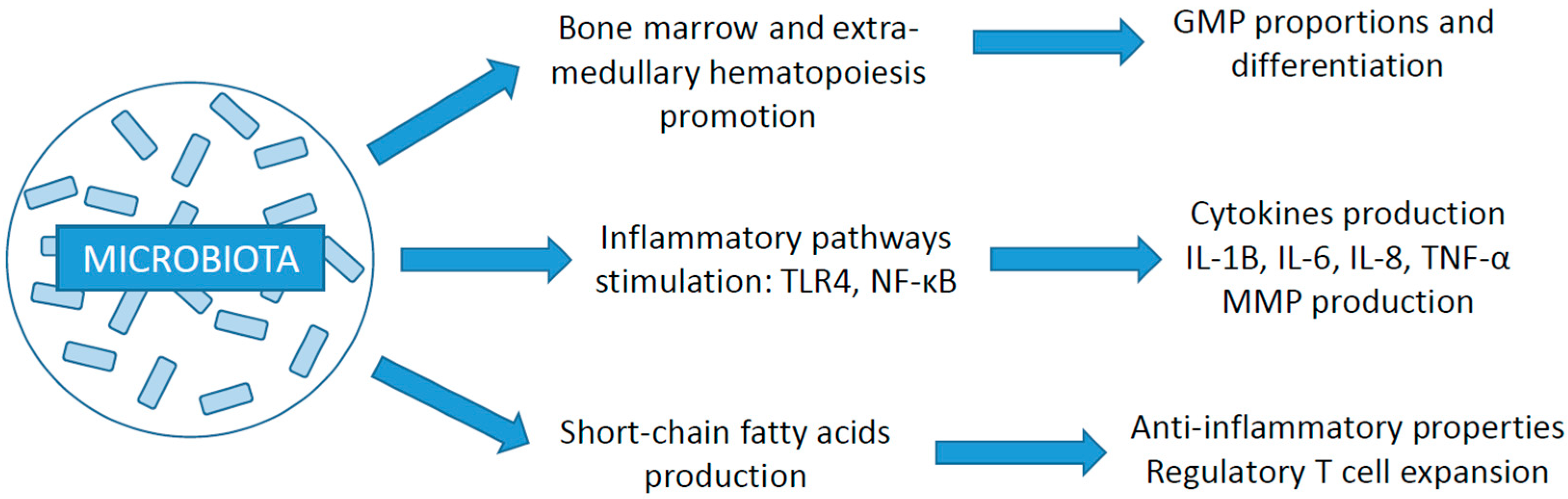
3. The Microbiota and the Immune System
3.1. Microbiota: Definition and Composition
3.2. Relation with the Immune System
3.3. Impact of Gut Microbiota on Cancer Development and ICI Efficacy
3.4. Focus on Akkermansia Muciniphila
3.5. Role of Other Organ-Specific Microbiomes
3.6. Treatment-Induced Microbiome Changes
4. Corticosteroids
4.1. Impact of Immunosuppression on Cancer Development and Microbiota Composition
4.2. Effect on ICI Treatment Efficacy
5. Antibiotics
5.1. Antibiotic-Induced Perturbations of the Microbiota
5.2. Impact of Antibiotics on ICI Response according to Histology
5.3. Specificities under Immunotherapy versus Chemotherapy
5.4. Importance of the Antibiotic Treatment Modality: Timing and Duration
5.5. Importance of the Antibiotic Treatment Modality: Molecule and Spectrum
6. Proton Pump Inhibitors
6.1. PPI-Induced Alterations of the Microbiota
6.2. Impact on ICI Efficacy
6.3. Differences in Histology
6.4. Importance of Timing
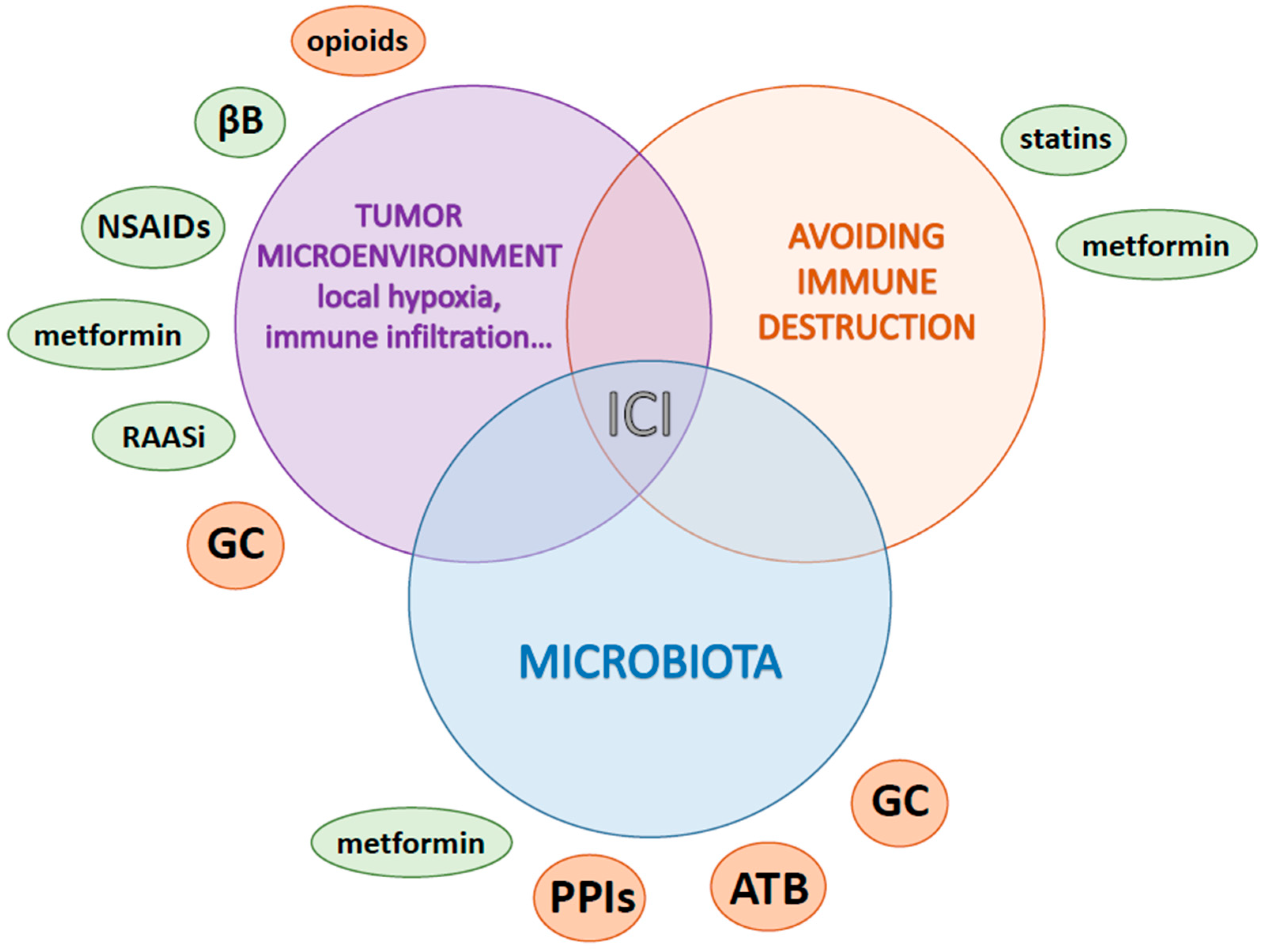
7. Other Medications according to Pathway Alterations
7.1. Metabolism and Hypoxia Lowering: Metformin
7.2. Local Inflammation: Aspirin and Nonsteroidal Anti-Inflammatory Drugs
7.3. Stress and Neuro-Oncology: Beta Blockers
7.4. Microenvironment Remodeling and Immune Modulation
7.4.1. Renin-Angiotensin-Aldosterone System Inhibitors
7.4.2. Opioids
7.4.3. Statins
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune Checkpoint Inhibitors in Melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. In Regulation of Cancer Immune Checkpoints; Xu, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1248, pp. 33–59. ISBN 9789811532658. [Google Scholar]
- Kalfeist, L.; Galland, L.; Ledys, F.; Ghiringhelli, F.; Limagne, E.; Ladoire, S. Impact of Glucocorticoid Use in Oncology in the Immunotherapy Era. Cells 2022, 11, 770. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between Drugs and the Gut Microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Guillaume, C.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Houessinon, A.; et al. Associations between Dysbiosis-Inducing Drugs, Overall Survival and Tumor Response in Patients Treated with Immune Checkpoint Inhibitors. Ther. Adv. Med. Oncol. 2021, 13, 175883592110005. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The Role of the Gut Microbiome in the Healthy Adult Status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef]
- MetaHIT Consortium (Additional Members); Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Pavel, F.M.; Vesa, C.M.; Gheorghe, G.; Diaconu, C.C.; Stoicescu, M.; Munteanu, M.A.; Babes, E.E.; Tit, D.M.; Toma, M.M.; Bungau, S. Highlighting the Relevance of Gut Microbiota Manipulation in Inflammatory Bowel Disease. Diagnostics 2021, 11, 1090. [Google Scholar] [CrossRef]
- Imdad, A.; Nicholson, M.R.; Tanner-Smith, E.E.; Zackular, J.P.; Gomez-Duarte, O.G.; Beaulieu, D.B.; Acra, S. Fecal Transplantation for Treatment of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2018, 2018, CD012774. [Google Scholar] [CrossRef]
- Olivares, M.; Neef, A.; Castillejo, G.; Palma, G.D.; Varea, V.; Capilla, A.; Palau, F.; Nova, E.; Marcos, A.; Polanco, I.; et al. The HLA-DQ2 Genotype Selects for Early Intestinal Microbiota Composition in Infants at High Risk of Developing Coeliac Disease. Gut 2015, 64, 406–417. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.K.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced Diversity of the Intestinal Microbiota during Infancy Is Associated with Increased Risk of Allergic Disease at School Age. J. Allergy Clin. Immunol. 2011, 128, 646–652.e5. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, Inflammation, and the Gut Microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J.; et al. Linking the Human Gut Microbiome to Inflammatory Cytokine Production Capacity. Cell 2016, 167, 1125–1136.e8. [Google Scholar] [CrossRef] [PubMed]
- Lambring, C.B.; Siraj, S.; Patel, K.; Sankpal, U.T.; Mathew, S.; Basha, R. Impact of the Microbiome on the Immune System. Crit. Rev. Immunol. 2019, 39, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut Microbiota Promote Hematopoiesis to Control Bacterial Infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef]
- Tabowei, G.; Gaddipati, G.N.; Mukhtar, M.; Alzubaidee, M.J.; Dwarampudi, R.S.; Mathew, S.; Bichenapally, S.; Khachatryan, V.; Muazzam, A.; Hamal, C.; et al. Microbiota Dysbiosis a Cause of Colorectal Cancer or Not? A Systematic Review. Cureus 2022, 14, e30893. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Ting, N.L.-N.; Lau, H.C.-H.; Yu, J. Cancer Pharmacomicrobiomics: Targeting Microbiota to Optimise Cancer Therapy Outcomes. Gut 2022, 71, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A Defined Commensal Consortium Elicits CD8 T Cells and Anti-Cancer Immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal Microbiota Is Altered in Patients with Colon Cancer and Modified by Probiotic Intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Ikeda, T.; Sakata, S.; Saruwatari, K.; Sato, R.; Iyama, S.; Jodai, T.; Akaike, K.; Ishizuka, S.; Saeki, S.; et al. Association of Probiotic Clostridium Butyricum Therapy with Survival and Response to Immune Checkpoint Blockade in Patients with Lung Cancer. Cancer Immunol. Res. 2020, 8, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1–Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Thomas, A.M.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia Muciniphila Predicts Clinical Response to PD-1 Blockade in Patients with Advanced Non-Small-Cell Lung Cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Desilets, A.; Daillère, R.; Terrisse, S.; Kroemer, G.; Zitvogel, L. Microbiota-Centered Interventions: The Next Breakthrough in Immuno-Oncology? Cancer Discov. 2021, 11, 2396–2412. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Yan, J.; Sheng, L.; Li, H. Akkermansia Muciniphila: Is It the Holy Grail for Ameliorating Metabolic Diseases? Gut Microbes 2021, 13, 1984104. [Google Scholar] [CrossRef]
