A Recent Review on Cancer Nanomedicine
Abstract
Simple Summary
Abstract
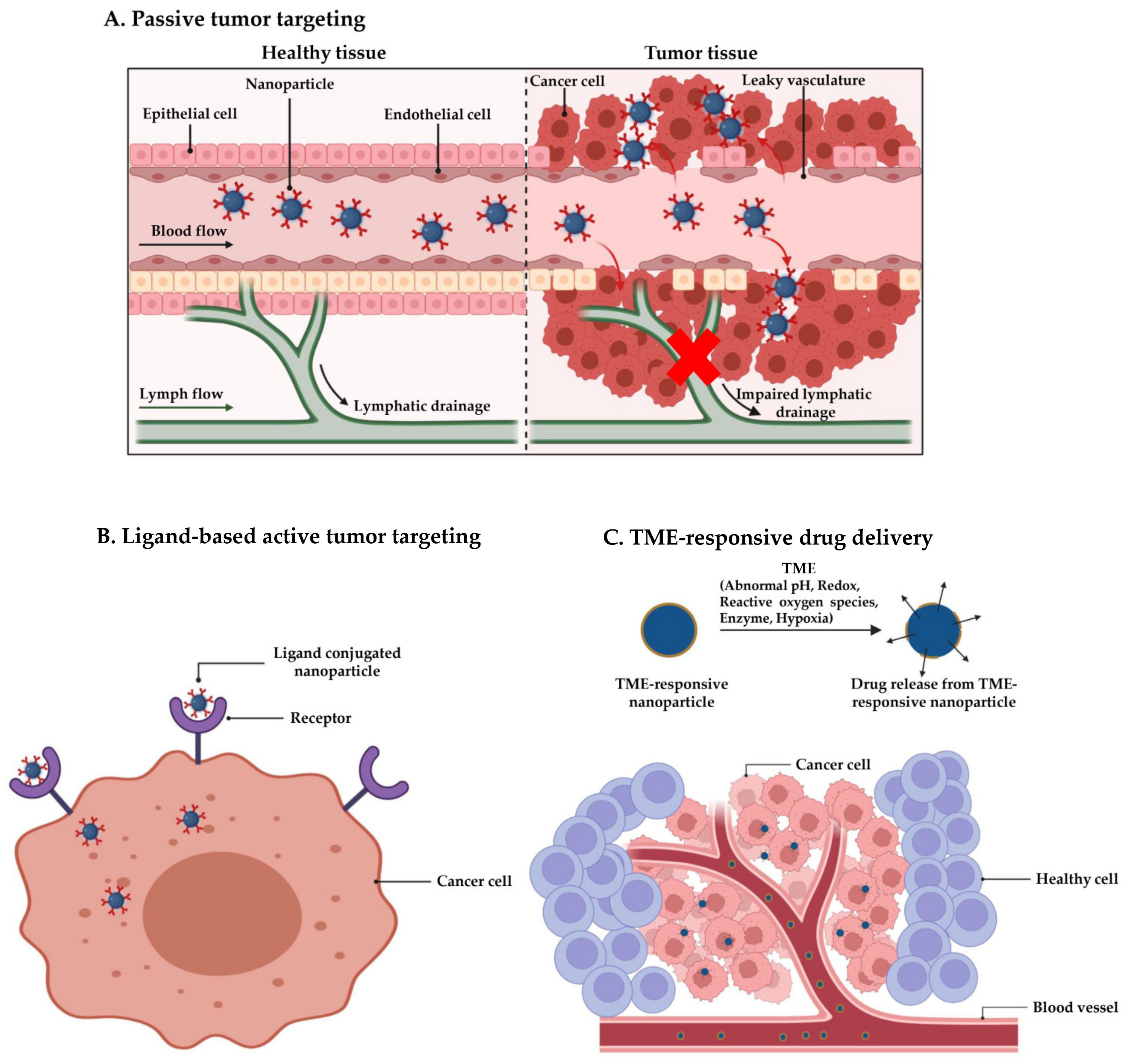
1. Introduction
2. Types of Nanocarriers
2.1. Lipid-Based Nanocarriers
2.1.1. Liposomes
2.1.2. Solid Lipid Nanoparticles (SLNs)
2.1.3. Nanostructured Lipid Carriers (NLCs)
2.2. Inorganic Nanocarriers
2.2.1. Iron Nanoparticles (FeNPs)
2.2.2. Gold Nanoparticles (AuNPs)
2.2.3. Mesoporous Silica Nanoparticles (MSNs)
2.2.4. Carbon Nanotubes (CNTs)
2.2.5. Graphene Oxide Nanoparticles (GONPs)
2.3. Polymeric Nanoparticles
2.4. Biological Nanocarriers
3. Current Status of Cancer Nanomedicine
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release Off. J. Control. Release Soc. 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46 Pt 1, 6387–6392. [Google Scholar] [PubMed]
- Salvioni, L.; Rizzuto, M.A.; Bertolini, J.A.; Pandolfi, L.; Colombo, M.; Prosperi, D. Thirty Years of Cancer Nanomedicine: Success, Frustration, and Hope. Cancers 2019, 11, 1855. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y.; et al. Nanoparticle-based medicines in clinical cancer therapy. Nano Today 2022, 45, 101512. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Prasanna, R.; Bunger, D.; Khan, M.A. Efficacy and safety of DoceAqualip in a patient with locally advanced cervical cancer: A case report. Mol. Clin. Oncol. 2018, 8, 296–299. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- El Hallal, R.; Lyu, N.; Wang, Y. Effect of Cetuximab-Conjugated Gold Nanoparticles on the Cytotoxicity and Phenotypic Evolution of Colorectal Cancer Cells. Molecules 2021, 26, 567. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Nayak, B.; Dey, R.K. PEGylation in anti-cancer therapy: An overview. Asian J. Pharm. Sci. 2016, 11, 337–348. [Google Scholar] [CrossRef]
- Layek, B.; Gidwani, B.; Tiwari, S.; Joshi, V.; Jain, V.; Vyas, A. Recent Advances in Lipid-based Nanodrug Delivery Systems in Cancer Therapy. Curr. Pharm. Des. 2020, 26, 3218–3233. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Arora, S.; Lamptey, R.; Chaulagain, B.; Singh, J.; Layek, B. A Summarized View of Lipid, Polyplex, Inorganic, and Carbon-Based Nanotherapeutics for Hepatocellular Carcinoma Treatment. In Nanotherapeutics for the Treatment of Hepatocellular Carcinoma; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; pp. 248–279. [Google Scholar]
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Nakhaei, P.; Margiana, R.; Bokov, D.O.; Abdelbasset, W.K.; Jadidi Kouhbanani, M.A.; Varma, R.S.; Marofi, F.; Jarahian, M.; Beheshtkhoo, N. Liposomes: Structure, Biomedical Applications, and Stability Parameters With Emphasis on Cholesterol. Front. Bioeng. Biotechnol. 2021, 9, 705886. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Layek, B.; Mukherjee, A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: An in vitro study. Int. J. Nanomed. 2007, 2, 213–225. [Google Scholar]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin. Cancer Biol. 2021, 69, 337–348. [Google Scholar] [CrossRef]
- Sharma, G.; Modgil, A.; Layek, B.; Arora, K.; Sun, C.; Law, B.; Singh, J. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: Biodistribution and transfection. J. Control. Release 2013, 167, 1–10. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K.; et al. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control. Release 2022, 351, 50–80. [Google Scholar] [PubMed]
- Zhou, S.; Li, J.; Yu, J.; Wang, Y.; Wang, Z.; He, Z.; Ouyang, D.; Liu, H.; Wang, Y. Tumor microenvironment adrenergic nerves blockade liposomes for cancer therapy. J. Control. Release 2022, 351, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shim, M.K.; Song, S.; Cho, H.; Choi, J.; Jeon, S.I.; Kim, W.J.; Um, W.; Park, J.H.; Yoon, H.Y.; et al. Liposome-mediated PD-L1 multivalent binding promotes the lysosomal degradation of PD-L1 for T cell-mediated antitumor immunity. Biomaterials 2022, 290, 121841. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Taléns-Visconti, R.; Díez-Sales, O.; de Julián-Ortiz, J.V.; Nácher, A. Nanoliposomes in Cancer Therapy: Marketed Products and Current Clinical Trials. Int. J. Mol. Sci. 2022, 23, 4249. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt. A, 28–51. [Google Scholar] [CrossRef]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged Circulation Time and Enhanced Accumulation in Malignant Exudates of Doxorubicin Encapsulated in Polyethylene-glycol Coated Liposomes1. Cancer Res. 1994, 54, 987–992. [Google Scholar]
- Luiz, M.T.; Dutra, J.A.P.; Ribeiro, T.D.C.; Carvalho, G.C.; Sábio, R.M.; Marchetti, J.M.; Chorilli, M. Folic acid-modified curcumin-loaded liposomes for breast cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128935. [Google Scholar] [CrossRef]
- Kim, Y.; Youn, Y.S.; Oh, K.T.; Kim, D.; Lee, E.S. Tumor-Targeting Liposomes with Transient Holes Allowing Intact Rituximab Internally. Biomacromolecules 2021, 22, 723–731. [Google Scholar] [CrossRef]
- Zalba, S.; Contreras, A.M.; Haeri, A.; ten Hagen, T.L.M.; Navarro, I.; Koning, G.; Garrido, M.J. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J. Control. Release 2015, 210, 26–38. [Google Scholar] [CrossRef]
