Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Extravasation of Tumor Cells through the Blood–Brain Barrier as A Key Step in Brain Metastasis
2.1. Molecular Mechanism Mediating Extravasation in Breast Cancer Brain Metastasis
2.1.1. Factors Mediating Rolling and Firm Adhesion of Breast Cancer Cells to the Brain Endothelium
2.1.2. Factors Affecting the BBB Permeability in Breast Cancer Brain Metastasis
2.1.3. Factors Mediating Trans-Endothelial Migration in Breast Cancer Brain Metastasis
2.1.4. Cytokines Mediating Extravasation in Breast Cancer Brain Metastasis
2.1.5. Endothelial-to-Mesenchymal Transition (EndMT) Mediating Extravasation in Breast Cancer Brain Metastasis
2.1.6. MicroRNA Mediating Extravasation in Breast Cancer Brain Metastasis
2.2. Molecular Mechanisms Mediating Extravasation in Melanoma Brain Metastasis
2.2.1. Factors Mediating Rolling and Firm Adhesion of Melanoma Cells to the Brain Endothelium
2.2.2. Factors Affecting the BBB Permeability in Melanoma Brain Metastasis
2.2.3. Factors Mediating Trans-Endothelial Migration in Melanoma Brain Metastasis
2.2.4. Cytokines Mediating Extravasation in Melanoma Brain Metastasis
2.2.5. EndMT Mediating Extravasation in Melanoma Brain Metastasis
2.2.6. MicroRNA Mediating Extravasation in Melanoma Brain Metastasis
2.3. Molecular Mechanisms Mediating Extravasation in Lung Cancer Brain Metastasis
2.3.1. Factors Mediating Rolling and Firm Adhesion of Lung Cancer Cells to the Brain Endothelium
2.3.2. Factors Affecting the BBB Permeability in Lung Cancer Brain Metastasis
2.3.3. Factors Mediating Trans-Endothelial Migration in Lung Cancer Brain Metastasis
2.3.4. Cytokines Mediating Extravasation in Lung Cancer Brain Metastasis
2.3.5. EndMT Mediating Extravasation in Lung Cancer Brain Metastasis
| Step of Extravasation | Factor | Effect | Regulation in BM | Reference |
|---|---|---|---|---|
| Adhesion | ALCAM | Increases tumor cell adhesion to BE. | Upregulation | [130] |
| CD15 | Interacts with E-selectin (CD62E) to mediate adhesion of tumor cells to BE and disrupts the BBB. | Upregulation | [133] | |
| CD15s (sLex) | Interacts with E-selectin (CD62E) to mediate adhesion of tumor cells to BE and disrupts the BBB. | Upregulation | [133] | |
| ADAM9 | Increases adhesion to brain tissues through upregulating integrin α3β1. | Upregulation | [134] | |
| Annexin A1 | Promotes tumor adhesion to BE. | Upregulation | [138] | |
| Alteration in the BBB permeability | Mfsd2a | Its downregulation in BE will promote permeability of BBB. | Downregulation | [54] |
| Claudin-5 | Its downregulation will promote permeability of BBB. | Downregulation | [135] | |
| Adenosine A2A receptor | Its downregulation will activate SDF-1/CXCR4 signaling pathway and inhibit the expression of claudin-5, occludin, and ZO-1. | Downregulation | [136] | |
| AKR1B10 | Degrades junctional proteins level (ZO-1 and VE- cadherin) to promote TEM. Upregulates MMP2 and MMP9 via MEK/ERK signaling pathway. | Upregulation | [137] | |
| Rho/ROCK | BE Rho/ROCK signaling pathway facilitate TEM with subsequent disruption of tight junction proteins (occludin, claudin-5, and ZO-1). | Upregulation | [139] | |
| PLGF | Disassembly of tight junction proteins (occludin and ZO-1) via subsequent activation of VEGFR-1, Rho/ROCK, and ERK signaling pathways. | Upregulation | [140] | |