- Ramírez-Labrada, A.G.; Isla, D.; Artal, A.; Arias, M.; Rezusta, A.; Pardo, J.; Gálvez, E.M. The Influence of Lung Microbiota on Lung Carcinogenesis, Immunity, and Immunotherapy. Trends Cancer 2020, 6, 86–97. [Google Scholar] [CrossRef]
- Hartmann, J.E.; Albrich, W.C.; Dmitrijeva, M.; Kahlert, C.R. The Effects of Corticosteroids on the Respiratory Microbiome: A Systematic Review. Front. Med. 2021, 8, 588584. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Santoni, M.; Ticinesi, A.; Buti, S. The Urinary Microbiome and Anticancer Immunotherapy: The Potentially Hidden Role of Unculturable Microbes. Target. Oncol. 2019, 14, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Burcher, K.M.; Burcher, J.T.; Inscore, L.; Bloomer, C.H.; Furdui, C.M.; Porosnicu, M. A Review of the Role of Oral Microbiome in the Development, Detection, and Management of Head and Neck Squamous Cell Cancers. Cancers 2022, 14, 4116. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.B.; Ahn, J.; Fan, X.; Peters, B.A.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.D.; Ganly, I.; Purdue, M.P.; et al. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.; Harrington, K.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Vokes, E.; Gillison, M.; Even, C.; et al. Abstract CT022: Evaluation of Oral Microbiome Profiling as a Response Biomarker in Squamous Cell Carcinoma of the Head and Neck: Analyses from CheckMate 141. Cancer Res. 2017, 77, CT022. [Google Scholar] [CrossRef]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The Influence of a Short-Term Gluten-Free Diet on the Human Gut Microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.-E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut Microbiota Associations with Common Diseases and Prescription Medications in a Population-Based Cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Ticinesi, A.; Milani, C.; Lauretani, F.; Nouvenne, A.; Mancabelli, L.; Lugli, G.A.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut Microbiota Composition Is Associated with Polypharmacy in Elderly Hospitalized Patients. Sci. Rep. 2017, 7, 11102. [Google Scholar] [CrossRef]
- Gemikonakli, G.; Mach, J.; Zhang, F.; Bullock, M.; Tran, T.; El-Omar, E.; Hilmer, S.N. Polypharmacy with High Drug Burden Index (DBI) Alters the Gut Microbiome Overriding Aging Effects and Is Reversible with Deprescribing. J. Gerontol. Ser. A 2022, 78, 213–222. [Google Scholar] [CrossRef]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Acuna, S.A. Etiology of Increased Cancer Incidence after Solid Organ Transplantation. Transplant. Rev. 2018, 32, 218–224. [Google Scholar] [CrossRef]
- Guba, M.; Graeb, C.; Jauch, K.-W.; Geissler, E.K. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 2004, 77, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.E.; Anver, M.R.; Schechter, S.L.; Bole, G.G. Prolonged Lifespan and High Incidence of Neoplasms in NZB/NZW Mice Treated with Hydrocortisone Sodium Succinate. Kidney Int. 1978, 14, 151–157. [Google Scholar] [CrossRef]
- Chong, P.P.; Koh, A.Y. The Gut Microbiota in Transplant Patients. Blood Rev. 2020, 39, 100614. [Google Scholar] [CrossRef]
- Tourret, J.; Willing, B.P.; Dion, S.; MacPherson, J.; Denamur, E.; Finlay, B.B. Immunosuppressive Treatment Alters Secretion of Ileal Antimicrobial Peptides and Gut Microbiota, and Favors Subsequent Colonization by Uropathogenic Escherichia coli. Transplantation 2017, 101, 74–82. [Google Scholar] [CrossRef]
- Wu, T.; Yang, L.; Jiang, J.; Ni, Y.; Zhu, J.; Zheng, X.; Wang, Q.; Lu, X.; Fu, Z. Chronic Glucocorticoid Treatment Induced Circadian Clock Disorder Leads to Lipid Metabolism and Gut Microbiota Alterations in Rats. Life Sci. 2018, 192, 173–182. [Google Scholar] [CrossRef]
- Huang, E.Y.; Inoue, T.; Leone, V.A.; Dalal, S.; Touw, K.; Wang, Y.; Musch, M.W.; Theriault, B.; Higuchi, K.; Donovan, S.; et al. Using Corticosteroids to Reshape the Gut Microbiome: Implications for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2015, 21, 963–972. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients with Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in Patients with Melanoma and Brain Metastases: An Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Chasset, F.; Pages, C.; Biard, L.; Roux, J.; Sidina, I.; Madelaine, I.; Basset-Seguin, N.; Viguier, M.; Madjlessi-EzrA, N.; Schneider, P.; et al. Single-Center Study under a French Temporary Authorization for Use (TAU) Protocol for Ipilimumab in Metastatic Melanoma: Negative Impact of Baseline Corticosteroids. Eur. J. Dermatol. 2015, 25, 36–44. [Google Scholar] [CrossRef]
- Hendriks, L.E.L.; Henon, C.; Auclin, E.; Mezquita, L.; Ferrara, R.; Audigier-Valette, C.; Mazieres, J.; Lefebvre, C.; Rabeau, A.; Le Moulec, S.; et al. Outcome of Patients with Non–Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J. Thorac. Oncol. 2019, 14, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Kaseb, A.; Wang, Y.; Saeed, A.; Szafron, D.; Jun, T.; Dharmapuri, S.; Naqash, A.R.; Muzaffar, M.; Navaid, M.; et al. Impact of Corticosteroid Therapy on the Outcomes of Hepatocellular Carcinoma Treated with Immune Checkpoint Inhibitor Therapy. J. Immunother. Cancer 2020, 8, e000726. [Google Scholar] [CrossRef]
- Umehara, K.; Yama, K.; Goto, K.; Wakamoto, A.; Hatsuyama, T.; Honjo, O.; Saikai, T.; Fujita, A.; Sato, H. Effect of Systemic Corticosteroid Therapy on the Efficacy and Safety of Nivolumab in the Treatment of Non-Small-Cell Lung Cancer. Cancer Control 2021, 28, 107327482098579. [Google Scholar] [CrossRef]
- Gaucher, L.; Adda, L.; Séjourné, A.; Joachim, C.; Chaby, G.; Poulet, C.; Liabeuf, S.; Gras-Champel, V.; Masmoudi, K.; Moreira, A.; et al. Impact of the Corticosteroid Indication and Administration Route on Overall Survival and the Tumor Response after Immune Checkpoint Inhibitor Initiation. Ther. Adv. Med. Oncol. 2021, 13, 175883592199665. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Huang, X.; Li, J.; Ma, H.; Zeng, R. Impact of Corticosteroid Use on Outcomes of Non–Small-cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. J. Clin. Pharm. Ther. 2021, 46, 927–935. [Google Scholar] [CrossRef]
