Simple Summary
Inflammation of the colon (colitis) can increase the risk of developing colorectal cancers. Gut microbiota may play a role in the development of this cancer. One potential way to prevent this cancer is to manipulate the community of bacteria in the colon. In a study using mice, we found that transferring the bacteria from the bedding of healthy mice to those with colitis increased the presence of Akkermansia, which may help reduce inflammation in the colon. Meanwhile, untreated mice had an increase in other types of bacteria, such as Anaeroplasma and Alistipes, which may contribute to inflammation. These findings suggest that certain types of bacteria may help reduce inflammation in the colon and prevent cancer.
Abstract
Chronic inflammation of the colon (colitis) is a known risk factor for inflammatory-driven colorectal cancers (id-CRCs), and intestinal microbiota has been implicated in the etiology of id-CRCs. Manipulation of the microbiome is a clinically viable therapeutic approach to limiting id-CRCs. To understand the microbiome changes that occur over time in id-CRCs, we used a mouse model of id-CRCs with the treatment of azoxymethane (AOM) and dextran sodium sulfate (DSS) and measured the microbiome over time. We included cohorts where the microbiome was restored using cage bedding swapping and where the microbiome was depleted using antibiotics to compare to untreated animals. We identified consistent increases in Akkermansia in mice receiving horizontal microbiome transfer (HMT) via cage bedding swapping, while the control cohort had consistent longitudinal increases in Anaeroplasma and Alistipes. Additionally, fecal lipocalin-2 (Lcn-2), a marker of intestinal inflammation, was elevated in unrestored animals compared to restored and antibiotic-treated counterparts following HMT. These observations suggest a potential role for Akkermansia, Anaeroplasma, and Alistipes in regulating colonic inflammation in id-CRCs.
1. Importance
Preclinical models of inflammation-driven colorectal cancer (id-CRC) have shown that the microbiome regulates intestinal inflammation. However, most of these studies have relied on short periods of longitudinal microbiome sampling and a single mouse strain to conclude bacterial genera influencing id-CRC pathology. Here we utilized a mouse model of id-CRC. We show that horizontal microbiome transfer (HMT) through fecal restoration with healthy animals can longitudinally alter the microbiome of mice from Balb/c and C57BL/6 backgrounds compared to untreated controls. The longitudinal compositional changes showed consistent enrichments of Akkermansia in HMT-treated animals and Anaeroplasma and Alistipes in untreated controls. Additionally, we show that HMT can transiently decrease the concentration of inflammatory stool marker lipocalin-2 (Lcn-2) compared to untreated controls. Further investigation is warranted through additional preclinical and clinical investigations into how Akkermansia, Alistipes, and Anaeroplasma potentially alter epithelial inflammation and how they can be manipulated.
2. Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death worldwide [1]. Inflammation of the colon has been shown to contribute to the development of CRC through various mechanisms, such as changing the local and systemic cytokine milieu through alteration of NF-kB and STAT3 signaling pathways promoting growth and survival [2,3]. Additionally, colitis increases the likelihood of induced gene mutations of the colonic epithelium and epigenetic alterations, providing conditions for growth [4,5,6]. Clinical observation also suggests a correlation between colonic neoplasia and increased mucosal prostaglandin, implicating a link between inflammation and CRC progression. Inflammatory- or colitis-driven CRC presents a unique microenvironment in which microbial dysbiosis has been implicated in driving or supporting tumor initiation and progression [7,8,9]. Dysbiosis of the microbiota can be loosely defined by a reduction in total microbial diversity and reduced abundances of commensal bacteria such as Firmicutes and Bacteroidetes. These commensal bacteria can provide the host with short-chain fatty acids such as butyrate, which has been shown to suppress colonic inflammation [10,11]. This is coupled with the increased abundance of pathobionts, such as members of the family Enterobacteriaceae [12,13]. However, these studies have yet to reach a consensus.
Many factors complicate determining causative associations between the microbiome and CRC pathogenesis. These factors include environmental, inter-individual, and dietary differences, variable experimental conditions (such as sampling parameters, i.e., stool or mucosa), longitudinal fluctuations, and lack of batch-to-batch reproducibility [14,15,16]. The advantages of using experimental mouse models include their homogenous genetic background, consistent environment and diet, and ability to perform longitudinal sampling, which can be temporally controlled. The most widely used model to induce colonic inflammation consists of administering a single intraperitoneal (IP) injection of azoxymethane (AOM), which is a carcinogen that causes DNA damage. This is followed by dextran sodium sulfate (DSS) administration via drinking water, which induces colonic inflammation [17]. This AOM/DSS model induces epithelial injury and promotes CRC development, mimicking human id-CRC [18].
Some studies have reported microbial composition and diversity alterations following AOM/DSS induction [19,20,21]. Others have shown that unique fecal compositions can be transplanted into germ-free mice and are associated with different tumor outcomes through the alteration of T-cell responses in AOM/DSS-induced models [22]. Fecal microbiota transplantation (FMT) of a CRC patient’s stool into APCmin/+ mice was also shown to lead to the accumulation of more intestinal tumors compared to healthy donor controls, suggesting a role for the tumor microbiome in CRC progression [23]. Restoration of fecal microbiota via FMT is an interesting approach to understanding microbial ecology. It is being explored in many colon diseases, including recurrent Clostridiodes difficile infection [24], irritable bowel disease, [25,26] and CRC [27]. Further, it has been shown that cohousing of animals can modulate the microbiome between mice through horizontal microbiome transfer (HMT) [28].
The longitudinal effects on the gut microbiota following HMT in AOM/DSS models of CRC have not been explored. In this study, we longitudinally analyzed the fecal microbiome of C57BL/6 and BALB/c mice that underwent swapping of used bedding from untreated mice to achieve HMT. In one group of mice, we homogenized the microbiome by swapping in bedding from untreated mice (restored microbiome) and compared it to mice that did not have their bedding swapped (unrestored microbiome). We observed significant and different early to late changes between restored and unrestored groups, consistent between C57BL/6 and BALB/c species following AOM/DSS administration.
3. Materials and Methods
3.1. Mice and Animal Husbandry
Three to four-week-old female C57BL/6 and BALB/c mice purchased from Jackson Laboratory were used in this study. On arrival at the animal facility, mice were randomly assigned to restored or unrestored groups (n = 5/group) and received an ear punch. Bedding on days 1–3 was pooled between experimental groups to normalize the baseline microbiome between restored and unrestored groups. Following baseline normalization, mice underwent AOM/DSS treatment, and stool samples were longitudinally collected 3x/week for 18 weeks. In week 18, mice were sacrificed and dissected. Mouse colons were fixed in 10% neutral buffered formalin and processed for histological analysis. All mice were housed in specific pathogen-free conditions in fully autoclaved cages for the duration of the experiment. The Institutional Animal Care and Use Committee approved all animal studies.