| Trans-endothelial migration | AKR1B10 | Degrades junctional proteins level (ZO-1 and VE- cadherin) to promote TEM. | Upregulation | [137] |
| annexin A1 | Promotes tumor adhesion to BE and thus facilitating TEM. | Upregulation | [138] | |
| Rho/ROCK | BE Rho/ROCK signaling pathway facilitated TEM with subsequent disruption of tight junction proteins (occludin, claudin-5, and ZO-1). | Upregulation | [139] | |
| PLGF | Promotes TEM. | Upregulation | [140] | |
| Visfatin | Upregulates CCL2 via PI3K/Akt signaling pathway, promoting TEM. | Upregulation | [142] | |
| miR-143-3p | Increases TEM through BBB. | Upregulation | [148] |
2.3.6. MicroRNA Mediating Extravasation in Lung Cancer Brain Metastasis
3. Comparison of the Molecular Mechanisms Mediating Extravasation in the Three Types of Cancer: Breast Cancer, Melanoma, and Lung Cancer
4. Potential Anti-Brain Metastasis Strategies Targeting the Extravasation Step and Future Research Lines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ALCAM | activated leukocyte cell adhesion molecule |
| BBB | blood–brain barrier |
| CD | cluster of differentiation |
| CNS | central nervous system |
| COX2 | cyclooxygenase-2 |
| EndMT | endothelial-to-mesenchymal transition |
| ERK | extracellular signal-regulated kinase |
| GBP1 | guanylate-binding protein 1 |
| GLUT1 | glucose transporter 1 |
| HER2 | human epidermal growth factor receptor 2 |
| ICAM-1 | intercellular adhesion molecule-1 |
| IGF2 | insulin-like growth factor 2 |
| IgSF | immunoglobulin superfamily |
| IL | interleukin |
| ILK | integrin-linked kinase |
| JAK | janus kinase |
| L1CAM | cell adhesion molecule L1 |
| LFA-1 | lymphocyte function-associated antigen 1 |
| MAPK | mitogen-activated protein kinase |
| Mfsd2a | major facilitator superfamily domain 2a |
| MIF | macrophage migration inhibitory factor |
| miR | micro-RNA |
| MMP | metalloproteinase |
| MRTF | myocardin-related transcription factor |
| NSCLC | non-small cell lung cancer |
| PECAM-1 | platelet endothelial cell adhesion molecule 1 |
| PSGL-1 | platelet selectin glycoprotein ligand- 1 |
| ROCK | Rho-associated protein kinase |
| SDF-1 | stromal cell-derived factor 1 |
| sLex | sialyl-Lewis X |
| SP | substance P |
| ST6GALNAC5 | α2, 6-sialyltransferase |
| TEM | trans-endothelial migration |
| TNFα | tumor necrosis factor α |
| uPAR | urokinase plasminogen activator receptor |
| VEGF | vascular endothelial growth factor |
| STAT3 | signal transducer and activator of transcription 3 |
| 3′UTR | 3′untranslated region |
| AKR1B10 | aldo-keto reductase family 1 B10 |
| Ang-2 | angiopoietin-2 |
| BCRP | breast cancer resistance protein |
| BE | brain endothelium |
| BEC | brain endothelial cells |
| BM | brain metastasis |
| BTB | blood–tumor barrier |
| CCL | CC chemokine ligand |
| Cx | connexins |
| CXCL | chemokine (C-X-C motif) ligand |
| CXCR | chemokine receptor |
| DHA | docosahexaenoic acid |
| ECM | extracellular matrix |
| EMT | epithelial-mesenchymal transition |
| ER | estrogen |
| ESG | endothelial surface glycocalyx |
| FUT | fucosyltransferases |
| GEF-H1 | guanine nucleotide exchange factor-H1 |
| GFAP | glial fibrillary acidic protein |
| HA | hylarunan |
| HB-EGFHB-EGF | heparin-binding epidermal growth factor-like growth factor |
| HPSE | heparanase |
| HRG | heregulin |