- Jessurun, C.A.C.; Hulsbergen, A.F.C.; de Wit, A.E.; Tewarie, I.A.; Snijders, T.J.; Verhoeff, J.J.C.; Phillips, J.G.; Reardon, D.A.; Mekary, R.A.; Broekman, M.L.D. The Combined Use of Steroids and Immune Checkpoint Inhibitors in Brain Metastasis Patients: A Systematic Review and Meta-Analysis. Neuro-Oncology 2021, 23, 1261–1272. [Google Scholar] [CrossRef]
- Korpela, K.; de Vos, W. Antibiotic Use in Childhood Alters the Gut Microbiota and Predisposes to Overweight. Microb. Cell 2016, 3, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early Infancy Microbial and Metabolic Alterations Affect Risk of Childhood Asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Collins, J.J.; Relman, D.A. Antibiotics and the Gut Microbiota. J. Clin. Investig. 2014, 124, 4212–4218. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLoS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Simin, J.; Fornes, R.; Liu, Q.; Olsen, R.S.; Callens, S.; Engstrand, L.; Brusselaers, N. Antibiotic Use and Risk of Colorectal Cancer: A Systematic Review and Dose–Response Meta-Analysis. Br. J. Cancer 2020, 123, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Gagnière, J. Gut Microbiota Imbalance and Colorectal Cancer. World J. Gastroenterol. 2016, 22, 501. [Google Scholar] [CrossRef]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative Association of Antibiotics on Clinical Activity of Immune Checkpoint Inhibitors in Patients with Advanced Renal Cell and Non-Small-Cell Lung Cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774. [Google Scholar] [CrossRef]
- Tinsley, N.; Zhou, C.; Tan, G.; Rack, S.; Lorigan, P.; Blackhall, F.; Krebs, M.; Carter, L.; Thistlethwaite, F.; Graham, D.; et al. Cumulative Antibiotic Use Significantly Decreases Efficacy of Checkpoint Inhibitors in Patients with Advanced Cancer. Oncologist 2020, 25, 55–63. [Google Scholar] [CrossRef]
- Cortellini, A.; Di Maio, M.; Nigro, O.; Leonetti, A.; Cortinovis, D.L.; Aerts, J.G.; Guaitoli, G.; Barbieri, F.; Giusti, R.; Ferrara, M.G.; et al. Differential Influence of Antibiotic Therapy and Other Medications on Oncological Outcomes of Patients with Non-Small Cell Lung Cancer Treated with First-Line Pembrolizumab versus Cytotoxic Chemotherapy. J. Immunother. Cancer 2021, 9, e002421. [Google Scholar] [CrossRef]
- Cortellini, A.; Ricciuti, B.; Facchinetti, F.; Alessi, J.V.M.; Venkatraman, D.; Dall’Olio, F.G.; Cravero, P.; Vaz, V.R.; Ottaviani, D.; Majem, M.; et al. Antibiotic-Exposed Patients with Non-Small-Cell Lung Cancer Preserve Efficacy Outcomes Following First-Line Chemo-Immunotherapy. Ann. Oncol. 2021, 32, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Naeem, M.; Pinato, D.J. Concomitant Medications and Immune Checkpoint Inhibitor Therapy for Cancer: Causation or Association? Hum. Vaccines Immunother. 2021, 17, 55–61. [Google Scholar] [CrossRef]
- Lurienne, L.; Cervesi, J.; Duhalde, L.; de Gunzburg, J.; Andremont, A.; Zalcman, G.; Buffet, R.; Bandinelli, P.-A. NSCLC Immunotherapy Efficacy and Antibiotic Use: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2020, 15, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X.; Wang, H.; Ge, W.; Cao, D. The Association between Antibiotics Use and Outcome of Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2020, 149, 102909. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, J.; Wu, S.; Xie, X. The Impact of Antibiotics on Efficacy of Immune Checkpoint Inhibitors in Malignancies: A Study Based on 44 Cohorts. Int. Immunopharmacol. 2021, 92, 107303. [Google Scholar] [CrossRef]
- Luo, Z.; Hao, S.; Li, Y.; Cheng, L.; Zhou, X.; Gunes, E.G.; Liu, S.; Chen, J. The Negative Effect of Antibiotics on RCC Patients with Immunotherapy: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 1065004. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumar, A.; Parikh, K.; Anwar, A.; Knoll, B.M.; Puccio, C.; Chun, H.; Fanucchi, M.; Lim, S.H. Use of Broad-Spectrum Antibiotics Impacts Outcome in Patients Treated with Immune Checkpoint Inhibitors. OncoImmunology 2018, 7, e1507670. [Google Scholar] [CrossRef]
- Chalabi, M.; Cardona, A.; Nagarkar, D.R.; Dhawahir Scala, A.; Gandara, D.R.; Rittmeyer, A.; Albert, M.L.; Powles, T.; Kok, M.; Herrera, F.G. Efficacy of Chemotherapy and Atezolizumab in Patients with Non-Small-Cell Lung Cancer Receiving Antibiotics and Proton Pump Inhibitors: Pooled Post Hoc Analyses of the OAK and POPLAR Trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef]
- Medik, Y.B.; Zhou, Y.; Kahn, L.M.; Patel, B.; Babcock, R.L.; Chrisikos, T.T.; Wan, X.; Dyevoich, A.; Ajami, N.J.; Wargo, J.A.; et al. Outcome of Concurrent Treatment with A-CTLA4 and Metronidazole in Murine Model of Colon Adenocarcinoma. J. Clin. Oncol. 2021, 39, e14566. [Google Scholar] [CrossRef]
- Cortellini, A.; Facchinetti, F.; Derosa, L.; Pinato, D.J. Antibiotic Exposure and Immune Checkpoint Inhibitors in Patients With NSCLC: The Backbone Matters. J. Thorac. Oncol. 2022, 17, 739–741. [Google Scholar] [CrossRef]
- Monge, B.M.C.; Xie, C.; Mabry-Hrones, D.; Wood, B.J.; Steinberg, S.M.; Kleiner, D.E.; Greten, T.F. Phase II Study of Nivolumab (Anti-PD1), Tadalafil, and Oral Vancomycin in Patients with Refractory Primary Hepatocellular Carcinoma or Liver Dominant Metastatic Cancer from Colorectal or Pancreatic Cancers. J. Clin. Oncol. 2020, 38, TPS4656. [Google Scholar] [CrossRef]