- Kim, D.-M.; Kim, M.; Park, H.-B.; Kim, K.-S.; Kim, D.-E. Anti-MUC1/CD44 Dual-Aptamer-Conjugated Liposomes for Cotargeting Breast Cancer Cells and Cancer Stem Cells. ACS Appl. Bio Mater. 2019, 2, 4622–4633. [Google Scholar] [CrossRef]
- Nunes, S.S.; Miranda, S.E.M.; de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; de Aguiar Ferreira, C.; Townsend, D.M.; Cassali, G.D.; Oliveira, M.C.; Branco de Barros, A.L. pH-responsive and folate-coated liposomes encapsulating irinotecan as an alternative to improve efficacy of colorectal cancer treatment. Biomed. Pharmacother. 2021, 144, 112317. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Su, J.; Wu, K.; Ma, W.; Wang, B.; Li, M.; Sun, P.; Shen, Q.; Wang, Q.; Fan, Q. Multifunctional Thermosensitive Liposomes Based on Natural Phase-Change Material: Near-Infrared Light-Triggered Drug Release and Multimodal Imaging-Guided Cancer Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10540–10553. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Islan, G.A.; de Bravo, M.G.; Durán, N.; Castro, G.R. Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy. Colloids Surf. B Biointerfaces 2017, 154, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Enhanced Anticancer Efficiency in Breast Cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: Synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109006. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.L.; Ré, M.I. Lipid Nanoparticles as Carriers for Cosmetic Ingredients: The First (SLN) and the Second Generation (NLC). In Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Beck, R., Guterres, S., Pohlmann, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 101–122. [Google Scholar]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Jain, P.; Rahi, P.; Pandey, V.; Asati, S.; Soni, V. Nanostructure lipid carriers: A modish contrivance to overcome the ultraviolet effects. Egypt. J. Basic Appl. Sci. 2017, 4, 89–100. [Google Scholar] [CrossRef]
- López-García, R.; Ganem-Rondero, A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 62. [Google Scholar] [CrossRef]
- Fang, C.L.; Al-Suwayeh, S.A.; Fang, J.Y. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat. Nanotechnol. 2013, 7, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Karn-Orachai, K.; Smith, S.M.; Phunpee, S.; Treethong, A.; Puttipipatkhachorn, S.; Pratontep, S.; Ruktanonchai, U.R. The effect of surfactant composition on the chemical and structural properties of nanostructured lipid carriers. J. Microencapsul. 2014, 31, 609–618. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Monteiro, L.O.F.; Leite, E.A.; Cassali, G.D.; Rubello, D.; Cardoso, V.N.; Ferreira, L.A.M.; Oliveira, M.C.; de Barros, A.L.B. Doxorubicin-loaded nanocarriers: A comparative study of liposome and nanostructured lipid carrier as alternatives for cancer therapy. Biomed. Pharmacother. 2016, 84, 252–257. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in design of nanostructured lipid carriers for cancer targeting and theranostic application. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Ramírez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Kaur Narang, J.; Lather, V.; Beg, S.; Sharma, T.; Singh, B.; Pandita, D. Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: Systematic development, characterization and pharmacokinetic evaluation. Colloids Surf. B Biointerfaces 2019, 181, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Arabi, L.; Badiee, A.; Mosaffa, F.; Jaafari, M.R. Targeting CD44 expressing cancer cells with anti-CD44 monoclonal antibody improves cellular uptake and antitumor efficacy of liposomal doxorubicin. J. Control. Release 2015, 220, 275–286. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lee, C.D.; Ahn, J.B.; Kim, D.-H.; Lee, J.K.; Lee, J.-Y.; Choi, J.-S.; Park, J.-S. Hyaluronic acid-coated solid lipid nanoparticles to overcome drug-resistance in tumor cells. J. Drug Deliv. Sci. Technol. 2019, 50, 365–371. [Google Scholar] [CrossRef]
- Jia, D.; Wang, F.; Yang, Y.; Hu, P.; Song, H.; Lu, Y.; Wang, R.; Li, G.; Liu, R.; Li, J.; et al. Coupling EGFR-Antagonistic Affibody Enhanced Therapeutic Effects of Cisplatin Liposomes in EGFR-expressing Tumor Models. J. Pharm. Sci. 2022, 111, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Chen, J.; Wang, L.; Li, Q.; Yang, Y.; Lv, Z.; Bao, H.; Li, Y.; Luan, X.; Li, Y.; et al. Co-delivery of epirubicin and paclitaxel using an estrone-targeted PEGylated liposomal nanoparticle for breast cancer. Int. J. Pharm. 2020, 573, 118806. [Google Scholar] [CrossRef]
- Soe, Z.C.; Thapa, R.K.; Ou, W.; Gautam, M.; Nguyen, H.T.; Jin, S.G.; Ku, S.K.; Oh, K.T.; Choi, H.-G.; Yong, C.S.; et al. Folate receptor-mediated celastrol and irinotecan combination delivery using liposomes for effective chemotherapy. Colloids Surf. B Biointerfaces 2018, 170, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Moraes, S.; Marinho, A.; Lima, S.; Granja, A.; Araújo, J.P.; Reis, S.; Sousa, C.T.; Nunes, C. Targeted nanostructured lipid carriers for doxorubicin oral delivery. Int. J. Pharm. 2021, 592, 120029. [Google Scholar] [CrossRef]