3.2. AOM/DSS Tumor Induction
Depending on the experimental design, female 3–4-week-old BALB/c or C57BL/6 mice were injected intraperitoneally with 10 mg azoxymethane (Sigma) per kg mouse weight. After five days, mice were treated with two cycles (1 week/cycle) of 2% dextran sodium sulfate (MP Biomedicals M.W. = 36,000–50,000) for five days through their drinking water, followed by a supply of regular water for the duration of the experiment. Mice were sacrificed in week 18 following AOM induction. Depending on the experimental setup, the antibiotics cocktail of 0.05 g/L of vancomycin (Boynton Health), 0.025 g/L of metronidazole (Boynton Health), and 0.2 g/L of streptomycin (Sigma) was administered in the drinking water of mice following the second cycle of DSS administration. During necropsy, colons were dissected and flushed with PBS to visualize tumors in the colon. Following visualization of tumors, tumor tissues were fixed in 10% neutral buffered formalin and sectioned.
3.3. Fecal Restoration and Stool Sample Collection
Mouse stool samples were longitudinally collected 3 times weekly for 18 weeks. Stool sample collection was conducted as follows: Mice were separated into individual autoclaved SPF cages lacking food and bedding and left for 1 h. On average, 3–5 fecal pellets were collected per mouse. Depending on group assignment, mice were removed from individual cages and returned to their respective cages. Stool samples were pooled by experimental groups and stored at −80 °C until processing for DNA isolation. During weeks 8, 9, and 10, the bedding from normal mice without AOM/DSS was distributed to cages for fecal restoration by HMT.
3.4. DNA Isolation and 16S rRNA Amplicon Sequencing and Analysis
Mouse fecal pellets of approximately 0.1 g were homogenized and processed using the DNA PowerSoil kit (Qiagen) with the QIACube automated platform following the manufacturer’s inhibitor removal technology (IRT) protocol. The V5-V6 hypervariable regions were amplified and sequenced using the BSF784/1064R primer set [29]. Sterile water and no template controls were carried through amplification and sequencing and did not produce amplicons. Samples underwent paired-end sequencing on a single run of a MiSeq platform (Illumina, Inc., San Diego, CA, USA) at a length of 301 nucleotides (nt). All sequence processing was performed using Mothur software version 1.35.1 [30]. Raw fastq files were trimmed to 150 nt sequences to remove low-quality regions and were paired-end merged using the fastq-join function [31]. Reads were further trimmed to remove reads with mean quality scores < 35 over a 50 nt window, homopolymers > 8 nt, ambiguous bases, and more that 2 nt mismatches to primer sequences. High-quality sequences were aligned to the SILVA database (version 132) [32] and subjected to a 2% pre-clustering step to remove likely errors [33]. Chimeric sequences were identified and removed using the UCHIME package [34]. Operational Taxonomic Units (OTUs) were assigned at 99% similarity using the complete-linkage clustering algorithm, and taxonomy was assigned using version 16 of the Ribosomal Database Project [35]. The raw fastq files are available at https://www.ncbi.nlm.nih.gov/sra/PRJNA915052.
3.5. Histopathological Analysis
Mouse colons were fixed overnight in 10% neutral buffered formalin at the experimental endpoint. Following fixation, solutions were changed to 70% ethanol before submission to the clinical and translational sciences institute at the University of Minnesota. Tissues were paraffin-embedded, and two sections per mouse were stained with hematoxylin and eosin. Board-certified pathologists reviewed tissue sections for dysplasia and acute and chronic inflammation.
3.6. Quantification of Fecal Lipocalin-2 (Lcn-2) by ELISA
Initially, 0.2 g of frozen fecal samples was reconstituted in PBS with 0.1% Tween 20 (100 mg/mL). Subsequently, 3 mm glass beads were added to the mixture and vortexed 3 times in 30 s bursts to obtain a homogenous fecal suspension. The samples were then centrifuged for 10 min at 12,000 rpm at 4 °C. Clear supernatants were collected and stored at −20 °C prior to analysis. Lcn-2 levels were approximated using a Duoset murine Lcn-2 ELISA kit (R&D Systems, Minneapolis, MN) following the manufacturer’s protocol. Four time points before and after fecal restoration (W2, W7, W10, W15) were selected for fecal Lcn-2 analysis. Samples for each experimental condition were run in triplicate. Calibration standard curves (500 pg/mL–7.81 pg/mL) were used to extrapolate Lcn-2 concentrations and averaged across experimental time points and conditions. Reagent blank 1.0% BSA in PBS was used as a negative control.
3.7. Statistics
Alpha diversity of microbial communities was assessed using Shannon and Chao1 indices. Bray–Curtis dissimilarity matrices [36] were calculated and used for ordination through principal coordinates analysis [37]. These matrices were also used to determine differences in beta diversity by analysis of similarity (ANOSIM) [38]. Bonferroni corrections for multiple comparisons were performed for ANOSIM analyses. The SplinectomeR R package [39] and its permuspliner functions were used to assess significant differences in the longitudinal abundances of bacterial genera between restored and unrestored groups using standard 999 permutations. One-way unpaired equal variance Student’s T-tests were conducted to assess statistically significant differences in concentrations of fecal Lcn-2. All statistics were evaluated at α = 0.05 unless otherwise corrected for multiple comparisons.
4. Results
4.1. Microbial Community Composition and Diversity between Restored and Unrestored Groups Are Longitudinally Different
We longitudinally compared the microbial community changes following AOM/DSS administration between unrestored (no HMT) and restored (HMT from normal Balb/c fecal bedding swap once weekly during weeks 8, 9, and 10) groups. Mouse fecal pellets were longitudinally collected three times weekly for 17 weeks from Balb/c mice in unrestored n = 5 and restored n = 5 groups (Figure 1A). The overall mean percentage of the relative abundance of bacterial genera in restored and unrestored groups was relatively stable and similar (Figure 1B). Longitudinally, the microbial communities in mice with restored microbiota had significantly lower Shannon index values than unrestored mice (p = 0.035; Figure 1C); however, no significant differences were observed in Chao1 (p = 0.084; Figure 1D).