- Nehra, A.K.; Alexander, J.A.; Loftus, C.G.; Nehra, V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin. Proc. 2018, 93, 240–246. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The Impact of Proton Pump Inhibitors on the Human Gastrointestinal Microbiome. Clin. Lab. Med. 2014, 34, 771–785. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton Pump Inhibitors Alter the Composition of the Gut Microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Leonard, J.; Marshall, J.K.; Moayyedi, P. Systematic Review of the Risk of Enteric Infection in Patients Taking Acid Suppression. Am. J. Gastroenterol. 2007, 102, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Goto, Y.; Sakata, S.; Imamura, K.; Minemura, A.; Oka, K.; Hayashi, A.; Jodai, T.; Akaike, K.; Anai, M.; et al. Clostridium Butyricum Therapy Restores the Decreased Efficacy of Immune Checkpoint Blockade in Lung Cancer Patients Receiving Proton Pump Inhibitors. OncoImmunology 2022, 11, 2081010. [Google Scholar] [CrossRef]
- Hopkins, A.M.; Kichenadasse, G.; Karapetis, C.S.; Rowland, A.; Sorich, M.J. Concomitant Proton Pump Inhibitor Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Clin. Cancer Res. 2020, 26, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Stokes, W.A.; Behera, M.; Jiang, R.; Gutman, D.; Giuste, F.; Burns, A.; Sebastian, N.; Ramalingam, S.; Sukhatme, V.; Lowe, M.C.; et al. Association of Proton Pump Inhibitors with Survival in Veterans with Non-Small Cell Lung Cancer Receiving Immunotherapy. J. Clin. Oncol. 2021, 39, e18729. [Google Scholar] [CrossRef]
- Baek, Y.; Kang, E.J.; Hong, S.; Park, S.; Kim, J.H.; Shin, J. Survival Outcomes of Patients with Nonsmall Cell Lung Cancer Concomitantly Receiving Proton Pump Inhibitors and Immune Checkpoint Inhibitors. Int. J. Cancer 2022, 150, 1291–1300. [Google Scholar] [CrossRef]
- Peng, K.; Chen, K.; Teply, B.A.; Yee, G.C.; Farazi, P.A.; Lyden, E.R. Impact of Proton Pump Inhibitor Use on the Effectiveness of Immune Checkpoint Inhibitors in Advanced Cancer Patients. Ann. Pharmacother. 2022, 56, 377–386. [Google Scholar] [CrossRef]
- Failing, J.J.; Finnes, H.D.; Kottschade, L.A.; Allred, J.B.; Markovic, S.N. Effects of Commonly Used Chronic Medications on the Outcomes of Ipilimumab Therapy in Patients with Metastatic Melanoma. Melanoma Res. 2016, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, C.; Yao, J.; Ge, Y.; An, G. The Association between Proton Pump Inhibitors Use and Clinical Outcome of Patients Receiving Immune Checkpoint Inhibitors Therapy. Int. Immunopharmacol. 2020, 88, 106972. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, H.; Mao, H.; Tong, J.; Yang, M.; Yan, X. An Up-To-Date Investigation Into the Correlation Between Proton Pump Inhibitor Use and the Clinical Efficacy of Immune Checkpoint Inhibitors in Advanced Solid Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 753234. [Google Scholar] [CrossRef]
- Chen, B.; Yang, C.; Dragomir, M.P.; Chi, D.; Chen, W.; Horst, D.; Calin, G.A.; Li, Q. Association of Proton Pump Inhibitor Use with Survival Outcomes in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Ther. Adv. Med. Oncol. 2022, 14, 175883592211117. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.; Merza, N.; Rahim, M.; Qatani, A.; Varughese, T.; Mohammad, A.; Masood, F.; Reza, F.; Shucenwan; Almas, T.; et al. Impact of Proton-Pump Inhibitors on the Efficacy of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Ann. Med. Surg. 2022, 78, 103752. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Martin-Castillo, B.; Menendez, J.A. Metformin as an Archetype Immuno-Metabolic Adjuvant for Cancer Immunotherapy. OncoImmunology 2019, 8, e1633235. [Google Scholar] [CrossRef]
- Shin, N.-R.; Lee, J.-C.; Lee, H.-Y.; Kim, M.-S.; Whon, T.W.; Lee, M.-S.; Bae, J.-W. An Increase in the Akkermansia Spp. Population Induced by Metformin Treatment Improves Glucose Homeostasis in Diet-Induced Obese Mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Munoz, L.E.; Huang, L.; Bommireddy, R.; Sharma, R.; Monterroza, L.; Guin, R.N.; Samaranayake, S.G.; Pack, C.D.; Ramachandiran, S.; Reddy, S.J.C.; et al. Metformin Reduces PD-L1 on Tumor Cells and Enhances the Anti-Tumor Immune Response Generated by Vaccine Immunotherapy. J. Immunother. Cancer 2021, 9, e002614. [Google Scholar] [CrossRef]
- Cha, J.-H.; Yang, W.-H.; Xia, W.; Wei, Y.; Chan, L.-C.; Lim, S.-O.; Li, C.-W.; Kim, T.; Chang, S.-S.; Lee, H.-H.; et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e7. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, C.; Zhang, K.; Lin, C.; Wu, F.; Tang, X.; Wu, D.; Dou, Y.; Han, R.; Wang, Y.; et al. Metformin Combining PD-1 Inhibitor Enhanced Anti-Tumor Efficacy in STK11 Mutant Lung Cancer Through AXIN-1-Dependent Inhibition of STING Ubiquitination. Front. Mol. Biosci. 2022, 9, 780200. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Mercado, R.R.; Shirai, K. Efficacy of Metformin in Combination with Immune Checkpoint Inhibitors (Anti-PD-1/Anti-CTLA-4) in Metastatic Malignant Melanoma. J. Immunother. Cancer 2018, 6, 64. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Dragnev, K.; Sarwar, T.; Shirai, K. Clinical Outcomes in Non-Small-Cell Lung Cancer Patients Receiving Concurrent Metformin and Immune Checkpoint Inhibitors. Lung Cancer Manag. 2019, 8, LMT11. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.H.; Jung, E.H.; Kim, S.; Suh, K.J.; Lee, J.Y.; Kim, J.; Kim, J.W.; Lee, J.; Kim, Y.J.; et al. The Effect of Metformin or Dipeptidyl Peptidase 4 Inhibitors on Clinical Outcomes in Metastatic Non-small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors. Thorac. Cancer 2023, 14, 52–60. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front. Immunol. 2021, 11, 586760. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Ninomiya, T.; Hotta, K.; Kozuki, T.; Toyooka, S.; Okada, H.; Fujiwara, T.; Udono, H.; Kiura, K. Study Protocol: Phase-Ib Trial of Nivolumab Combined With Metformin for Refractory/Recurrent Solid Tumors. Clin. Lung Cancer 2018, 19, e861–e864. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Murray, L.J.; Cardwell, C.R.; McShane, C.M.; McMenamin, Ú.C.; Cantwell, M.M. PTGS2 (Cyclooxygenase-2) Expression and Survival among Colorectal Cancer Patients: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1490–1497. [Google Scholar] [CrossRef]