- Dumont, N.; Merrigan, S.; Turpin, J.; Lavoie, C.; Papavasiliou, V.; Geretti, E.; Espelin, C.W.; Luus, L.; Kamoun, W.S.; Ghasemi, O.; et al. Nanoliposome targeting in breast cancer is influenced by the tumor microenvironment. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 71–81. [Google Scholar] [CrossRef] [PubMed]
- D’Avanzo, N.; Torrieri, G.; Figueiredo, P.; Celia, C.; Paolino, D.; Correia, A.; Moslova, K.; Teesalu, T.; Fresta, M.; Santos, H.A. LinTT1 peptide-functionalized liposomes for targeted breast cancer therapy. Int. J. Pharm. 2021, 597, 120346. [Google Scholar] [CrossRef]
- Cohen, L.; Assaraf, Y.G.; Livney, Y.D. Novel Selectively Targeted Multifunctional Nanostructured Lipid Carriers for Prostate Cancer Treatment. Pharmaceutics 2021, 14, 88. [Google Scholar] [CrossRef]
- Akanda, M.; Getti, G.; Nandi, U.; Mithu, M.S.; Douroumis, D. Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy. Int. J. Pharm. 2021, 599, 120416. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, Y.; Fan, T.; Lin, Y.; Zhang, H.; Mei, L. Inorganic nano-carriers based smart drug delivery systems for tumor therapy. Smart Mater. Med. 2020, 1, 32–47. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Majhi, K.C.; Yadav, M. Chapter 5—Synthesis of inorganic nanomaterials using carbohydrates. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, Boddula , R., Ahamed, M.I., Asiri, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–135. [Google Scholar]
- Paul, W.; Sharma, C.P. 8—Inorganic nanoparticles for targeted drug delivery. In Biointegration of Medical Implant Materials; Sharma, C.P., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 204–235. [Google Scholar]
- Kashapov, R.; Ibragimova, A.; Pavlov, R.; Gabdrakhmanov, D.; Kashapova, N.; Burilova, E.; Zakharova, L.; Sinyashin, O. Nanocarriers for Biomedicine: From Lipid Formulations to Inorganic and Hybrid Nanoparticles. Int. J. Mol. Sci. 2021, 22, 7055. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kim, Y.-J.; Im, G.-B.; Zhu, J.; Wu, Y.; Liu, Y.; Bhang, S.H. Inorganic Nanoparticles Applied as Functional Therapeutics. Adv. Funct. Mater. 2021, 31, 2008171. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, Y.; Lee, D.S. Recent Advances of pH-Induced Charge-Convertible Polymer-Mediated Inorganic Nanoparticles for Biomedical Applications. Macromol. Rapid Commun. 2020, 41, 2000106. [Google Scholar] [CrossRef] [PubMed]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Ngema, L.M.; Adeyemi, S.A.; Marimuthu, T.; Choonara, Y.E. A review on engineered magnetic nanoparticles in Non-Small-Cell lung carcinoma targeted therapy. Int. J. Pharm. 2021, 606, 120870. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Schneider-Futschik, E.K.; Reyes-Ortega, F. Advantages and Disadvantages of Using Magnetic Nanoparticles for the Treatment of Complicated Ocular Disorders. Pharmaceutics 2021, 13, 1157. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: A combinational approach for enhanced delivery of nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Illés, E.; Szekeres, M.; Tóth, I.Y.; Farkas, K.; Földesi, I.; Szabó, Á.; Iván, B.; Tombácz, E. PEGylation of Superparamagnetic Iron Oxide Nanoparticles with Self-Organizing Polyacrylate-PEG Brushes for Contrast Enhancement in MRI Diagnosis. Nanomaterials 2018, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.; Lee, H.; Kim, H.; Hwang, S.; Hadadian, Y.; Mohanty, A.; Park, I.K.; Cho, B.; Yoon, J.; Lee, J.Y. Highly Optimized Iron Oxide Embedded Poly(Lactic Acid) Nanocomposites for Effective Magnetic Hyperthermia and Biosecurity. Int. J. Nanomed. 2022, 17, 31–44. [Google Scholar] [CrossRef]
- Hajikarimi, Z.; Khoei, S.; Khoee, S.; Mahdavi, S.R. Evaluation of the cytotoxic effects of PLGA coated iron oxide nanoparticles as a carrier of 5- fluorouracil and mega-voltage X-ray radiation in DU145 prostate cancer cell line. IEEE Trans. Nanobioscience 2014, 13, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S. Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles for Doxorubicin Delivery: Synthesis and Anticancer Effect against Human Ovarian Cancer Cells. Chem. Biol. Drug Des. 2013, 82, 296–306. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, J.; Bajpai, A.K.; Mongre, R.K.; Lee, M.-S. Encapsulation of cytarabine into casein coated iron oxide nanoparticles (CCIONPs) and study of in vitro drug release and anticancer activities. J. Drug Deliv. Sci. Technol. 2020, 55, 101396. [Google Scholar] [CrossRef]
- Hedayatnasab, Z.; Dabbagh, A.; Abnisa, F.; Wan Daud, W.M.A. Polycaprolactone-coated superparamagnetic iron oxide nanoparticles for in vitro magnetic hyperthermia therapy of cancer. Eur. Polym. J. 2020, 133, 109789. [Google Scholar] [CrossRef]
- Specht, J.M.; Lee, S.; Turtle, C.; Berger, C.; Veatch, J.; Gooley, T.; Mullane, E.; Chaney, C.; Riddell, S.; Maloney, D.G. Phase I study of immunotherapy for advanced ROR1+ malignancies with autologous ROR1-specific chimeric antigen receptor-modified (CAR)-T cells. J. Clin. Oncol. 2018, 36 (Suppl. 5), TPS79. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Wang-Rodriguez, J.; Zhang, L.; Cui, B.; Frankel, W.; Wu, R.; Kipps, T.J. The Onco-Embryonic Antigen ROR1 Is Expressed by a Variety of Human Cancers. Am. J. Pathol. 2012, 181, 1903–1910. [Google Scholar] [CrossRef]