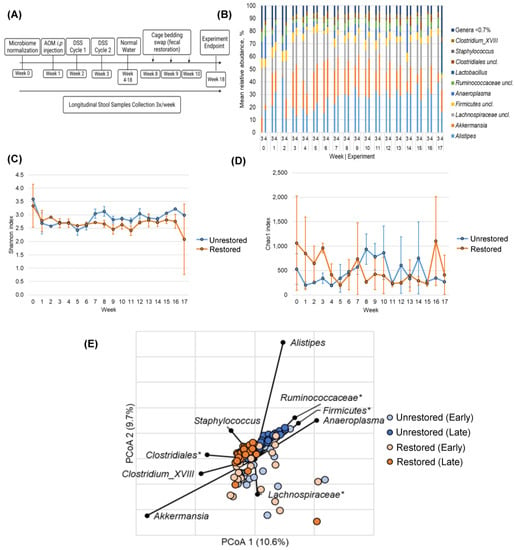
Figure 1.
Microbiome composition and diversity in Balb/c mice. (A) Percentage of the relative abundance of bacterial genera in Balb/c mice following AOM/DSS administration between (3) unrestored and (4) restored groups. Genera that accounted for <0.7% of mean sequence reads were consolidated for clarity. (B) Longitudinal Shannon index between no restoration (blue) and restoration (orange). Error bars reflect standard deviation. (C) Longitudinal Chao1 index between no restoration (blue) and restoration (orange). (D) Principal coordinates analysis (E) Correlated taxa associated with ordination position on either axis (Spearman p > 0.05) are overlaid on the PCoA plot. (*) Indicates genera that could not be further classified.
Next, we sought to investigate the difference in community composition between restored and unrestored groups using Bray–Curtis dissimilarity. Samples in both treatment groups were considered as “early” (weeks 0–7; before microbiota restoration) and “late” (weeks 8–18; at the time of and following HMT). Principal coordinates analysis (PCoA) showed significant early and late changes in the microbiome of Balb/c mice treated with AOM/DSS (Figure 1E). The unrestored group had significant early and late changes (ANOSIM R = 0.499, p < 0.001) within the group (Figure 1E). Similarly, we also observed significant early and late changes in the restored group (ANOSIM R = 0.261, p < 0.001). Microbiota composition also differed significantly between experimental groups among the later “late” samples, but not samples collected prior to week 8 (R = 0.530 and 0.081, p < 0.001 and p = 0.01; Bonferroni-corrected α = 0.008; Figure 1E)
Spearman correlation was used to identify bacterial genera associated with the late differences in the microbial community between unrestored and restored groups (Figure 1E). Akkermansia, Clostridium_clade XVIII, and members of the order Clostridiales that could not be further classified were compositionally correlated with the restored group (Figure 1E). This was an interesting observation as Akkermansia and Clostridium_XVIII have been implicated as having probiotic properties and are associated with reduced intestinal inflammation through production of short-chain fatty acids such as butyrate [40,41].
In contrast, microbial communities in the restored group were correlated with greater relative abundances of the bacterial genera Alistipes, Anaeroplasma, and Ruminococcaceae, as shown in Figure 1E. We were unable to classify Rumminococcaceae further in our 16S rRNA analysis. Interestingly, Ruminococcus gnavus is associated with Crohn’s disease, likely through the ability of R. gnavus to synthesize and secrete glucorhamnan polysaccharides, which can lead to TNFα secretion by dendritic cells [42]. Additionally, the increased abundance of potentially pathogenic Alistipes is consistent with recent investigations of AOM/DSS-induced cancer [43]. These data suggest that HMT altered the microbial composition in the restored group compared to the unrestored group, potentially shifting toward a less inflammatory community composition.
4.2. Longitudinal Differences in the Microbiome between Restored and Unrestored Groups Is Significant
Next, we sought to investigate longitudinal changes in bacterial genera between restored and unrestored groups using the SplinectomeR permuspliner function, which can be used to assess longitudinal microbiome data [39]. Significant longitudinal differences were observed between groups among the genera Alistipes (p = 0.032), Akkermansia (p = 0.001), Anaeroplasma (p = 0.032), Ruminococaceae (further unclassified) (p = 0.001), Clostridiales (further unclassified) (p = 0.001), and Clostridium_XVIII (p = 0.001) (Supplemental Figure S1A–F). Significant longitudinal differences in the Shannon index (p = 0.035) between groups were also observed (Supplemental Figure S1G). Notably, the longitudinal significant difference between unrestored and restored is a somewhat conflicting result as previous work has shown that alpha diversity has decreased longitudinally in AOM/DSS models of intestinal inflammation.
The longitudinal abundance of Akkermansia was significantly different between unrestored and restored groups following fecal restoration (Supplemental Figure S1B). For Akkermansia, the mean relative abundance was significantly greater in the restored group compared to the unrestored in weeks 9–17. This was also reflected in our Bray–Curtis dissimilarity and Spearman correlation analysis in Figure 1E. Many bacterial genera identified from Spearman correlations were also longitudinally altered between unrestored and restored groups in SplinectomeR permuspliner analysis.
4.3. Intestinal Inflammation Marker Lipocalin-2 Is Decreased in Restored Balb/c Mice
To assess differences in intestinal inflammation before and after HMT in AOM/DSS-treated animals, we utilized a semi-quantitative sandwich ELISA (R&D systems, Minneapolis, MN) to probe fecal concentrations of lipocalin-2 (Lcn-2) [44,45]. Fecal Lcn-2 concentrations are a non-invasive marker of intestinal inflammation. Two time points were used before HMT (weeks 2 and 7), and two time points post-HMT (weeks 10 and 15) were selected to quantify fecal concentrations of Lcn-2 in restored and unrestored groups. In weeks 2 and 7, we did not observe significant differences in fecal Lcn-2 concentrations (week 2, 136.1 ± 8.0 pg/mL and 123.7 ± 9.8 pg/mL, p = 0.082, n = 3) (week 7, 171.1 ± 21.7 pg/mL and 154.2 ± 17.2 pg/mL, p = 0.154, n = 3) between unrestored and restored animals (Figure 2A). Post-HMT, we did observe significant decreases in fecal Lcn-2 concentrations (week 10, 206.1 ± 20.9 pg/mL and 139.4 ± 17.8 pg/mL, p = 0.007, n = 3) (Week 15, 335.4 ± 16.2 pg/mL and 252.4 ± 27.3 pg/mL, p = 0.005, n = 3) in unrestored and restored animals (Figure 2A). These data suggest that HMT could potentially reduce Lcn-2 concentrations and could have transiently reduced intestinal inflammation in restored animals compared to unrestored counterparts.