- Pi, C.; Jing, P.; Li, B.; Feng, Y.; Xu, L.; Xie, K.; Huang, T.; Xu, X.; Gu, H.; Fang, J. Reversing PD-1 Resistance in B16F10 Cells and Recovering Tumour Immunity Using a COX2 Inhibitor. Cancers 2022, 14, 4134. [Google Scholar] [CrossRef] [PubMed]
- Riesenberg, B.P.; Ansa-Addo, E.A.; Gutierrez, J.; Timmers, C.D.; Liu, B.; Li, Z. Cutting Edge: Targeting Thrombocytes to Rewire Anticancer Immunity in the Tumor Microenvironment and Potentiate Efficacy of PD-1 Blockade. J. Immunol. 2019, 203, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kumar, P.; Adashek, J.J.; Skelton, W.P.; Li, J.; Vosoughi, A.; Chahoud, J.; Manley, B.J.; Spiess, P.E. Adding Cyclooxygenase Inhibitors to Immune Checkpoint Inhibitors Did Not Improve Outcomes in Metastatic Renal Cell Carcinoma. Cells 2022, 11, 2505. [Google Scholar] [CrossRef]
- Aiad, M.; Tahir, A.; Fresco, K.; Prenatt, Z.; Ramos-Feliciano, K.; Walia, J.; Stoltzfus, J.; Albandar, H.J. Does the Combined Use of Aspirin and Immunotherapy Result in Better Outcomes in Non-Small Cell Lung Cancer Than Immunotherapy Alone? Cureus 2022, 14, e25891. [Google Scholar] [CrossRef] [PubMed]
- Kanai, O.; Ito, T.; Saito, Z.; Yamamoto, Y.; Fujita, K.; Okamura, M.; Hashimoto, M.; Nakatani, K.; Sawai, S.; Mio, T. Effect of Cyclooxygenase Inhibitor Use on Immunotherapy Efficacy in Non-small Cell Lung Cancer. Thorac. Cancer 2021, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated Analysis of Concomitant Medications and Oncological Outcomes from PD-1/PD-L1 Checkpoint Inhibitors in Clinical Practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Chen, S.; Li, Z.; Chen, J.; Li, W. The Effect of Concomitant Use of Statins, NSAIDs, Low-Dose Aspirin, Metformin and Beta-Blockers on Outcomes in Patients Receiving Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. OncoImmunology 2021, 10, 1957605. [Google Scholar] [CrossRef]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of Cancer: The Role of β-Adrenergic Receptor Signaling in Various Tumor Environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef]
- Yan, X.; Liu, P.; Li, D.; Hu, R.; Tao, M.; Zhu, S.; Wu, W.; Yang, M.; Qu, X. Novel Evidence for the Prognostic Impact of β-Blockers in Solid Cancer Patients Receiving Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2022, 113, 109383. [Google Scholar] [CrossRef]
- Fjæstad, K.Y.; Rømer, A.M.A.; Goitea, V.; Johansen, A.Z.; Thorseth, M.-L.; Carretta, M.; Engelholm, L.H.; Grøntved, L.; Junker, N.; Madsen, D.H. Blockade of Beta-Adrenergic Receptors Reduces Cancer Growth and Enhances the Response to Anti-CTLA4 Therapy by Modulating the Tumor Microenvironment. Oncogene 2022, 41, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta Blocker Use Correlates with Better Overall Survival in Metastatic Melanoma Patients and Improves the Efficacy of Immunotherapies in Mice. OncoImmunology 2018, 7, e1405205. [Google Scholar] [CrossRef] [PubMed]
- Mellgard, G.; Patel, V.G.; Zhong, X.; Joshi, H.; Qin, Q.; Wang, B.; Parikh, A.; Jun, T.; Alerasool, P.; Garcia, P.; et al. Effect of Concurrent Beta-Blocker Use in Patients Receiving Immune Checkpoint Inhibitors for Advanced Solid Tumors. J. Cancer Res. Clin. Oncol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Guzner, A.; Wainwright, D.A.; Mohindra, N.A.; Chae, Y.K.; Behdad, A.; Villaflor, V.M. The Impact of Beta Blockers on Survival Outcomes in Patients With Non–Small-Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, e57–e62. [Google Scholar] [CrossRef]
- Gandhi, S.; Pandey, M.R.; Attwood, K.; Ji, W.; Witkiewicz, A.K.; Knudsen, E.S.; Allen, C.; Tario, J.D.; Wallace, P.K.; Cedeno, C.D.; et al. Phase I Clinical Trial of Combination Propranolol and Pembrolizumab in Locally Advanced and Metastatic Melanoma: Safety, Tolerability, and Preliminary Evidence of Antitumor Activity. Clin. Cancer Res. 2021, 27, 87–95. [Google Scholar] [CrossRef]
- Nakamura, K.; Yaguchi, T.; Ohmura, G.; Kobayashi, A.; Kawamura, N.; Iwata, T.; Kiniwa, Y.; Okuyama, R.; Kawakami, Y. Involvement of Local Renin-Angiotensin System in Immunosuppression of Tumor Microenvironment. Cancer Sci. 2018, 109, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Cheng, T.; Lin, J.; Zhang, L.; Zheng, J.; Liu, Y.; Xie, G.; Wang, B.; Yuan, Y. Local Angiotensin II Contributes to Tumor Resistance to Checkpoint Immunotherapy. J. Immunother. Cancer 2018, 6, 88. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Chen, I.X.; Tong, R.; Ng, M.R.; Martin, J.D.; Naxerova, K.; Wu, M.W.; Huang, P.; Boucher, Y.; Kohane, D.S.; et al. Reprogramming the Microenvironment with Tumor-Selective Angiotensin Blockers Enhances Cancer Immunotherapy. Proc. Natl. Acad. Sci. USA 2019, 116, 10674–10680. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin Inhibition Enhances Drug Delivery and Potentiates Chemotherapy by Decompressing Tumour Blood Vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, P.V.; Adib, E.; Weise, N.; Curran, C.; Stewart, T.; Freeman, D.; Nassar, A.H.; Abou Alaiwi, S.; Bakouny, Z.; McGregor, B.A.; et al. Impact of Renin-Angiotensin System Inhibitors on Outcomes in Patients with Metastatic Renal Cell Carcinoma Treated with Immune-Checkpoint Inhibitors. Clin. Genitourin. Cancer 2022, 20, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Michielin, O.; Quinaglia, T.; Zlotoff, D.A.; Zubiri, L.; Gilman, H.K.; Supraja, S.; Merkely, B.; Muller, V.; Sullivan, R.J.; et al. Renin–Angiotensin–Aldosterone System Inhibitors and Survival in Patients with Hypertension Treated with Immune Checkpoint Inhibitors. Eur. J. Cancer 2022, 163, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Medjebar, S.; Truntzer, C.; Perrichet, A.; Limagne, E.; Fumet, J.-D.; Richard, C.; Elkrief, A.; Routy, B.; Rébé, C.; Ghiringhelli, F. Angiotensin-Converting Enzyme (ACE) Inhibitor Prescription Affects Non-Small-Cell Lung Cancer (NSCLC) Patients Response to PD-1/PD-L1 Immune Checkpoint Blockers. OncoImmunology 2020, 9, 1836766. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, R.A.; Metselaar-Albers, M.; Engels, F. Concomitant Use of Analgesics and Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: A Pharmacodynamics Perspective. Eur. J. Pharmacol. 2021, 906, 174284. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, L.; Song, B. Impact of Opioid Analgesics on the Efficacy of Immune Checkpoint Inhibitors in a Lung Cancer Population. BMC Pulm. Med. 2022, 22, 431. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Tamiya, A.; Matsuda, Y.; Adachi, Y.; Enomoto, T.; Azuma, K.; Kouno, S.; Tokoro, A.; Atagi, S. Opioids Impair Nivolumab Outcomes: A Retrospective Propensity Score Analysis in Non-Small-Cell Lung Cancer. BMJ Support. Palliat. Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Cirillo, A.; Pomati, G.; Cerbelli, B.; Scagnoli, S.; Roberto, M.; Gelibter, A.; Mammone, G.; Calandrella, M.L.; Cerbelli, E.; et al. The Role of Opioids in Cancer Response to Immunotherapy. J. Transl. Med. 2021, 19, 119. [Google Scholar] [CrossRef]