- Layek, B.; Singh, J. Amino Acid Grafted Chitosan for High Performance Gene Delivery: Comparison of Amino Acid Hydrophobicity on Vector and Polyplex Characteristics. Biomacromolecules 2013, 14, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Shirangi, A.; Mottaghitalab, F.; Dinarvand, S.; Atyabi, F. Theranostic silk sericin/SPION nanoparticles for targeted delivery of ROR1 siRNA: Synthesis, characterization, diagnosis and anticancer effect on triple-negative breast cancer. Int. J. Biol. Macromol. 2022, 221, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, Z.; Deng, Y.; Liao, T.; Kuang, Y.; Liu, J.; Duan, J.; Xu, Z.; Jiang, B.; Li, C. A novel intratumoral pH/redox-dual-responsive nanoplatform for cancer MR imaging and therapy. J. Colloid Interface Sci. 2020, 573, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.-l.; Huang, Z.-j.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Acosta, R.; Iriarte-Mesa, C.; Alvarez-Alminaque, D.; Hassannia, B.; Wiernicki, B.; Díaz-García, A.M.; Vandenabeele, P.; Vanden Berghe, T.; Pardo Andreu, G.L. Novel Iron Oxide Nanoparticles Induce Ferroptosis in a Panel of Cancer Cell Lines. Molecules 2022, 27, 3970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Coradduzza, D.; Zoroddu, M.A. Gold nanoparticles and cancer: Detection, diagnosis and therapy. Semin. Cancer Biol. 2021, 76, 27–37. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Herizchi, R.; Abbasi, E.; Milani, M.; Akbarzadeh, A. Current methods for synthesis of gold nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 44, 596–602. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Wang, J.-Q.; Ashby, C.R.; Zeng, L.; Fan, Y.-F.; Chen, Z.-S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of Various Size Gold Nanoparticles by Chemical Reduction Method with Different Solvent Polarity. Nanoscale Res. Lett. 2020, 15, 140. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Kim, G.-D.J.C.; Biointerfaces, S.B. Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerfaces 2018, 172, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef] [PubMed]
- D’Acunto, M.; Cioni, P.; Gabellieri, E.; Presciuttini, G. Exploiting gold nanoparticles for diagnosis and cancer treatments. Nanotechnology 2021, 32, 192001. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.S.; Tajon, C.A.; Bischof, T.S.; Iafrati, J.; Fernandez-Bravo, A.; Garfield, D.J.; Chamanzar, M.; Maharbiz, M.M.; Sohal, V.S.; Schuck, P.J. Energy-looping nanoparticles: Harnessing excited-state absorption for deep-tissue imaging. ACS Nano 2016, 10, 8423–8433. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Park, S.; Lee, W.J.; Park, S.; Choi, D.; Kim, S.; Park, N. Reversibly pH-responsive gold nanoparticles and their applications for photothermal cancer therapy. Sci. Rep. 2019, 9, 20180. [Google Scholar] [CrossRef]
- Mulens-Arias, V.; Nicolás-Boluda, A.; Pinto, A.; Balfourier, A.; Carn, F.; Silva, A.K.A.; Pocard, M.; Gazeau, F. Tumor-Selective Immune-Active Mild Hyperthermia Associated with Chemotherapy in Colon Peritoneal Metastasis by Photoactivation of Fluorouracil–Gold Nanoparticle Complexes. ACS Nano 2021, 15, 3330–3348. [Google Scholar] [CrossRef]
- Khoobchandani, M.; Katti, K.K.; Karikachery, A.R.; Thipe, V.C.; Bloebaum, P.L.; Katti, K.V. Targeted phytochemical-conjugated gold nanoparticles in cancer treatment. In Biotechnology Products in Everyday Life; Springer: Berlin/Heidelberg, Germany, 2019; pp. 37–52. [Google Scholar]
- Lee, C.S.; Kim, T.W.; Kang, Y.; Ju, Y.; Ryu, J.; Kong, H.; Jang, Y.S.; Oh, D.E.; Jang, S.J.; Cho, H.; et al. Targeted drug delivery nanocarriers based on hyaluronic acid-decorated dendrimer encapsulating gold nanoparticles for ovarian cancer therapy. Mater. Today Chem. 2022, 26, 101083. [Google Scholar] [CrossRef]
- Tunç, C.Ü.; Aydin, O. Co-delivery of Bcl-2 siRNA and doxorubicin through gold nanoparticle-based delivery system for a combined cancer therapy approach. J. Drug Deliv. Sci. Technol. 2022, 74, 103603. [Google Scholar] [CrossRef]
- Moodley, T.; Singh, M. Current Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer Therapy. Pharmaceutics 2021, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hu, J.; Wang, L.; Li, Y.; Zhu, C.; Chen, C.; Shi, M.; Ju, Z.; Cao, X.; Zhang, Z. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci. Rep. 2020, 10, 14447. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Song, F.X.; Zhang, L.; Yang, W.; Wang, H.X.; Chen, Q.L. A pH-sensitive drug delivery system based on folic acid-targeted HBP-modified mesoporous silica nanoparticles for cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124470. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: Optimization of length and PEGylation degree. Colloids Surf. B Biointerfaces 2018, 168, 43–49. [Google Scholar] [CrossRef]
- Hassan, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and chances. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Han, Z.; Wu, J.; Song, K.; Wu, J.; Gao, H.; Mi, Y. Synthesis and electrochemical performance of vertical carbon nanotubes on few-layer graphene as an anode material for Li-ion batteries. Mater. Chem. Phys. 2018, 205, 359–365. [Google Scholar] [CrossRef]