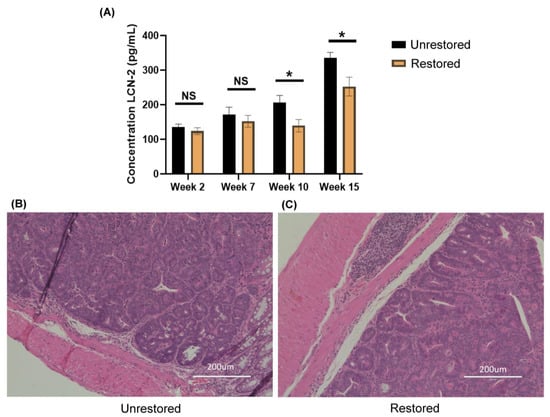
Figure 2.
Fecal restoration alters colonic inflammation in Balb/c mice. (A) Fecal concentrations of Lcn-2 in unrestored (black) restored (orange) groups at weeks 2, 7, 10, and 15 (left to right) (* p < 0.05) (n = 3) (+/− SD). (B) Hematoxylin and eosin staining of the colon tissues of unrestored (C) Restored Balb/c mice.
Despite significant differences in fecal Lcn-2 concentrations, we did not observe any pathological differences at the experiment endpoint when looking at the hematoxylin and eosin staining of fixed colon tissues (Figure 2B). Both restored and unrestored animals at the experiment endpoint presented histology ranging from high-grade dysplasia to high-grade intramucosal adenocarcinoma. Additionally, restored and unrestored animals presented with acute and chronic inflammation ranging from focally mild to focally moderate and focally marked. These observations also align with attempted colonoscopy of unrestored and restored animals in the early stage. However, a colonoscopy was impossible at late stages due to bloody stool and inflammation within the colon. Although there were no significant histological differences at the experiment endpoint, we observed significant differences post-HMT in fecal Lcn-2 concentrations. This observation led us to consider that the protective effects of HMT were likely transient, and more frequent treatment with HMT could impact pathology between groups.
4.4. Bacterial Community Compositions Are Similar between Balb/c and C57BL/6 in Late Restored Groups
We previously observed altered longitudinal fecal microbiomes of restored Balb/c mice compared to unrestored mice. Considering the variable fluctuations in microbiome analysis, we questioned whether our observations were specific to Balb/c. We used 3–4-week-old Balb/c and C57BL/6 mice in the AOM/DSS model to test this. Additionally, we included a group of 3–4-week-old C57BL/6 mice treated with an antibiotics cocktail of vancomycin (0.05 g/L), metronidazole (0.025 g/L), and streptomycin (0.2 g/L) to deplete mouse microbiomes as a control to ensure our longitudinal data acquisition and analysis pipelines were working appropriately. Interestingly, antibiotics-treated mice observed lower-grade dysplasia compared to unrestored and restored counterparts, which was in line with previous studies [20]. Fecal restoration via HMT was again performed during weeks 8, 9, and 10.
Longitudinally stable mean relative abundances of genera between Group 6A (Balb/c), Group 6B (C57BL/6 unrestored), Group 7 (C57BL/6 +Antibiotics), and Group 8 (C57BL/6 restored) were observed (Figure 3A,B). These data from C57BL/6 were consistent with our previous observation in Balb/c mice. As expected, we observed depleted alpha diversity in Shannon and Chao1 indices for mice receiving antibiotics compared to restored and unrestored groups (Figure 3C and Supplemental Figure S2). Similar alpha diversity was observed between the restored and unrestored groups, which was not significant when compared using ANOSIM. Interestingly, we did not notice decreasing alpha diversity in restored C57BL/6 mice compared to unrestored mice, as was observed in Balb/c mice.
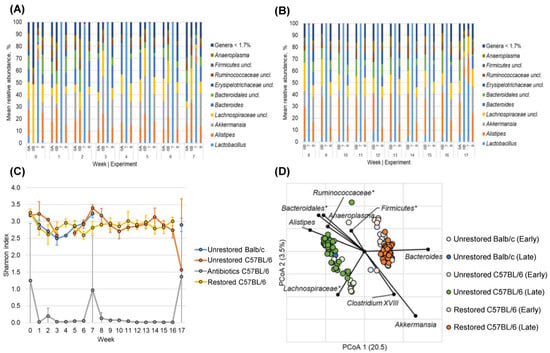
Figure 3.
Microbiome composition and diversity in C57BL/6 mice. (A) Percentage of the relative abundance of bacterial genera (W0-W7) in EXP6A (Balb/c unrestored), EXP6B (C57BL/6 unrestored), EXP7 (C57BL/6 + ABX), and EXP8 (C57BL/6 C57BL/6 restored) following AOM/DSS administration. Genera that accounted for <0.7% of mean sequence reads were consolidated for clarity. (B) Percentage of the relative abundance of bacterial genera (W8-17). (C) Longitudinal average Shannon index, with groups shown in blue (unrestored Balb/c), orange (unrestored C57BL/6), yellow (restored C57BL/6), and gray (antibiotics C57BL/6). (D) Principal coordinates analysis with correlated taxa associated with ordination position on either axis (Spearman p > 0.05) are overlaid on the PCoA plot. (*) Indicates genera that could not be further classified.
Next, we used Bray–Curtis dissimilarity and PCoA to compare restored and unrestored groups’ early and late microbial compositions. The early compositional signature between Balb/c and C57BL/6 unrestored animals differed significantly (ANOSIM R = 0.833, p < 0.001; Figure 3D). However, unrestored mice showed more similarity in late microbial compositions between Balb/c and C57BL/6 mice (ANOSIM R = −0.101, p = 0.723; Figure 3D). These observations suggested that even though C57BL/6 and Balb/c unrestored mice had significantly different initial microbial compositions after AOM/DSS treatment, similar compositions were observed between strains of mice when left untreated.
Furthermore, we also observed significant differences between late restored C57BL/6 and unrestored C57BL/6 mice (R = 0.897, p < 0.001; Figure 3D). Spearman correlation was used to investigate bacterial genera associated with differences in ordination position. Interestingly, we noticed similar taxa (i.e., Bacteroides, Clostridium_XVIII, and Akkermansia) were correlated with late compositions of the restored group (Figure 3D), similar to our prior observations in Balb/c mice. Additionally, unrestored C57BL/6 communities were associated with a greater relative abundance of Anaeroplasma, Alistipes, Lachnospiraceae, Bacteroidales (not further classified), and Ruminococcaceae (not further classified). Notably, the genera Ruminococcaceae, Alistipes, and Anaeroplasma were found in greater abundance in late samples among untreated Balb/c and C57BL/6 mice. Similarly, Akkermansia and Clostridium_XVIII were found at greater relative abundances in the restored microbiota of both mouse genotypes.