- Mao, Z.; Jia, X.; Jiang, P.; Wang, Q.; Zhang, Y.; Li, Y.; Fu, X.; Jiao, M.; Jiang, L.; Liu, Z.; et al. Effect of Concomitant Use of Analgesics on Prognosis in Patients Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 861723. [Google Scholar] [CrossRef] [PubMed]
- Shwe, T.H.; Pothacharoen, P.; Phitak, T.; Wudtiwai, B.; Kongtawelert, P. Atorvastatin Attenuates Programmed Death Ligand-1 (PD-L1) Induction in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 8755. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.-J.; Lee, C.-H.; Bae, J.-H.; Park, J.-M.; Park, S.-S.; Baek, M.-C. Atorvastatin Enhances the Efficacy of Immune Checkpoint Therapy and Suppresses the Cellular and Extracellular Vesicle PD-L1. Pharmaceutics 2022, 14, 1660. [Google Scholar] [CrossRef]
- Lim, W.-J.; Lee, M.; Oh, Y.; Fang, X.-Q.; Lee, S.; Lim, C.-H.; Park, J.; Lim, J.-H. Statins Decrease Programmed Death-Ligand 1 (PD-L1) by Inhibiting AKT and β-Catenin Signaling. Cells 2021, 10, 2488. [Google Scholar] [CrossRef] [PubMed]
- Cantini, L.; Pecci, F.; Hurkmans, D.P.; Belderbos, R.A.; Lanese, A.; Copparoni, C.; Aerts, S.; Cornelissen, R.; Dumoulin, D.W.; Fiordoliva, I.; et al. High-Intensity Statins Are Associated with Improved Clinical Activity of PD-1 Inhibitors in Malignant Pleural Mesothelioma and Advanced Non-Small Cell Lung Cancer Patients. Eur. J. Cancer 2021, 144, 41–48. [Google Scholar] [CrossRef]
- Rossi, A.; Filetti, M.; Taurelli Salimbeni, B.; Piras, M.; Rizzo, F.; Giusti, R.; Marchetti, P. Statins and Immunotherapy: Togetherness Makes Strength The Potential Effect of Statins on Immunotherapy for NSCLC. Cancer Rep. 2021, 4, e1368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Tian, J.; Sui, L.; Chen, X. Concomitant Statins and the Survival of Patients with Non-Small-Cell Lung Cancer Treated with Immune Checkpoint Inhibitors: A Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Buti, S.; Bersanelli, M.; Perrone, F.; Tiseo, M.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; et al. Effect of Concomitant Medications with Immune-Modulatory Properties on the Outcomes of Patients with Advanced Cancer Treated with Immune Checkpoint Inhibitors: Development and Validation of a Novel Prognostic Index. Eur. J. Cancer 2021, 142, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dussart, C.; Decaux-Tramoni, B.; Quesada, S.; Thomas, Q.D.; Benzerouale, O.; Nicolas, E.; Fiteni, F. Combinaisons d’inhibiteurs de points de contrôle immunitaires en oncologie: état de l’art et perspectivesCombination strategies for checkpoint inhibition: Current practices and perspectives. Bull. Cancer, 2023; in press. [Google Scholar] [CrossRef]
| Author (Year) | Type of Cancer | ICI | GC Regimen and Indication | n Patients/Total (%) | Compared Arms | ORR [CI 95%] | PFS [CI 95%] | OS [CI 95%] |
|---|---|---|---|---|---|---|---|---|
| Margolin (2012) [54] | Melanoma with BM | Ipi | Systemic GC for symptomatic BM | 21/72 (29) | No statistical comparison | - | 1.3 m vs. 2.7 m * | 3.7 m vs. 7 m * |
| Chasset (2015) [55] | Melanoma | Ipi | ≥10 mg pred baseline multiple indications | 12/45 (27) | Overall | - | - | 4 m vs. 11 m HR: 5.82 [2.45, 13.8], p < 0.001 |
| Among BM | - | - | 4 m vs. 7 m, p = 0.043 | |||||
| Arbour (2018) [51] | NSCLC | Multiple | ≥10 mg pred baseline multiple indications | 90/640 (14) | Overall | 7% vs. 18%, p = 0.05 | HR: 1.31 [1.03, 1.67], p = 0.03 | HR: 1.66 [1.28, 2.16], p < 0.001 |
| Scott (2018) [52] | NSCLC | Nivo | (A) ≥10 mg pred in first 30 d | 25/210 (12) | A vs. non A | - | - | HR: 2.30 [1.27, 4.16], p = 0.006 |
| (B) ≥10 mg pred for irAEs | 31/210 (15) | B vs. non B | - | - | 16.1 m vs. 10.5 m, p = 0.50 | |||
| Hendriks (2019) [56] | NSCLC BM+ or BM− prospective | Multiple | (A) GC at baseline (overall) | 141/1025 (14) | A vs. non A | - | HR: 1.31 [1.07, 1.62], p = 0.01 | HR: 1.46 [1.16, 1.84], p = 0.001 |
| (B) GC at baseline among BM+ | 69/255 (27) | B vs. BM + non B | - | HR: 2.78 [1.90, 4.08], p < 0.001 | HR: 2.37 [1.54, 3.63], p < 0.001 | |||
| Ricciuti (2019) [53] | NSCLC | Multiple | (A) ≥10 mg pred baseline: SC | 56/640 (10) | A + B vs. C | 10.8% vs. 19.7%, p = 0.04 | 2.0 m vs. 3.4 m HR: 1.36 [1.08, 1.73], p = 0.01 | 4.9 m vs. 11.2 m HR: 1.68 [1.30, 2.17], p < 0.001 |
| (B) ≥10 mg pred baseline: non SC | 27/640 (4) | A vs. C | 6.1% vs. 19.7%, p = 0.01 | 1.4 m vs. 3.4 m HR: 1.87 [1.43, 2.45], p < 0.001 | 2.2 m vs. 11.2 m HR: 2.38 [1.78, 3.19], p = 0.001 | |||
| (C) 0 to <10 mg pred baseline | 557/640 (86) | B vs. C | 22.2% vs. 19.7% * | 4.6 m vs. 3.4 m HR: 0.77 [0.50, 1.19], p = 0.24 | 10.7 m vs. 11.2 m HR: 0.93 [0.59, 1.48], p = 0.77 | |||
| Pinato (2020) [57] | HCC | Multiple | (A) ≥10 mg pred baseline | 14/304 (5) | A vs. B + C | p = 0.62 | 6.7 m vs. 5.8 m, p = 0.37 | 10.4 m vs. 12.2 m, p = 0.48 |
| (B) ≥10 mg pred during ICI | 64/304 (20) | B vs. A + C | p = 0.62 | 8.1 m vs. 10.7 m, p = 0.46 | 16.1 m vs. 11.7 m, p = 0.25 | |||
| (C) no GC at all | 226/304 (75) | Among A + B: SC vs. non | SC: “more ICI refractory” p = 0.05 | 1.6 m vs. 8.8 m, p < 0.01 | 4.9 m vs. 15.4 m, p = 0.05 | |||