| HSPG | heparan sulfate proteoglycan |
| JAM | unction adhesion molecules |
| MTf | melanotransferrin |
| MUC1 | mucin-1 |
| NRP1 | neuropilin-1 |
| PAI-1 | plasminogen activator inhibitor-1 |
| PDPK1 | 3-phosphoinositide-dependent protein kinase-1 gene |
| PI3K | hosphatidylinositol 3-kinase |
| PLEKHA5 | pleckstrin homology domain-containing family A member 5 |
| PR | progesterone receptors |
| RAGE | receptor for advanced glycation end product |
| S100A4 | EF-hand calcium-binding protein |
| SCLC | small cell lung cancer |
| Sdcs-1 | syndecan-1 |
| Sema4D | semaphorin 4D |
| SMA | α-smooth muscle actin |
| TGF-β | transforming growth factor |
| VCAM-1 | vascular cell adhesion protein 1 |
| VE-cadherin | vascular endothelial cadherin |
| VLA-4 | very late antigen-4 |
| YAP | yes-associated protein |
| ZO | zonula occluden |
References
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of Brain Metastases According to Molecular Subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Bollig-Fischer, A.; Michelhaugh, S.; Ali-Fehmi, R.; Mittal, S. The Molecular Genomics of Metastatic Brain Tumours. OA Mol. Oncol. 2013, 1. [Google Scholar] [CrossRef]
- Cruz-Muñoz, W.; Kerbel, R.S. Preclinical Approaches to Study the Biology and Treatment of Brain Metastases. Semin. Cancer Biol. 2011, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Custódio-Santos, T.; Videira, M.; Brito, M.A. Brain Metastasization of Breast Cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 132–147. [Google Scholar] [CrossRef]
- Wilhelm, I.; Fazakas, C.; Molnár, K.; Végh, A.G.; Haskó, J.; Krizbai, I.A. Foe or Friend? Janus-Faces of the Neurovascular Unit in the Formation of Brain Metastases. J. Cereb. Blood Flow Metab. 2018, 38, 563–587. [Google Scholar] [CrossRef]
- Eichler, A.F.; Chung, E.; Kodack, D.P.; Loeffler, J.S.; Fukumura, D.; Jain, R.K. The Biology of Brain Metastases-Translation to New Therapies. Nat. Rev. Clin. Oncol. 2011, 8, 344–356. [Google Scholar] [CrossRef]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rüger, R. Dissection of the Process of Brain Metastasis Reveals Targets and Mechanisms for Molecular-Based Intervention. Cancer Genom. Proteom. 2016, 13, 245–258. [Google Scholar]
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and Cellular Mechanisms Underlying Brain Metastasis of Breast Cancer. Cancer Metastasis Rev. 2020, 39, 711–720. [Google Scholar] [CrossRef]
- Fidler, I.J. The Biology of Brain Metastasis: Challenges for Therapy. Cancer J. 2015, 21, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood-Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.M.A.; Gong, C.; Xu, Y.G.; Chang, Y.; Shi, H. Factors Controlling Permeability of the Blood-Brain Barrier. Cell. Mol. Life Sci. 2016, 73, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R. The Blood-Brain Barrier in Health and Disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Brain Endothelial Cell-Cell Junctions: How to “Open” the Blood Brain Barrier. Curr. Neuropharmacol. 2008, 6, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Dejana, E. Endothelial Cell-to-Cell Junctions: Molecular Organization and Role in Vascular Homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The Blood-Brain Barrier: An Overview: Structure, Regulation, and Clinical Implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Petty, M.A.; Lo, E.H. Junctional Complexes of the Blood-Brain Barrier: Permeability Changes in Neuroinflammation. Prog. Neurobiol. 2002, 68, 311–323. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Stoletov, K.; Kato, H.; Zardouzian, E.; Kelber, J.; Yang, J.; Shattil, S.; Klemke, R. Visualizing Extravasation Dynamics of Metastatic Tumor Cells. J. Cell Sci. 2010, 123, 2332–2341. [Google Scholar] [CrossRef]