4.5. Inflammatory and Pathological Signatures between Balb/c Mice and C57BL/6 Mice Are Different
We again used ELISA to probe fecal concentrations of Lcn-2 pre-HMT (weeks 2 and 7) and post-HMT (weeks 10 and 15) in restored, unrestored, and antibiotics-treated animals to observe alterations in intestinal inflammation between groups. We did not observe significant differences in fecal Lcn-2 concentrations pre-HMT (week 2, 115.3 ± 27.4 pg/mL and 110.1 ± 19.3 pg/mL, p = 0.400, n = 3) (week 7, 178.6 ± 26.0 pg/mL and 208.6 ±35.1 pg/mL, p = 0.150, n = 3) between unrestored and restored animals (Figure 4A). We did observe significant differences in fecal Lcn-2 concentrations post-HMT (week 10, 200.8 ± 15.3 pg/mL and 131.4 pg/mL ± 24.5 pg/mL, p = 0.007, n = 3) between unrestored and restored groups (Figure 4A). This result was consistent with our previous investigation in Balb/c mice post-HMT. However, we did observe a difference in fecal concentration of Lcn-2 (188.8 ± 29.5 pg/mL and 152.5 ± 15.1 pg/mL, p = 0.065, n = 3) between unrestored and restored groups in week 15, but it did not reach statistical significance (p = 0.065). Interestingly, for all four time points, antibiotics-treated animals had statistically significantly lower concentrations of fecal Lcn-2 (44.2 ± 9.7 pg/mL, p = 0.003, n = 3) (83.5 ± 18.9 pg/mL, p = 0.003, n = 3) (78.1 ± 22.1 pg/mL, p = 0.024, n = 3) (97.9 ± 23.8 pg/mL, p = 0.014, n = 3) than both unrestored and restored animals (Figure 4A). This was in line with previous investigations in C57BL/6 animals treated with antibiotics in AOM/DSS models of intestinal inflammation. Additionally, in general, Balb/c mice presented with elevated levels of fecal Lcn-2 across all treatment conditions compared to C57BL/6 mice, which was also recapitulated in H and E stains of fixed colon tissues, as shown in Figure 2.
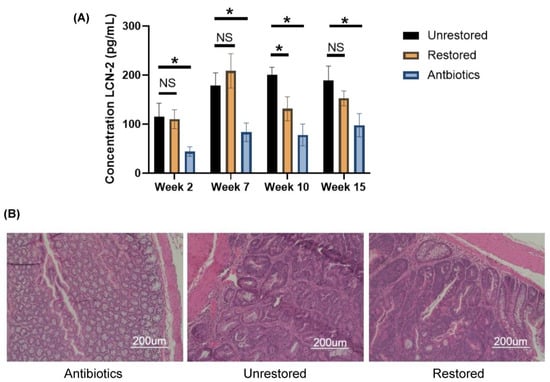
Figure 4.
Fecal restoration modulates intestinal inflammation in C57BL/6 mice. (A) Fecal concentrations of Lcn-2 in unrestored (black), restored (orange), and antibiotics-treated mice (blue) in weeks 2, 7, 10, and 15 (left to right) (* p < 0.05) (n = 3) (+/− SD). (* p < 0.05) (NS p > 0.05) (B) Hematoxylin and eosin staining of the colon tissues of unrestored, antibiotics-treated, and restored C57BL/6 mice.
Even though we observed a statistically significant decrease in fecal Lcn-2 concentrations in week 10 following HMT, this did not influence the pathological observations at the experimental endpoint between restored and unrestored animals (Figure 4B). Mice in restored and unrestored groups at the experiment endpoint presented with high-grade dysplasia to high-grade intramucosal adenocarcinoma. Furthermore, both restored and unrestored animals presented with acute inflammation, ranging from minimal to focally moderate, and chronic inflammation, ranging from mild to focally moderate. These observations suggested that any beneficial effect from HMT, as determined by concentrations of fecal Lcn-2, was likely transient and did not influence overall pathological outcomes between unrestored and restored groups. However, mice receiving antibiotics were absent of dysplasia and showed only minimal acute inflammation and mild chronic inflammation. This was consistent with fecal Lcn-2 concentrations as antibiotics-treated animals had significantly reduced levels of Lcn-2 compared to restored and unrestored animals throughout all sampled time points. These data further support a role or niche of the gut microbiota in regulating intestinal inflammation as mice treated with antibiotics presented with less advanced-stage disease than unrestored and restored groups.
4.6. Alistipes, Akkermansia, and Anaeroplasma Are Longitudinally Altered in C57BL/6 Restored and C57BL/6 Unrestored Mice
Next, we investigated longitudinal changes in bacterial genera between C57BL/6 restored and unrestored groups using the SplinectomeR permuspliner function [39]. We observed significant longitudinal differences in Alistipes (p= 0.001), Akkermansia (p = 0.001), Bacteroides (p = 0.001), Erysipelotrichaeae (p = 0.001), and Anaeroplasma (p = 0.001) between unrestored and restored groups (Figure 5 and Supplemental Figure S3). These bacterial genera were also identified in our Bray–Curtis dissimilarity analysis and were correlated using Spearman correlation. Additionally, we observed longitudinally significant differences in Bacteroides between unrestored C57BL/6 mice and restored C57BL/6 mice. However, this was not observed in Balb/c mice. Compared to healthy controls, Bacteroides are commonly observed in patients with irritable bowel disease [46,47]. When comparing Balb/c and C57BL/6 from all experiments, we consistently saw longitudinal increases in Akkermansia in the restored group compared to the unrestored, along with consistent observations of an increased abundance of Alitipes and Anaeroplasma in unrestored compared to restored Balb/c and C57BL/6 mice.
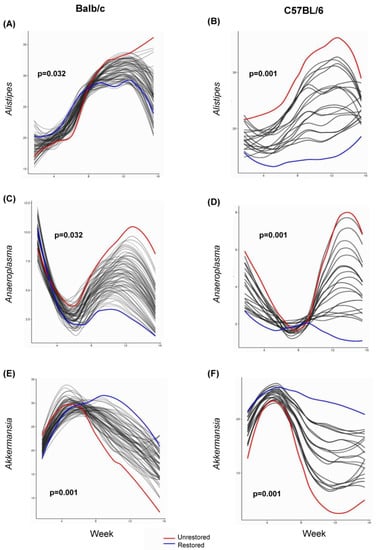
Figure 5.
Longitudinally significant genera conserved between mouse genotypes. Longitudinal differences in the relative abundance of genera significantly differ between no restoration (red) and restoration (blue) groups as determined by SplinectomeR. (A) Alistipes, Balb/c (p = 0.032); (B) Alistipes, C57BL/6 (p = 0.001); (C) Anaeroplasma, Balb/c (p = 0.032); (D) Anaeroplasma, C57BL/6 (p = 0.001); (E) Akkermansia, Balb/c (p = 0.001); (F) Akkermansia, C57BL/6 (p = 0.001).