- Ravi Kiran, A.V.V.V.; Kusuma Kumari, G.; Krishnamurthy, P.T. Carbon nanotubes in drug delivery: Focus on anticancer therapies. J. Drug Deliv. Sci. Technol. 2020, 59, 101892. [Google Scholar] [CrossRef]
- Elhissi, A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.; D’Emanuele, A. Carbon nanotubes in cancer therapy and drug delivery. J. Drug Deliv. 2012, 2012, 867327. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.; Stassin, F.; Pagnoulle, C.; Jérôme, R. Noncovalent functionalization of multi-walled carbon nanotubes by pyrene containing polymers. Chem. Commun. 2003, 23, 2904–2905. [Google Scholar] [CrossRef] [PubMed]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized carbon nanotubes in drug design and discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar] [CrossRef]
- Radzi, M.R.M.; Johari, N.A.; Zawawi, W.F.A.W.M.; Zawawi, N.A.; Latiff, N.A.; Malek, N.A.N.N.; Wahab, A.A.; Salim, M.I.; Jemon, K. In vivo evaluation of oxidized multiwalled-carbon nanotubes-mediated hyperthermia treatment for breast cancer. Biomater. Adv. 2022, 134, 112586. [Google Scholar] [CrossRef]
- Suo, X.; Eldridge, B.N.; Zhang, H.; Mao, C.; Min, Y.; Sun, Y.; Singh, R.; Ming, X. P-Glycoprotein-Targeted Photothermal Therapy of Drug-Resistant Cancer Cells Using Antibody-Conjugated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 33464–33473. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.H.; Shang, W.T.; Deng, H.; Han, Z.Y.; Hu, M.; Liang, X.Y.; Fang, C.H.; Zhu, X.H.; Fan, Y.F.; Tian, J. Targeting carbon nanotubes based on IGF-1R for photothermal therapy of orthotopic pancreatic cancer guided by optical imaging. Biomaterials 2019, 195, 13–22. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Alemi, F.; Zarezadeh, R.; Sadigh, A.R.; Hamishehkar, H.; Rahimi, M.; Majidinia, M.; Asemi, Z.; Ebrahimi-Kalan, A.; Yousefi, B.; Rashtchizadeh, N. Graphene oxide and reduced graphene oxide: Efficient cargo platforms for cancer theranostics. J. Drug Deliv. Sci. Technol. 2020, 60, 101974. [Google Scholar] [CrossRef]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna; Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Zhou, S.; Bongiorno, A. Origin of the Chemical and Kinetic Stability of Graphene Oxide. Sci. Rep. 2013, 3, 2484. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef]
- Martín, C.; Ruiz, A.; Keshavan, S.; Reina, G.; Murera, D.; Nishina, Y.; Fadeel, B.; Bianco, A. A Biodegradable Multifunctional Graphene Oxide Platform for Targeted Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1901761. [Google Scholar] [CrossRef]
- Mallick, A.; Nandi, A.; Basu, S. Polyethylenimine Coated Graphene Oxide Nanoparticles for Targeting Mitochondria in Cancer Cells. ACS Appl. Bio Mater. 2019, 2, 14–19. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Mandal, S. Natural polysaccharides for controlled delivery of oral therapeutics: A recent update. Carbohydr. Polym. 2020, 230, 115617. [Google Scholar] [CrossRef] [PubMed]
- Wafa, E.I.; Geary, S.M.; Goodman, J.T.; Narasimhan, B.; Salem, A.K. The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 2017, 50, 417–427. [Google Scholar] [CrossRef]
- Pagels, R.F.; Prud’homme, R.K. Polymeric nanoparticles and microparticles for the delivery of peptides, biologics, and soluble therapeutics. J. Control. Release 2015, 219, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Markwalter, C.E.; Pagels, R.F.; Wilson, B.K.; Ristroph, K.D.; Prud’homme, R.K. Flash NanoPrecipitation for the Encapsulation of Hydrophobic and Hydrophilic Compounds in Polymeric Nanoparticles. J. Vis. Exp. 2019, 143, e58757. [Google Scholar]
- Sánchez, A.; Mejía, S.P.; Orozco, J. Recent Advances in Polymeric Nanoparticle-Encapsulated Drugs against Intracellular Infections. Molecules 2020, 25, 3760. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. 1—Biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–37. [Google Scholar]
- Rehman, U.; Parveen, N.; Sheikh, A.; Abourehab, M.A.S.; Sahebkar, A.; Kesharwani, P. Polymeric nanoparticles-siRNA as an emerging nano-polyplexes against ovarian cancer. Colloids Surf. B Biointerfaces 2022, 218, 112766. [Google Scholar] [CrossRef]
- Cai, J.; Qian, K.; Zuo, X.; Yue, W.; Bian, Y.; Yang, J.; Wei, J.; Zhao, W.; Qian, H.; Liu, B. PLGA nanoparticle-based docetaxel/LY294002 drug delivery system enhances antitumor activities against gastric cancer. J. Biomater. Appl. 2019, 33, 1394–1406. [Google Scholar] [CrossRef]
- Yadav, B.; Chauhan, M.; Shekhar, S.; Kumar, A.; Mehta, A.; Kumar Nayak, A.; Dutt, R.; Garg, V.; Kailashiya, V.; Muthu, M.S.; et al. RGD-decorated PLGA nanoparticles improved effectiveness and safety of cisplatin for lung cancer therapy. Int. J. Pharm. 2023, 633, 122587. [Google Scholar] [CrossRef]