- Fan, J.; Fu, B.M. Quantification of Malignant Breast Cancer Cell MDA-MB-231 Transmigration Across Brain and Lung Microvascular Endothelium. Ann. Biomed. Eng. 2016, 44, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.F.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-Time Imaging Reveals the Single Steps of Brain Metastasis Formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Heinävaara, S.; Sarkeala, T.; Anttila, A. Impact of Organised Mammography Screening on Breast Cancer Mortality in a Case-Control and Cohort Study. Br. J. Cancer 2016, 114, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Daoud, G.H.; Mohamed, A.; Harati, R. New Insights into the Therapeutic Applications of CRISPR/Cas9 Genome Editing in Breast Cancer. Genes 2021, 12, 723. [Google Scholar] [CrossRef]
- Pedrosa, R.M.S.M.; Mustafa, D.A.; Soffietti, R.; Kros, J.M. Breast Cancer Brain Metastasis: Molecular Mechanisms and Directions for Treatment. Neuro Oncol. 2018, 20, 1439–1449. [Google Scholar] [CrossRef]
- Lorger, M.; Felding-Habermann, B. Capturing Changes in the Brain Microenvironment during Initial Steps of Breast Cancer Brain Metastasis. Am. J. Pathol. 2010, 176, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.S.; Serres, S.; Anthony, D.C.; Sibson, N.R. Functional Role of Endothelial Adhesion Molecules in the Early Stages of Brain Metastasis. Neuro Oncol. 2014, 16, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.H.; Gajjar, K.A.; Pearson, R.M.; Launiere, C.A.; Eddington, D.T.; Hong, S. Direct Measurements on CD24-Mediated Rolling of Human Breast Cancer MCF-7 Cells on E-Selectin. Anal. Chem. 2011, 83, 1078–1083. [Google Scholar] [CrossRef]
- Kang, S.-A.; Hasan, N.; Mann, A.P.; Zheng, W.; Zhao, L.; Morris, L.; Zhu, W.; Zhao, Y.D.; Suh, K.S.; Dooley, W.C.; et al. Blocking the Adhesion Cascade at the Premetastatic Niche for Prevention of Breast Cancer Metastasis. Mol. Ther. 2015, 23, 1044–1054. [Google Scholar] [CrossRef]
- Chaudhry, G.-E.-S.; Akim, A.; Naveed Zafar, M.; Safdar, N.; Sung, Y.Y.; Muhammad, T.S.T. Understanding Hyaluronan Receptor (CD44) Interaction, HA-CD44 Activated Potential Targets in Cancer Therapeutics. Adv. Pharm. Bull. 2021, 11, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Hamester, F.; Stürken, C.; Legler, K.; Eylmann, K.; Möller, K.; Roßberg, M.; Gorzelanny, C.; Bauer, A.T.; Windhorst, S.; Schmalfeldt, B.; et al. Key Role of Hyaluronan Metabolism for the Development of Brain Metastases in Triple-Negative Breast Cancer. Cells 2022, 11, 3275. [Google Scholar] [CrossRef]
- Geng, Y.; Yeh, K.; Takatani, T.; King, M.R. Three to Tango: MUC1 as a Ligand for Both E-Selectin and ICAM-1 in the Breast Cancer Metastatic Cascade. Front. Oncol. 2012, 2, 76. [Google Scholar] [CrossRef]
- Wai Wong, C.; Dye, D.E.; Coombe, D.R. The Role of Immunoglobulin Superfamily Cell Adhesion Molecules in Cancer Metastasis. Int. J. Cell Biol. 2012, 2012, 340296. [Google Scholar] [CrossRef]