5. Discussion
Interactions between the microbiota that contribute to host intestinal inflammation are increasingly being investigated. Associations have been reported between bacterial genera and disease phenotypes in inflammatory bowel diseases and CRC [7,48,49]. Many variables render causative associations between the microbiome and disease pathology challenging [14,15,16]. Our study aimed to use an established mouse model of id-CRC [17,18] to longitudinally track shifts in the microbiome of AOM/DSS-treated mice and assess how fecal restoration influences longitudinal microbial signature and disease pathology. Our study demonstrated that longitudinal compositional changes over 17 weeks are similar between Balb/c and C57BL/6 mice following AOM/DSS treatments (Figure 1 and Figure 3). Additionally, we demonstrated that horizontal microbiome transfer (HMT) restoration from untreated mice through bedding swaps led to consistent longitudinal increases in Akkermansia in Balb/c and C57BL/6 mice compared to their respective unrestored controls, along with consistent observations of an increased abundance of Anaeroplasma and Alistipes in unrestored Balb/c and C57BL/6 mice.
Clinical investigations have reported that the relative abundance of Akkermansia is decreased in stool samples of patients with active ulcerative colitis compared to patients with quiescent ulcerative colitis and healthy controls [50]. We reason that observing consistent longitudinal increases in Akkermansia in restored mice compared to unrestored mice may represent a more quiescent phenotype following fecal restoration. Additionally, it has been reported that oral administration of A. muciniphila strain BAA-835 significantly ameliorated intestinal inflammation following DSS-induced inflammation and was dependent on NLRP3 expression [51]. Others have shown that A. muciniphila strain BAA-835 can reduce inflammatory cytokine expression and reduce mucosal inflammation in DSS models of inflammation [39,40]. Knowing that Akkermansia can reduce inflammatory cytokine expression could explain why we observed decreased fecal Lcn-2 concentrations in week 10 following HMT in both genotypes of mice (Figure 2 and Figure 4). Nevertheless, these temporal increases in Akkermansia in restored groups did not yield a difference in pathological outcomes between restored and unrestored groups, so this effect was likely transient. More frequent administration of HMT could potentially yield alterations in pathological outcomes; however, without further investigation, the overall impact of HMT on disease pathology remains unknown.
Additionally, mice treated with antibiotics observed less dysplasia and inflammation than restored and unrestored animals. Our observations corroborate previous investigations and suggest that microbiota regulates intestinal inflammation, which can be seen in mice lacking a microbiome exhibiting less aggressive disease [20]. Furthermore, our Lcn-2 data support different pathological outcomes in antibiotics-treated mice as, throughout all sampled time points observed, there were significantly lower concentrations of Lcn-2 compared to unrestored and restored animals (Figure 4). We also observed significantly lower concentrations of Lcn-2 in C57BL/6 mice compared to Balb/c mice across treatment conditions. This supports our observations from hematoxylin and eosin staining, where Balb/c mice tended to present more frequently with intramucosal adenocarcinoma and focally marked inflammation than C57BL/6 counterparts. In general, C57BL/6 mice presented with less aggressive disease than Balb/c mice. Our studies suggest that these observations should be considered in future investigations of id-CRCs, as choosing an appropriate model can influence pathological outcomes. Nevertheless, these observations suggest that fecal restoration could contribute to an altered colonic inflammatory response through longitudinal increases in Akkermansia.
We also observed significant longitudinal increases in Alistipes and Anaeroplasma in the unrestored compared to the restored group in Balb/c and C57BL/6 mice (Figure 1 and Figure 3 and Supplemental Figures S1 and S3). The observation of an increased abundance of potentially pathogenic Alistipes is consistent with recent investigations of AOM/DSS-induced CRCs [43]. Additionally, more mechanistic insight has been gained by studies showing that Alistipes abundance is sufficient to induce colitis in IL10 -/- mice and can lead to tumorigenesis [52]. Alistipes has also been identified as a predominant genus of CRC patients from metagenome-wide association studies and is more abundant in carcinomas compared to adenomas and healthy controls [53]. Our observation of an increased relative abundance of Alistipes in unrestored groups compared to restored groups could reflect an increased abundance of Alistipes contributing to id-CRCs. Furthermore, Alistipes abundance has been implicated in the response to immune checkpoint inhibitors (ICIs) in preclinical models [54]. Iida et al. showed that antibiotic-treated animals bearing MC38 tumors observed resistance to anti-IL10 antibody treatment [55]. Additionally, Alistipes and Ruminoccocus were positively correlated with TNF-dependent anti-tumor immune activation in response to immunotherapy [55]. Routy et al. also implicated Alistipes indistinctus in response to ICIs and other commensal gut microbes in reversing resistance to ICIs [56]. Routy et al. administered Alistipes indistinctus to tumor-bearing animals and reversed resistance to ICIs [56]. Further studies are needed to fully illuminate how genera can modulate immune responses in the context of ICI resistance to enhance response rates in different malignancies so this knowledge can be translated into clinics.
We also observed consistent increases in the relative abundance of Anaeroplasma in unrestored compared to restored groups for both murine backgrounds. It has been reported that Anaeroplasma is enriched in mice receiving AOM/DSS compared to healthy controls, [21] and our work demonstrates a similar profile. However, conflicting results have also been reported. For instance, Anaeroplasma was shown to be decreased in the stool of mice with a common CRC driver gene APC mutation compared to the wild type [57]. However, different inflammatory niches between models could explain this observed difference in the abundance of Anaeroplasma. Further, it has been reported that Anaeroplasma strongly correlates with intestinal IgA and TGF-B secretion and regulates intestinal inflammation [58]. This is also conflicting as IgA increases in patients with IBD [59], while TGF-B is a known immunosuppressive cytokine [60].
Lastly, we also observed significant longitudinal increases in Ruminococcaceae in Balb/c restored mice compared to unrestored Balb/c but not C57BL/6 mice. Interestingly, Ruminococcus gnavus is associated with Crohn’s disease, likely through the ability of R. gnavus to synthesize and secrete glucorhamnan polysaccharides, which can lead to TNFα secretion by dendritic cells [42]; it is also enriched in CRC patients compared to normal controls [61]. We did not see this recapitulated in Balb/c and C57BL/6 mice. However, we reason this as evidence that fecal restoration longitudinally shifted the microbiome and modulated fecal Lcn-2 concentrations between restored and unrestored mice between genotypes.