- Al-Nemrawi, N.K.; Altawabeyeh, R.M.; Darweesh, R.S. Preparation and Characterization of Docetaxel-PLGA Nanoparticles Coated with Folic Acid-chitosan Conjugate for Cancer Treatment. J. Pharm. Sci. 2022, 111, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Haldar, M.K.; Sharma, G.; Lipp, L.; Mallik, S.; Singh, J. Hexanoic Acid and Polyethylene Glycol Double Grafted Amphiphilic Chitosan for Enhanced Gene Delivery: Influence of Hydrophobic and Hydrophilic Substitution Degree. Mol. Pharm. 2014, 11, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Lipp, L.; Singh, J. APC targeted micelle for enhanced intradermal delivery of hepatitis B DNA vaccine. J. Control. Release 2015, 207, 143–153. [Google Scholar] [CrossRef]
- Helmi, O.; Elshishiny, F.; Mamdouh, W. Targeted doxorubicin delivery and release within breast cancer environment using PEGylated chitosan nanoparticles labeled with monoclonal antibodies. Int. J. Biol. Macromol. 2021, 184, 325–338. [Google Scholar] [CrossRef]
- Li, Q.; Lv, X.; Tang, C.; Yin, C. Co-delivery of doxorubicin and CRISPR/Cas9 or RNAi-expressing plasmid by chitosan-based nanoparticle for cancer therapy. Carbohydr. Polym. 2022, 287, 119315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly(Ethylene Glycol)–Polylactide Micelles for Cancer Therapy. Front. Pharmacol. 2018, 9, 902. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Maghzi, P.; Hasanzadeh, F.; Sadeghi, H.; Mirian, M.; Rostami, M. PLGA-PEG-RA-based polymeric micelles for tumor targeted delivery of irinotecan. Pharm. Dev. Technol. 2018, 23, 41–54. [Google Scholar] [CrossRef]
- Kim, J.; Shamul, J.G.; Shah, S.R.; Shin, A.; Lee, B.J.; Quinones-Hinojosa, A.; Green, J.J. Verteporfin-Loaded Poly(ethylene glycol)-Poly(beta-amino ester)-Poly(ethylene glycol) Triblock Micelles for Cancer Therapy. Biomacromolecules 2018, 19, 3361–3370. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Osada, K.; Chen, Q.; Tockary, T.A.; Machitani, K.; Osawa, S.; Liu, X.; Ishii, T.; Miyata, K.; Oba, M.; et al. Optimized rod length of polyplex micelles for maximizing transfection efficiency and their performance in systemic gene therapy against stroma-rich pancreatic tumors. Biomaterials 2014, 35, 5359–5368. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, W.; Hong, T.; Miyazaki, T.; Dirisala, A.; Kataoka, K.; Cabral, H. Nanocarriers escaping from hyperacidified endo/lysosomes in cancer cells allow tumor-targeted intracellular delivery of antibodies to therapeutically inhibit c-MYC. Biomaterials 2022, 288, 121748. [Google Scholar] [CrossRef]
- Gillies, E.R.; Fréchet, J.M.J. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Zenze, M.; Daniels, A.; Singh, M. Dendrimers as Modifiers of Inorganic Nanoparticles for Therapeutic Delivery in Cancer. Pharmaceutics 2023, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, K.; Deręgowska, A.; Betlej, G.; Walczak, M.; Wnuk, M.; Lewińska, A.; Wołowiec, S. Cytarabine and dexamethasone-PAMAM dendrimer di-conjugate sensitizes human acute myeloid leukemia cells to apoptotic cell death. J. Drug Deliv. Sci. Technol. 2023, 81, 104242. [Google Scholar] [CrossRef]
- Narayanan, P.; Anitha, A.K.; Ajayakumar, N.; Kumar, K.S. Poly-Lysine Dendritic Nanocarrier to Target Epidermal Growth Factor Receptor Overexpressed Breast Cancer for Methotrexate Delivery. Materials 2022, 15, 800. [Google Scholar] [CrossRef]
- Xie, F.; Li, R.; Shu, W.; Zhao, L.; Wan, J. Self-assembly of Peptide dendrimers and their bio-applications in theranostics. Mater. Today Bio 2022, 14, 100239. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sahebkar, A.; Kesharwani, P. Poly (propylene imine) dendrimer as an emerging polymeric nanocarrier for anticancer drug and gene delivery. Eur. Polym. J. 2021, 158, 110683. [Google Scholar] [CrossRef]
- Soltani, A.; Faramarzi, M.; Farjadian, F.; Parsa, S.A.M.; Panahi, H.A. pH-responsive glycodendrimer as a new active targeting agent for doxorubicin delivery. Int. J. Biol. Macromol. 2022, 221, 508–522. [Google Scholar] [CrossRef]
- Pooja, D.; Srinivasa Reddy, T.; Kulhari, H.; Kadari, A.; Adams, D.J.; Bansal, V.; Sistla, R. N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur. J. Pharm. Biopharm. 2020, 154, 377–386. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Jangid, A.K.; Patel, K.; Joshi, U.; Patel, S.; Singh, A.; Pooja, D.; Saharan, V.A.; Kulhari, H. PEGylated G4 dendrimers as a promising nanocarrier for piperlongumine delivery: Synthesis, characterization, and anticancer activity. Eur. Polym. J. 2022, 179, 111547. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Thiruvengadam, M.; Venkidasamy, B.; Alomary, M.N.; Salawi, A.; Chung, I.-M.; Shariati, M.A.; Rebezov, M. Exosome-based nanomedicine for cancer treatment by targeting inflammatory pathways: Current status and future perspectives. Semin. Cancer Biol. 2022, 86, 678–696. [Google Scholar] [CrossRef]
- Keller, S.; Ridinger, J.; Rupp, A.K.; Janssen, J.W.; Altevogt, P. Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med. 2011, 9, 86. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef]