- Fan, J.; Cai, B.; Zeng, M.; Hao, Y.; Giancotti, F.G.; Fu, B.M. Integrin Β4 Signaling Promotes Mammary Tumor Cell Adhesion to Brain Microvascular Endothelium by Inducing ErbB2-Mediated Secretion of VEGF. Ann. Biomed. Eng. 2011, 39, 2223–2241. [Google Scholar] [CrossRef]
- Wu, M.; Gong, Y.; Jiang, L.; Zhang, M.; Gu, H.; Shen, H.; Dang, B. VEGF Regulates the Blood-brain Barrier through MMP-9 in a Rat Model of Traumatic Brain Injury. Exp. Ther. Med. 2022, 24, 728. [Google Scholar] [CrossRef]
- Ippolitov, D.; Arreza, L.; Munir, M.N.; Hombach-Klonisch, S. Brain Microvascular Pericytes-More than Bystanders in Breast Cancer Brain Metastasis. Cells 2022, 11, 1263. [Google Scholar] [CrossRef]
- Molnár, K.; Mészáros, Á.; Fazakas, C.; Kozma, M.; Győri, F.; Reisz, Z.; Tiszlavicz, L.; Farkas, A.E.; Nyúl-Tóth, Á.; Haskó, J.; et al. Pericyte-Secreted IGF2 Promotes Breast Cancer Brain Metastasis Formation. Mol. Oncol. 2020, 14, 2040–2057. [Google Scholar] [CrossRef] [PubMed]
- Er, E.E.; Valiente, M.; Ganesh, K.; Zou, Y.; Agrawal, S.; Hu, J.; Griscom, B.; Rosenblum, M.; Boire, A.; Brogi, E.; et al. Pericyte-like Spreading by Disseminated Cancer Cells Activates YAP and MRTF for Metastatic Colonization. Nat. Cell Biol. 2018, 20, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Hamilla, S.M.; Stroka, K.M.; Aranda-Espinoza, H. VE-Cadherin-Independent Cancer Cell Incorporation into the Vascular Endothelium Precedes Transmigration. PLoS ONE 2014, 9, e109748. [Google Scholar] [CrossRef] [PubMed]
- Herman, H.; Fazakas, C.; Haskó, J.; Molnár, K.; Mészáros, Á.; Nyúl-Tóth, Á.; Szabó, G.; Erdélyi, F.; Ardelean, A.; Hermenean, A.; et al. Paracellular and Transcellular Migration of Metastatic Cells through the Cerebral Endothelium. J. Cell. Mol. Med. 2019, 23, 2619–2631. [Google Scholar] [CrossRef]
- Wu, K.; Fukuda, K.; Xing, F.; Zhang, Y.; Sharma, S.; Liu, Y.; Chan, M.D.; Zhou, X.; Qasem, S.A.; Pochampally, R.; et al. Roles of the Cyclooxygenase 2 Matrix Metalloproteinase 1 Pathway in Brain Metastasis of Breast Cancer. J. Biol. Chem. 2015, 290, 9842–9854. [Google Scholar] [CrossRef]
- Liu, H.; Kato, Y.; Erzinger, S.A.; Kiriakova, G.M.; Qian, Y.; Palmieri, D.; Steeg, P.S.; Price, J.E. The Role of MMP-1 in Breast Cancer Growth and Metastasis to the Brain in a Xenograft Model. BMC Cancer 2012, 12, 583. [Google Scholar] [CrossRef]
- Conrad, C.; Götte, M.; Schlomann, U.; Roessler, M.; Pagenstecher, A.; Anderson, P.; Preston, J.; Pruessmeyer, J.; Ludwig, A.; Li, R.; et al. ADAM8 Expression in Breast Cancer Derived Brain Metastases: Functional Implications on MMP-9 Expression and Transendothelial Migration in Breast Cancer Cells. Int. J. Cancer 2018, 142, 779–791. [Google Scholar] [CrossRef]
- Rodriguez, P.L.; Jiang, S.; Fu, Y.; Avraham, S.; Avraham, H.K. The Proinflammatory Peptide Substance P Promotes Blood-Brain Barrier Breaching by Breast Cancer Cells through Changes in Microvascular Endothelial Cell Tight Junctions. Int. J. Cancer 2014, 134, 1034–1044. [Google Scholar] [CrossRef]
- Avraham, H.K.; Jiang, S.; Fu, Y.; Nakshatri, H.; Ovadia, H.; Avraham, S. Angiopoietin-2 Mediates Blood-Brain Barrier Impairment and Colonization of Triple-Negative Breast Cancer Cells in Brain. J. Pathol. 2014, 232, 369–381. [Google Scholar] [CrossRef]