6. Conclusions
This study investigated how HMT through cage bedding swapping with untreated animals could influence the microbiome and pathological outcomes of Balb/c and C57BL/6 mice treated with AOM/DSS to mimic id-CRC. We observed consistent and significant increases in Akkermansia in restored groups compared to unrestored counterparts in both genotypes. At the same time, we observed consistent increases in Anaeroplasma and Alistipes in unrestored animals compared to restored counterparts across genotypes. These observations were correlated with reduced fecal Lcn-2 concentrations post-HMT in restored mice compared to unrestored counterparts. We also observed differences in dysplasia and inflammatory signatures between Balb/c and C57BL/6 mice, which should be considered in future investigations.
In comparison, fecal restoration through HMT did not yield pathologically different outcomes between restored and unrestored animals. Temporal differences in fecal Lcn-2 concentrations were observed post-HMT. Future studies should seek to illuminate the direct microbiota–host mechanisms of Akkermansia, Anaeroplasma, and Alistipes for their roles in regulating intestinal inflammation and CRC development in different model systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15082260/s1, Supplementary Figure S1: Longitudinally significant genera. Longitudinal differences in the relative abundance of genera significantly differ between no restoration (red) and restoration (blue) groups as determined by SplinectomeR. (A) Alistipes (p= 0.032); (B) Akkermansia (p = 0.001); (C) Anaeroplasma (p = 0.032); (D) Ruminococacea_uncl (p = 0.001); (E) Clostridiales_uncl (p = 0.001); (F) Clostridium_XVIII (p = 0.001); (G) Shannon index (p = 0.035). Supplemental Figure S2: Alpha diversity analysis: Longitudinal average Chao1 index between unrestored Balb/c (blue), unrestored C57BL/6 (orange), restored C57BL/6 (yellow), and antibiotics C57BL/6 (gray) groups. Supplemental Figure S3: Longitudinally significant genera: Longitudinal analysis between unrestored C57BL/6 (red) and restored C57BL/6 (blue) mice using SplinectomeR. (A) Alistipes (p = 0.001); (B) Akkermansia (p = 0.001); (C) Bacteroides (p = 0.001); (D) Erysipelotrichaeae (p = 0.001); (E) Anaeroplasma (p = 0.001).
Author Contributions
All authors provided meaningful intellectual contributions to this work and were involved in its preparation and editing. T.J.G., C.Y., T.K.S., C.S. and S.S. conceived the study. T.J.G., C.Y., T.K.S, C.S., M.S., T.K. and S.S. generated and analyzed the data. A.C. and A.C.N. carried out the histopathological analysis. T.J.G., C.Y., T.K.S., C.S., M.S., T.K. and S.S. wrote the manuscript, and all authors edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by research grants from the Masonic Cancer Center ChainBreaker Fund, the Mezin Koats colorectal cancer research fund, Minnesota Colorectal Cancer Funds, and research funds from the Department of Surgery and CTSI, University of Minnesota. The Minnesota Colorectal Cancer Research Foundation supports T.J.G. through a graduate fellowship.
Institutional Review Board Statement
This study was conducted in accordance with the University of Minnesota Institutional Animal Care and Use Committee protocol code 2101-38805A 12-20-21.
Acknowledgments
We thank the Masonic Cancer Center’s core facilities, the University of Minnesota Genomics Center, and the Clinical and Translational Sciences Institute for microbiome sequencing and supporting histological services. This study is supported by research grants from the Masonic Cancer Center ChainBreaker Fund, the Mezin Koats colorectal cancer research fund, Minnesota Colorectal Cancer Funds, and research funds from the Department of Surgery and CTSI, University of Minnesota. The Minnesota Colorectal Cancer Research Foundation supports T.J.G. through a graduate fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, A.M.; Laroui, H.; Merlin, D.; Yang, V.W. Genetic deletion of Klf4 in the mouse intestinal epithelium ameliorates dextran sodium sulfate-induced colitis by modulating the NF-κB pathway inflammatory response. Inflamm. Bowel Dis. 2014, 20, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Yang, V.W.; Liu, Y.; Kim, J.; Shroyer, K.R.; Bialkowska, A.B. Increased Genetic Instability and Accelerated Progression of Colitis-Associated Colorectal Cancer through Intestinal Epithelium-specific Deletion of Klf4. Mol. Cancer Res. 2019, 17, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, A.M.; Wei, B.; Braun, J.; Schiestl, R.H. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009, 69, 4827–4834. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, R.; Wu, W.; Qin, H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013, 34, 1285–1300. [Google Scholar] [CrossRef]
- Gao, R.; Gao, Z.; Huang, L.; Qin, H. Gut microbiota and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 757–769. [Google Scholar] [CrossRef]
- Fachi, J.L.; Felipe, J.S.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; de Sales, E.S.É.; et al. Butyrate Protects Mice from Clostridium difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761.e757. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van de Wiele, T. Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Miyata, N.; Winter, M.G.; Arenales, A.; Hughes, E.R.; Spiga, L.; Kim, J.; Sifuentes-Dominguez, L.; Starokadomskyy, P.; Gopal, P.; et al. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J. Exp. Med. 2019, 216, 2378–2393. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.K.; Kelly, S.A.; Legge, R.; Ma, F.; Low, S.J.; Kim, J.; Zhang, M.; Oh, P.L.; Nehrenberg, D.; Hua, K.; et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18933–18938. [Google Scholar] [CrossRef]
- Schloss, P.D. Identifying and Overcoming Threats to Reproducibility, Replicability, Robustness, and Generalizability in Microbiome Research. mBio 2018, 9, e00525-18. [Google Scholar] [CrossRef]
- Staley, C.; Kaiser, T.; Khoruts, A. Clinician Guide to Microbiome Testing. Dig. Dis. Sci. 2018, 63, 3167–3177. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef]
- Parang, B.; Barrett, C.W.; Williams, C.S. AOM/DSS Model of Colitis-Associated Cancer. Methods Mol. Biol. 2016, 1422, 297–307. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Fischer, A.; Siegmund, B.; Kupz, A.; Niebergall, J.; Fuchs, D.; Jahn, H.-K.; Freudenberg, M.; Loddenkemper, C.; Batra, A.; et al. Shift Towards Pro-inflammatory Intestinal Bacteria Aggravates Acute Murine Colitis via Toll-like Receptors 2 and 4. PLoS ONE 2007, 2, e662. [Google Scholar] [CrossRef]