- Sharma, A. Chemoresistance in cancer cells: Exosomes as potential regulators of therapeutic tumor heterogeneity. Nanomedicine 2017, 12, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Ford, S.; Zhang, V.; Chung, J. Exosomes in Cancer Diagnostics. Cancers 2017, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Rupp, A.K.; Rupp, C.; Keller, S.; Brase, J.C.; Ehehalt, R.; Fogel, M.; Moldenhauer, G.; Marmé, F.; Sültmann, H.; Altevogt, P. Loss of EpCAM expression in breast cancer derived serum exosomes: Role of proteolytic cleavage. Gynecol. Oncol. 2011, 122, 437–446. [Google Scholar] [CrossRef]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Die Pharm. 2021, 76, 61–67. [Google Scholar]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Blaese, R.M. Suicide Genes. In Encyclopedia of Cancer, 2nd ed.; Bertino, J.R., Ed.; Academic Press: New York, NY, USA, 2002; pp. 285–296. [Google Scholar]
- Li, D.; Yao, S.; Zhou, Z.; Shi, J.; Huang, Z.; Wu, Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr. Res. 2020, 493, 108032. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.O.; Jo, H.; Yu, J.H.; Gambhir, S.S.; Pratx, G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018, 177, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Falagan-Lotsch, P.; Grzincic, E.M.; Murphy, C.J. New Advances in Nanotechnology-Based Diagnosis and Therapeutics for Breast Cancer: An Assessment of Active-Targeting Inorganic Nanoplatforms. Bioconjugate Chem. 2017, 28, 135–152. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Kwon, Y.J. Cancer nanotechnology: Current status and perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]







| Target | Nanomaterial Description | Payloads | Cancer Type | Model | Reference |
|---|---|---|---|---|---|
| CD44 receptor | Anti-CD44 monoclonal antibody conjugated PEGylated liposomes; size: 107 ± 3.1 nm; ZP: −15.6 ± 0.3 mV | Doxorubicin | Colon carcinoma | In vitro: C-26 mouse colon adenocarcinoma cells In vivo: BALB/c mice bearing C-26 tumor | [54] |
| Hyaluronic acid-coated SLNs; size: 224 ± 16 nm; ZP: −17.1 ± 0.73 mV | Docetaxel | Breast cancer | In vitro: MCF-7, MCF-7/ADR, and MDA-MBA-231 triple-negative human breast cancer cells | [55] | |
| Epidermal growth factor receptor (EGFR) | EGFR-antagonistic affibody (ZEGFR)-conjugated PEGylated liposomes; size: 140.01 ± 0.84 nm; ZP: −13.40 ± 0.8 mV | Cisplatin | Epidermoid carcinoma | In vitro: A431 human squamous carcinoma cells In vivo: BALB/c nude mice bearing A431 tumor grafts | [56] |
| Estrogen receptor | Estrone-conjugated PEGylated liposomes; size: 129.53 ± 1.19 nm; ZP: −5.74 ± 0.51 mV | Epirubicin and paclitaxel | Breast cancer | In vitro: MCF-7 cells In vivo: MCF-7 tumor-bearing BALB/c nude mice | [57] |
| Folate receptor | Folic acid conjugated liposomes; size: 174.0 ± 0.9 nm; ZP: −8.5 mV | Celastrol and irinotecan | Breast and lung cancers | In vitro: MCF-7, MDA-MB-231, and A549 cells In vivo: MDA-MB-231 xenograft tumor-bearing BALB/c nude mice | [58] |
| Folic acid conjugated NLCs; * NLC(Gel-DOX-PEG-FA) size: 220 ± 46 nm and ZP: −24.5 ± 1.7 mV * NLC(Pal-DOX-PEG-FA) size: 281 ± 18 nm and ZP: −28.0 ± 0.9 mV | Doxorubicin | Breast cancer | In vitro: MDA-MB-231 cells | [59] | |
| Her2 receptor | MM-302 conjugated PEGylated liposomes; size: ~100 nm | Doxorubicin | Breast cancer | In vivo: HER2 expressing murine and human breast cancer mice models | [60] |
| p32 protein | LinTT1 peptide-functionalized liposomes; size: 146 ± 4 nm; ZP: −32.6 ± 2.3 mV | Doxorubicin and sorafenib | Breast cancer | In vitro: MCF-7 and MDA-MB-231 cells, MDA-MB-231 spheroids | [61] |
| Prostate-specific membrane antigen (PSMA) | Glutamate-Urea-Lysine conjugated PEGylated NLCs; size: 129 ± 3 nm; ZP: −36.3 ± 0.3 mV | Cabazitaxel | Prostate cancer | In vitro: LNCaP human prostate cancer cells | [62] |
| Transferrin receptor | Transferrin conjugated SLNs; size: 231.4 ± 2.5 nm; ZP: −8.36 ± 0.1 mV | Curcumin | Prostate cancer | In vitro: LNCaP cells In vivo: BALB/c nude mice bearing LNCaP tumors | [63] |
| Product | Nanocarrier | Drug | Indication | Manufacturer | Initial Approval Year |
|---|---|---|---|---|---|
| Doxil (Caelyx) | PEGylated liposome | Doxorubicin | Kaposi’s sarcoma, breast cancer, ovarian cancer, multiple myeloma | Janssen | FDA (1995) EMA (1996) |
| DaunoXome | Liposome | Daunorubicin | Kaposi’s sarcoma | Galen | FDA (1996) |
| Lipo-Dox | PEGylated liposome | Doxorubicin | Kaposi’s sarcoma, breast cancer, ovarian cancer | Taiwan Liposome | Taiwan (1998) |
| DepoCyt | Liposome | Cytarabine | Lymphomatous meningitis | Pacira Pharmaceuticals | FDA (1999) |
| Myocet | Liposome | Doxorubicin | Metastatic breast cancer | Teva UK | EMA (2000) |
| Abraxane | Albumin nanoparticle | Paclitaxel | Advanced NSCLC, metastatic breast cancer, metastatic pancreatic cancer | Abraxis BioScience/Celgene | FDA (2005) EMA (2008) |
| Oncaspar | Polymer protein conjugate | L-asparaginase | Acute lymphoblastic leukemia | Enzon-Sigma-Tau | FDA (2006) |
| Lipusu | Liposome | Paclitaxel | NSCLC, ovarian cancer, and breast cancer | Luye Pharma | State Food and Drug Administration of China (2006) |
| Genexol-PM | PEG-b-PLA polymeric micelle | Paclitaxel | Breast cancer, ovarian cancer, and NSCLC | Samyang Biopharmaceuticals | South Korea (2007) |
| Mepact | Liposome | Mifamurtide | Osteosarcoma | Takeda | EMA (2009) |