| Umehara (2021) [58] | NSCLC | Nivo | (A) GC at baseline multiple indications | 12/109 (11) | A vs. C | 8% vs. 14%, p = 0.03 | 0.9 m vs. 3.3 m, p < 0.01 | 2.2 m vs. 11.9 m, p < 0.01 |
| (B) GC during ICI: irAE or no | 19/109 (17) 14/109 (13) | B vs. C | 36% vs. 14%, p = 0.02 | 3.6 m vs. 3.3 m, p = 0.23 | 12.5 m vs. 11.9 m, p = 0.72 | |||
| (C) no GC at all | 64/109 (59) | Among B: irAE vs. no | 47% vs. 21%, p = 0.13 | 5.1 m vs. 2.2 m, p = 0.17 | 13.5 m vs. 12.5 m, p = 0.30 | |||
| Gaucher (2021) [59] | Multiple | Multiple | (A) concomitant GC: irAE | 21/372 (6) | A + B vs. C | 16.9% vs. 27.8%, p = 0.025 | - | HR: 1.25 [0.91, 1.71], p = 0.16 |
| (B) concomitant GC: other indication | 56/372 (15) | A vs. B + C | 28.6% vs. 27.8%, p = 0.30 | - | HR: 1.04 [0.56, 1.95], p = 0.90 | |||
| (C) no GC at all | 295/372 (79) | B vs. A + C | 12.5% vs. 27.8%, p = 0.008 | - | HR: 1.34 [1.05, 2.03], p = 0.046 |
| Author (Year) | Type of Cancer | ICI | GC Regimen | GC Indication | n Studies (n Patients) | PFS: HR, [95% CI] | OS: HR, [95% CI] |
|---|---|---|---|---|---|---|---|
| Petrelli (2020) [42] | Multiple | Multiple | Multiple | Overall | 16 (4045) | 1.34 [1.02, 1.76], p = 0.03 | 1.54 [1.24, 1.91], p = 0.0001 |
| SC | 3 (836) | - | 2.5 [1.41, 4.43], p < 0.01 | ||||
| BM | 3 (1164) | - | 1.51 [1.22, 1.87], p < 0.01 | ||||
| irAEs | 9 (926) | - | 1.08 [0.79, 1.49], p = 0.62 | ||||
| Zhang (2021) [60] | NSCLC | Multiple | Multiple | Overall | 14 (5461) | 1.69 [1.51, 2.04], p = 0.009 | 1.82 [1.51, 2.18], p = 0.003 |
| SC | NS | 1.55 [1.26, 1.92] * | 1.94 [1.57, 2.20] * | ||||
| BM | NS | 1.56 [1.23, 1.97] * | 1.62 [1.41, 1.86] * | ||||
| Jessurun (2021) [61] | Multiple with BM | Multiple | Multiple | Overall BM | 15 (1113) | 2.00 [1.37, 2.91], p = 0.007 | 1.84 [1.22, 2.77], p = 0.007 |
| NSCLC BM | 4 (505) | - | 2.43 [0.38, 15.77] * | ||||
| Melanoma BM | NS | - | 1.67 [1.49, 1.87] * |
| Author (Year) | Type of Cancer | Treatment | ATB Regimen | n Patients/Total (%) | Subgroup | ORR [CI 95%] | PFS [CI 95%] | OS [CI 95%] |
|---|---|---|---|---|---|---|---|---|
| Routy (2018) [26] | NSCLC RCC UC | ICI (multiple) | Within 2 m before or 1 m after ICI initiation | 69/246 (28) | Overall | - | 3.5 m vs. 4.1 m, p = 0.017 | 11.5 m vs. 20.6 m, p < 0.001 |
| 37/140 (26) | NSCLC | - | 3.5 m vs. 2.8 m, p = 0.57 | 8.3 m vs. 15.3 m, p = 0.001 | ||||
| 20/67 (30) | RCC | - | 4.3 m vs. 7.4 m, p = 0.012 | 23.4 m vs. 27.9 m, p = 0.15 | ||||
| 12/42 (29) | UC | - | 1.8 m vs. 4.3 m, p = 0.049 | 11.5 m vs. NR, p = 0.098 | ||||
| 68/239 (28) | Validation cohort | - | 2.6 m vs. 3.6 m, p = 0.24 | 9.8 m vs. 21.9 m, p = 0.002 | ||||
| Derosa (2018) [68] | RCC NSCLC | ICI (multiple) +/− TT | Within 30 d before ICI initiation | 16/121 (13) | RCC | 13% vs. 26%, p < 0.01 | 1.9 m vs. 7.4 m HR: 3.1 [1.4, 6.9], p < 0.01 | 17.3 m vs. 30.6 m HR: 3.5 [1.1, 10.8], p = 0.03 |
| 48/239 (20) | NSCLC | 13% vs. 23%, p = 0.26 | 1.9 m vs. 3.8 m HR: 1.5 [1.0, 2.2], p = 0.03 | 7.9 m vs. 24.6 m HR: 4.4 [2.6, 7.7], p < 0.01 | ||||
| Pinato (2019) [69] | Multiple | ICI (multiple) | (A) within 30 d before ICI initiation | 29/196 (15) | A: overall | 8% vs. 43%, p < 0.01 | - | 2 m vs. 26 m HR: 3.4 [1.9, 6.1], p < 0.01 |
| 6/107 (6) | A: NSCLC | - | - | 2.5 m vs. 26 m HR: 9.3 [4.3, 19], p < 0.01 | ||||
| 17/38 (45) | A: melanoma | - | - | 3.9 m vs. 14 m HR: 7.5 [1.7, 30.4], p < 0.001 | ||||
| (B) concomitant | 68/196 (35) | B | - | - | NR vs. 26 m HR: 0.9 [0.5, 1.4], p = 0.65 | |||
| Tinsley (2019) [70] | Multiple | ICI (multiple) | Between 2 w before and 6 w after ICI initiation: single vs. cumulative course | 92/291 (32) | Overall | 3.1 m vs. 6.3 m HR: 1.40 [1.03, 1.92], p = 0.033 | 10.4 m vs. 21.7 m HR: 1.47 [1.04, 2.11], p = 0.033 | |
| NS | Single course | - | 3.7 m vs. 6.3 m HR: 1.32 [0.80, 2.20], p = 0.28 | 17.7 m vs. 21.7 m HR: 1.26 [0.82, 1.93], p = 0.29 | ||||
| NS | Cumulative courses | - | 2.8 m vs. 6.3 m HR: 2.63 [1.25, 6.13], p = 0.026 | 6.3 m vs. 21.7 m HR: 1.90 [1.18, 2.08], p = 0.009 | ||||
| Cortellini (2021) [71] | NSCLC TPS > 50% | Pembro (A) vs. CT (B) | Within 30 d before initiation | (A) 131/950 (14) | A | 30.1% vs. 44.4% OR: 0.57 [0.37, 0.87], p = 0.01 | 4.8 m vs. 7.5 m HR: 1.29 [1.04, 1.59], p = 0.02 | 10.4 m vs. 17.2 m HR: 1.42 [1.13, 1.79], p = 0.002 |
| (B) 87/595 (15) | B | 33.3% vs. 37.6%, p = 0.50 | 5.1 m vs. 5.9 m HR: 1.10 [0.86, 1.40], p = 0.42 | 13.2 m vs. 14.9 m HR: 1.23 [0.95, 1.61], p = 0.11 | ||||
| Cortellini (2021) [72] | NSCLC | CT + ICI 1st line | (A) within 30 d before ICI initiation | 47/302 (16) | A: overall | 42.6% vs. 57.4% OR: 0.83 [0.42, 1.64], p = 0.60 | 5.6 m vs. 6.3 m HR: 1.12 [0.76, 1.63], p = 0.56 | 11.2 m vs. 16.6 m HR: 1.42 [0.91, 2.22], p = 0.12 |
| 17/302 (6) | A: ATB > 7 d | - | HR: 1.31 [0.73, 2.31] * | HR: 1.76 [0.83, 3.71] * | ||||
| 20/302 (7) | A: ATB IV | - | HR: 1.67 [0.88, 3.17] * | HR: 1.44 [0.69, 3.09] * | ||||
| 12/76 (16) | A: among TPS > 50% | - | 7.0 m vs. 9.8 m HR: 1.48 [0.62, 3.53], p = 0.37 | 16.3 m vs. 25.9 m HR: 1.61 [0.57, 4.49], p = 0.36 | ||||
| (B) concomitant | 117/302 (39) | B | - | HR: 1.20 [0.89, 1.63], p = 0.22 | HR: 1.29 [0.91, 1.84], p = 0.15 |
| Author (Year) | Type of Cancer | ICI | ATB Regimen | Subgroup | n Studies (n Patients) | ORR: OR, [95% CI] | PFS: HR, [95% CI] | OS: HR, [95% CI] |
|---|---|---|---|---|---|---|---|---|
| Lurienne (2020) [74] | NSCLC | Multiple +/− CT or TT | Multiple | Overall | 23 (2208) | - | 1.47 [1.13, 1.90], p < 0.01 | 1.69 [1.25, 2.29], p < 0.01 |
| Within 90 d before ICI | 4 (708) | - | 1.56 [0.78, 3.13] * | 2.49 [0.95, 6.51] * | ||||
| Within 60 d before ICI | 3 (325) | - | 2.00 [1.34, 2.99] * | 2.94 [1.60, 5.40] * | ||||
| 60 d before to 60 d after ICI initiation | 12 (1624) | - | 1.72 [1.30, 2.27] * | 2.04 [1.49, 2.79] * | ||||
| Within 90 d before ICI and during ICI treatment | 5 (645) | - | 0.97 [0.44, 2.17] * | 1.24 [0.56, 2.76] * | ||||
| Xu (2020) [75] | Multiple | Multiple +/− CT or TT | Multiple | Overall | 20 (4331) | - | 1.53 [1.30, 1.79], p < 0.01 | 1.90 [1.55, 2.34], p < 0.01 |