- Zackular, J.P.; Baxter, N.T.; Iverson, K.D.; Sadler, W.D.; Petrosino, J.F.; Chen, G.Y.; Schloss, P.D. The gut microbiome modulates colon tumorigenesis. mBio 2013, 4, e00692-13. [Google Scholar] [CrossRef]
- Ibrahim, A.; Hugerth, L.W.; Hases, L.; Saxena, A.; Seifert, M.; Thomas, Q.; Gustafsson, J.; Engstrand, L.; Williams, C. Colitis-induced colorectal cancer and intestinal epithelial estrogen receptor beta impact gut microbiota diversity. Int. J. Cancer 2019, 144, 3086–3098. [Google Scholar] [CrossRef]
- Yu, A.I.; Zhao, L.; Eaton, K.A.; Ho, S.; Chen, J.; Poe, S.; Becker, J.; Gonzalez, A.; McKinstry, D.; Hasso, M.; et al. Gut Microbiota Modulate CD8 T Cell Responses to Influence Colitis-Associated Tumorigenesis. Cell Rep. 2020, 31, 107471. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Zhong, W.; Yang, M.; Xu, M.; Sun, Y.; Ma, J.; Liu, T.; Song, X.; Dong, W.; et al. Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in Apc(min/+) mice. EBioMedicine 2019, 48, 301–315. [Google Scholar] [CrossRef]
- Khoruts, A.; Staley, C.; Sadowsky, M.J. Faecal microbiota transplantation for Clostridioides difficile: Mechanisms and pharmacology. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 67–80. [Google Scholar] [CrossRef]
- Crothers, J.W.; Chu, N.D.; Nguyen, L.T.T.; Phillips, M.; Collins, C.; Fortner, K.; Del Rio-Guerra, R.; Lavoie, B.; Callas, P.; Velez, M.; et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: Results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021, 21, 281. [Google Scholar] [CrossRef]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Daca, A.; Fic, M.; van de Wetering, T.; Folwarski, M.; Makarewicz, W. Therapeutic methods of gut microbiota modification in colorectal cancer management—Fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes 2020, 11, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Ono, M.; Bunker, M.E.; Núñez, G.; Inohara, N. Dynamic and Asymmetric Changes of the Microbial Communities after Cohousing in Laboratory Mice. Cell Rep. 2019, 27, 3401–3412.e3403. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Wang, Q.; O’Sullivan, O.; Greene-Diniz, R.; Cole, J.R.; Ross, R.P.; O’Toole, P.W. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 2010, 38, e200. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Env. MicroBiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Aronesty, E. Comparison of sequencing utility programs. Open Bioinform. J. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Huse, S.M.; Welch, D.M.; Morrison, H.G.; Sogin, M.L. Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Env. MicroBiol. 2010, 12, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- CLARKE, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Shields-Cutler, R.R.; Al-Ghalith, G.A.; Yassour, M.; Knights, D. SplinectomeR Enables Group Comparisons in Longitudinal Microbiome Studies. Front. MicroBiol. 2018, 9, 785. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. MicroBiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.-H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e132. [Google Scholar] [CrossRef]
- Chassaing, B.; Srinivasan, G.; Delgado, M.A.; Young, A.N.; Gewirtz, A.T.; Vijay-Kumar, M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE 2012, 7, e44328. [Google Scholar] [CrossRef]
- Mills, R.H.; Dulai, P.S.; Vázquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Malfavon, M.; Zhu, Q.; Weldon, K.; et al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 2022, 7, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ishikawa, D.; Okahara, K.; Ito, S.; Haga, K.; Takahashi, M.; Arakawa, A.; Shibuya, T.; Osada, T.; Kuwahara-Arai, K.; et al. Bacteroidetes Species Are Correlated with Disease Activity in Ulcerative Colitis. J. Clin. Med. 2021, 10, 1749. [Google Scholar] [CrossRef]
- Priya, S.; Burns, M.B.; Ward, T.; Mars, R.A.T.; Adamowicz, B.; Lock, E.F.; Kashyap, P.C.; Knights, D.; Blekhman, R. Identification of shared and disease-specific host gene–microbiome associations across human diseases using multi-omic integration. Nat. Microbiol. 2022, 7, 780–795. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Earley, H.; Lennon, G.; Balfe, Á.; Coffey, J.C.; Winter, D.C.; O’Connell, P.R. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci. Rep. 2019, 9, 15683. [Google Scholar] [CrossRef]
- Qu, S.; Fan, L.; Qi, Y.; Xu, C.; Hu, Y.; Chen, S.; Liu, W.; Liu, W.; Si, J. Akkermansia muciniphila Alleviates Dextran Sulfate Sodium (DSS)-Induced Acute Colitis by NLRP3 Activation. MicroBiol. Spectr 2021, 9, e0073021. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Gerner, R.R.; Wang, J.; Klepsch, V.; Adolph, T.E.; Reider, S.J.; Hackl, H.; Pfister, A.; Schilling, J.; Moser, P.L.; et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe 2016, 19, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liang, S.; Jia, H.; Stadlmayr, A.; Tang, L.; Lan, Z.; Zhang, D.; Xia, H.; Xu, X.; Jie, Z.; et al. Gut microbiome development along the colorectal adenoma–carcinoma sequence. Nat. Commun. 2015, 6, 6528. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Placencio-Hickok, V.; Guan, M.; Hendifar, A.; Salgia, R. The gut microbiome and response to immune checkpoint inhibitors: Preclinical and clinical strategies. Clin. Transl. Med. 2019, 8, 9. [Google Scholar] [CrossRef]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Vitali, F.; Tortora, K.; Di Paola, M.; Bartolucci, G.; Menicatti, M.; De Filippo, C.; Caderni, G. Intestinal microbiota profiles in a genetic model of colon tumorigenesis correlates with colon cancer biomarkers. Sci. Rep. 2022, 12, 1432. [Google Scholar] [CrossRef]
- Beller, A.; Kruglov, A.; Durek, P.; von Goetze, V.; Werner, K.; Heinz, G.A.; Ninnemann, J.; Lehmann, K.; Maier, R.; Hoffmann, U.; et al. Specific microbiota enhances intestinal IgA levels by inducing TGF-β in T follicular helper cells of Peyer’s patches in mice. Eur. J. Immunol. 2020, 50, 783–794. [Google Scholar] [CrossRef]
- Lin, R.; Chen, H.; Shu, W.; Sun, M.; Fang, L.; Shi, Y.; Pang, Z.; Wu, W.; Liu, Z. Clinical significance of soluble immunoglobulins A and G and their coated bacteria in feces of patients with inflammatory bowel disease. J. Transl. Med. 2018, 16, 359. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Wang, T.; Cai, G.; Qiu, Y.; Fei, N.; Zhang, M.; Pang, X.; Jia, W.; Cai, S.; Zhao, L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012, 6, 320–329. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).