| NanoTherm | Iron oxide nanoparticle | Thermal ablation of glioblastoma, prostate cancer | MagForce Nano | EMA (2010) FDA (2018) | |
| Onivyde | PEGylated liposome | Irinotecan | Metastatic pancreatic cancer | Merrimack Pharmaceuticals | FDA (2015) |
| DHP107 | Lipid nanoparticle | Paclitaxel | Gastric cancer | Daehwa Pharmaceutical | South Korea (2016) |
| Vyxeos | Liposome | Daunorubicin: cytarabine (1:5 molar ratio) | Acute myeloid leukemia | Jazz Pharmaceuticals | FDA (2017) EMA (2018) |
| Apealea | Micelle | Paclitaxel | Ovarian, peritoneal, and fallopian tube cancer | Oasmia Pharmaceutical | EMA (2018) |
| Hensify | Hafnium oxide nanoparticle | Locally-advanced soft tissue sarcoma | Nanobiotix | CE mark (2019) |
| Product (Development Phase) | Sponsor | Active Ingredient | Nanoplatform | Indication | Status | Clinical Trial Number |
|---|---|---|---|---|---|---|
| Docetaxel-PNP (Phase 1) | Samyang Biopharmaceuticals Corporation | Docetaxel | Polymeric nanoparticles | Advanced solid malignancies | Completed | NCT01103791 |
| ABT-888 (Phase 2) | AbbVie (prior sponsor, Abbott) | Temozolomide and lipo somal and doxorubicin | PEGylated liposomes | Ovarian cancer | Completed | NCT01113957 |
| BIND-014 (Phase 2) | BIND Therapeutics | Docetaxel | Polymeric micelles | Second-line therapy for KRAS-positive or squamous cell NSCLC patients | Completed | NCT02283320 |
| CPX-351 (Phase 2) | M.D. Anderson Cancer Center | Cytarabine and daunorubicin at 5:1 ratio | Liposomes | Acute myeloid leukemia | Completed | NCT02286726 |
| LipoVNB (Phase 1/2) | Taiwan Liposome Company | Vinorelbine tartrate | Liposomes | Advanced malignancy | Completed | NCT02925000 |
| NU-0129 (Early Phase 1) | Northwestern University | Small interfering RNAs (siRNAs) targeting the Bcl-2-like protein 12 (BCL2L12) sequence | Gold nanoparticles | Recurrent glioblastoma multiforme (GBM) or gliosarcoma | Completed | NCT03020017 |
| iExosomes (Phase 1) | M.D. Anderson Cancer Center | KRAS G12D siRNA | Exosomes | Metastatic pancreas cancer with KrasG12D mutation | Recruiting | NCT03608631 |
| CPC634 (CriPec®) (Phase 2) | Cristal Therapeutics | Docetaxel | Polymeric micelles | Ovarian cancer | Completed | NCT03742713 |
| Cetuximab nanoparticles (Phase 1) | Ahmed A. H. Abdellatif | Cetuximab | Ethylcellulose nanoparticles | Colon cancer | Recruiting | NCT03774680 |
| FF-10850 (Phase 1) | Fujifilm Pharmaceuticals | Topotecan | Liposomes | Advanced solid tumors | Recruiting | NCT04047251 |
| Quantum dots coated with veldoreotide (Phase 1) | Al-Azhar University | Veldoreotide | Quantum dots | Breast cancer, skin cancer | Recruiting | NCT04138342 |
| LY01610 (Phase 2) | Luye Pharma Group Ltd. | Irinotecan hydrochloride | Liposomes | Small cell lung cancer | Unknown | NCT04381910 |
| INT-1B3 (Phase 1) | InteRNA | microRNA (miR-193a-3p) | Lipid nano-particles | Advanced solid tumors | Recruiting | NCT04675996 |
| PRECIOUS-01 (Phase 1) | Radboud University Medical Center | Tumor antigen NY-ESO-1 and the iNKT cell activator threitolceramide-6 (ThrCer6, IMM60) | PLGA nanoparticles | Advanced solid tumor | Recruiting | NCT04751786 |
| Mitoxantrone hydrochloride liposome injection (Phase 2) | CSPC ZhongQi Pharmaceutical Technology Co., Ltd. | Mitoxantrone hydrochloride | Liposomes | Breast cancer | Recruiting | NCT04927481 |
| Liposomal bupivacaine (Phase 4) | Samaritan Health Services | Bupivacaine hydrochloride | Liposomes | Benign neoplasm | Recruiting | NCT05082441 |
| WGI-0301 (Phase 1) | Zhejiang Haichang Biotech Co., Ltd. | AKT-1 antisense oligonucleotide | Lipid nanoparticles | Advanced solid tumors | Recruiting | NCT05267899 |
| MagTrace (Phase 1/2) | Sahlgrenska University Hospital | Superparamagnetic iron oxide | Iron oxide nanoparticles | Deiagnostic test: Sentinel lymph node detection in breast cancer | Recruiting | NCT05359783 |
| CDK-004 (Phase 1) | Codiak BioSciences | Antisense oligonucleotide targeting STAT6 | Exosomes | Advanced hepatocellular carcinoma, gastric cancer metastatic to liver | Recruiting | NCT05375604 |
| Nano-QUT (Phase 2) | Cairo University | Quercetin | PLGA-PEG nanoparticles | Oral cancer | Not yet recruiting | NCT05456022 |
| OTX-2002 (Phase 1/2) | Omega Therapeutics | Biscistronic mRNA downregulate c-Myc expression | Lipid nanoparticles | Hepatocellular carcinoma | Recruiting | NCT05497453 |
| Liposome doxorubicin (Phase 3) | Sun Yat-sen University | Doxorubicin | Liposomes | Desmoid tumor | Recruiting | NCT05561036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giri, P.M.; Banerjee, A.; Layek, B. A Recent Review on Cancer Nanomedicine. Cancers 2023, 15, 2256. https://doi.org/10.3390/cancers15082256
Giri PM, Banerjee A, Layek B. A Recent Review on Cancer Nanomedicine. Cancers. 2023; 15(8):2256. https://doi.org/10.3390/cancers15082256
Chicago/Turabian StyleGiri, Paras Mani, Anurag Banerjee, and Buddhadev Layek. 2023. "A Recent Review on Cancer Nanomedicine" Cancers 15, no. 8: 2256. https://doi.org/10.3390/cancers15082256
APA StyleGiri, P. M., Banerjee, A., & Layek, B. (2023). A Recent Review on Cancer Nanomedicine. Cancers, 15(8), 2256. https://doi.org/10.3390/cancers15082256