| NSCLC | 12 (1880) | - | 1.39 [1.16, 1.67], p < 0.01 | 1.73 [1.26, 2.38], p < 0.01 | ||||
| NSCLC: ATB within 6 m before ICI | 3 (515) | - | - | 1.81 [0.91, 3.63], p = 0.09 | ||||
| NSCLC: ATB within 1 m before ICI or during ICI | 7 (1365) | - | - | 2.09 [1.31, 3.32], p = 0.002 | ||||
| Wu (2021) [76] | Multiple | Multiple +/− CT or TT | Multiple | Overall | 44 (12492) | 0.61 [0.42, 0.90], p = 0.01 | 1.18 [1.11, 1.25], p < 0.01 | 1.20 [1.15, 1.25], p < 0.01 |
| RCC | 4 (367) | 0.30 [0.14, 0.67], p < 0.01 | 1.29 [1.19, 1.40], p < 0.01 | 1.12 [1.01, 1.25], p = 0.028 | ||||
| NSCLC | 9 (1276) | 0.84 [0.50, 1.42], p = 0.51 | 1.13 [1.04, 1.23], p < 0.01 | 1.26 [1.15, 1.38], p < 0.01 | ||||
| Melanoma | 2 (182) | 0.37 [0.12, 1.10], p = 0.07 | 1.75 [1.34, 2.29], p < 0.01 | 1.36 [1.06, 1.75], p = 0.017 | ||||
| ATB before ICI | 8 (1060) | 0.47 [0.32, 0.71], p < 0.01 | 1.23 [1.14, 1.32], p < 0.01 | 1.39 [1.26, 1.54], p < 0.01 | ||||
| ATB before or after ICI within 1 m | 9 (1010) | 0.63 [0.32, 1.26], p = 0.19 | 1.16 [1.06, 1.26], p < 0.01 | 1.17 [1.10, 1.24], p < 0.01 | ||||
| Luo (2022) [77] | RCC | Multiple +/− TT | Multiple | Overall | 6 (1104) | 0.58 [0.41, 0.84] * | 1.77 [1.25, 2.50] * | 1.69 [1.34, 2.12] * |
| 60 d before to 60 d after ICI initiation | 4 (NS) | - | 1.86 [1.18, 2.95] * | 1.66 [1.30, 2.11] * | ||||
| Within 90 d before ICI | 2 (NS) | - | 1.75 [0.40, 7.55] * | 0.66 [0.13, 3.35] * |
| Author (Year) | Type of Cancer | Treatment | PPI Regimen | n Patients/Total (%) | Subgroup | ORR [CI 95%] | PFS [CI 95%] | OS [CI 95%] |
|---|---|---|---|---|---|---|---|---|
| Hopkins (2020) [88] | UC | Atezo or CT (IMvigor210, 211) | Within 30 d before (A) or after (B) ICI initiation | 286/896 (32) | Pooled atezo | OR: 0.51 [0.32, 0.82], p = 0.006 | HR: 1.38 [1.18, 1.62], p < 0.001 | HR: 1.52 [1.27, 1.83], p < 0.001 |
| 185/464 (40) | CT | OR: 1.04 [0.64, 1.71], p = 0.2 | HR: 1.11 [0.89, 1.37], p = 0.35 | HR: 1.16 [0.93, 1.47], p = 0.2 | ||||
| 272 | Atezo + PPI: A vs. B | - | HR: 0.71 [0.49, 1.03], p = 0.07 | HR: 0.65 [0.44, 0.97], p = 0.033 | ||||
| Chalabi (2020) [79] | NSCLC | Atezo or CT (OAK, POPLAR) | Within 30 d before or after ICI initiation | 234/757 (31) | Pooled atezo | - | 1.9 m vs. 2.8 m HR: 1.30 [1.10, 1.53], p = 0.001 | 9.6 m vs. 14.5 m HR: 1.45 [1.20, 1.75], p < 0.001 |
| 260/755 (34) | CT | 3.5 m vs. 3.9 m HR: 1.04 [0.89, 1.22] * | 9.1 m vs. 11.0 m HR: 1.17 [0.97, 1.40] * | |||||
| 74/757 (10) | Pooled atezo: PPI + ATB | - | 1.7 m vs. 2.8 m HR: 1.48 [1.16, 1.91] * | 6.6 m vs. 14.1 m HR: 1.89 [1.42, 2.52] * | ||||
| Stokes (2021) [89] | NSCLC (US veterans) | ICI (multiple) +/− CT | Within 90 d of ICI initiation | 2159/3634 (59) | Overall | - | - | 10 m vs. 10 m HR: 0.98 [0.90, 1.06], p = 0.59 |
| Baek (2022) [90] | NSCLC | Multiple (L2+) | Within 30 d before ICI initiation (new users or not) | 936/2963 (32) | Overall | - | - | 5.1 m vs. 8.0 m HR: 1.28 [1.13, 1.46], p < 0.001 |
| 168/2963 (6) | New PPI users | - | - | 3.8 m vs. 8.4 m HR: 1.64 [1.25, 2.17], p < 0.001 | ||||
| Peng (2022) [91] | Multiple | Nivo or pembro +/− CT | Within 30 d before or after ICI initiation | 89/233 (38) | Overall | HR: 1.05 [0.76, 1.45] * | HR: 1.22 [0.80, 1.86] * | |
| 46/117 (39) | NSCLC | - | HR: 1.33 [0.86, 2.04] * | HR: 1.18 [0.79, 2.01] * |
| Author (Year) | Type of Cancer | ICI | PPI Regimen | Subgroup | n Studies (n Patients) | PFS: HR, [95% CI] | OS: HR, [95% CI] |
|---|---|---|---|---|---|---|---|
| Li (2020) [93] | Multiple | Multiple | Prior or within | Overall | 7 (1482) | 0.90 [0.66, 1.23], p = 0.51 | 1.05 [0.79, 1.40], p = 0.73 |
| NSCLC | 4 (NS) | 1.17 [1.05, 1.31], p = 0.006 | 1.24 [1.00, 1.55], p = 0.05 | ||||
| Melanoma | 2 (NS) | 0.50 [0.28, 0.91], p = 0.02 | 0.67 [0.30, 1.52], p = 0.34 | ||||
| Liu (2022) [94] | Multiple | Multiple +/− TT | Prior or within | Overall | 17 (9978) | 1.19 [0.98, 1.44] * | 1.29 [1.10, 1.50] * |
| 30 d before and/or after ICI initiation | 5 (NS) | 1.23 [1.06, 1.43], p = 0.007 | 1.38 [1.18, 1.62], p < 0.001 | ||||
| Any time after ICI initiation | 7 (NS) | 0.72 [0.40, 1.28], p = 0.18 | 1.27 [1.01, 1.59], p = 0.038 | ||||
| NSCLC | 6 (NS) | 1.27 [1.10, 1.47], p = 0.001 | 1.19 [0.92, 1.54], p = 0.18 | ||||
| Melanoma | 2 (NS) | 0.48 [0.25, 0.90], p = 0.023 | 0.70 [0.31, 1.56], p = 0.38 | ||||
| Chen (2022) [95] | Multiple | Multiple | Prior or within | Overall | 33 (15,957) | 1.30 [1.17, 1.46], p < 0.001 | 1.31 [1.19, 1.44], p < 0.001 |
| At baseline | 3 (2194) | 1.29 [1.15, 1.44], p < 0.001 | 1.43 [1.21, 1.69], p < 0.001 | ||||
| Within 60 d before ICI initiation | 20 (7742) | 1.33 [1.20, 1.48], p < 0.001 | 1.35 [1.22, 1.51], p < 0.001 | ||||
| After ICI initiation | 12 (>4900) | 1.19 [0.65, 2.17], p = 0.58 | 1.18 [0.98, 1.41], p = 0.09 | ||||
| NSCLC | 13 (9200) | 1.33 [1.17, 1.51], p < 0.001 | 1.33 [1.15, 1.54], p < 0.001 | ||||
| RCC | 6 (433) | 1.11 [0.89, 1.38], p = 0.37 | 1.01 [0.77, 1.33], p = 0.92 | ||||
| Dar (2022) [96] | NSCLC | Multiple +/− TT | NS | Overall | 4 (2940) | 1.31 [1.17, 1.47], p < 0.01 | 1.46 [1.27, 1.67], p < 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colard-Thomas, J.; Thomas, Q.D.; Viala, M. Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications. Cancers 2023, 15, 2276. https://doi.org/10.3390/cancers15082276
Colard-Thomas J, Thomas QD, Viala M. Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications. Cancers. 2023; 15(8):2276. https://doi.org/10.3390/cancers15082276
Chicago/Turabian StyleColard-Thomas, Julien, Quentin Dominique Thomas, and Marie Viala. 2023. "Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications" Cancers 15, no. 8: 2276. https://doi.org/10.3390/cancers15082276
APA StyleColard-Thomas, J., Thomas, Q. D., & Viala, M. (2023). Comedications with Immune Checkpoint Inhibitors: Involvement of the Microbiota, Impact on Efficacy and Practical Implications. Cancers, 15(8), 2276. https://doi.org/10.3390/cancers15082276