- Lee, T.-H.; Avraham, H.K.; Jiang, S.; Avraham, S. Vascular Endothelial Growth Factor Modulates the Transendothelial Migration of MDA-MB-231 Breast Cancer Cells through Regulation of Brain Microvascular Endothelial Cell Permeability. J. Biol. Chem. 2003, 278, 5277–5284. [Google Scholar] [CrossRef]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of Tumour- and Stroma-Supplied Proteolytic Networks Reveals a Brain-Metastasis-Promoting Role for Cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sullivan, P.; Suyama, J.; Marchetti, D. Epidermal Growth Factor-Induced Heparanase Nucleolar Localization Augments DNA Topoisomerase I Activity in Brain Metastatic Breast Cancer. Mol. Cancer Res. 2010, 8, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Ilan, N.; Elkin, M.; Vlodavsky, I. Regulation, Function and Clinical Significance of Heparanase in Cancer Metastasis and Angiogenesis. Int. J. Biochem. Cell Biol. 2006, 38, 2018–2039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sullivan, P.S.; Goodman, J.C.; Gunaratne, P.H.; Marchetti, D. MicroRNA-1258 Suppresses Breast Cancer Brain Metastasis by Targeting Heparanase. Cancer Res. 2011, 71, 645–654. [Google Scholar] [CrossRef]
- Ridgway, L.D.; Wetzel, M.D.; Ngo, J.A.; Erdreich-Epstein, A.; Marchetti, D. Heparanase-Induced GEF-H1 Signaling Regulates the Cytoskeletal Dynamics of Brain Metastatic Breast Cancer Cells. Mol. Cancer Res. 2012, 10, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, S.; Morales, J.E.; Kwiatkowski, S.C.; Lang, F.F.; Rao, G.; McCarty, J.H. Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci. Rep. 2018, 8, 8267. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, L.A.; Yeo, Y.K. Health Benefits of Docosahexaenoic Acid (DHA). Pharmacol. Res. 1999, 40, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Wu, T.; Lim, K. Omega-3 Polyunsaturated Fatty Acids and Cancer. Anticancer Agents Med. Chem. 2013, 13, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Tsuta, K.; Ono, M.; Shimizu, C.; Hirakawa, A.; Hasegawa, T.; Hatanaka, Y.; Narita, Y.; Shibui, S.; Fujiwara, Y. Disruption of the Blood Brain Barrier by Brain Metastases of Triple-Negative and Basal-Type Breast Cancer but Not HER2/Neu-Positive Breast Cancer. Cancer 2010, 116, 302–308. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.-F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes That Mediate Breast Cancer Metastasis to the Brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef]
- Mustafa, D.A.M.; Pedrosa, R.M.S.M.; Smid, M.; van der Weiden, M.; de Weerd, V.; Nigg, A.L.; Berrevoets, C.; Zeneyedpour, L.; Priego, N.; Valiente, M.; et al. T Lymphocytes Facilitate Brain Metastasis of Breast Cancer by Inducing Guanylate-Binding Protein 1 Expression. Acta Neuropathol. 2018, 135, 581–599. [Google Scholar] [CrossRef]
- Klotz, R.; Thomas, A.; Teng, T.; Han, S.M.; Iriondo, O.; Li, L.; Restrepo-Vassalli, S.; Wang, A.; Izadian, N.; MacKay, M.; et al. Circulating Tumor Cells Exhibit Metastatic Tropism and Reveal Brain Metastasis Drivers. Cancer Discov. 2020, 10, 86–103. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Buhamrah, A.; Schneider, A.; Lin, Y.-L.; Zhou, H.; Bugshan, A.; Basile, J.R. Semaphorin 4D Promotes Skeletal Metastasis in Breast Cancer. PLoS ONE 2016, 11, e0150151. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.S.; Jonason, A.; Reilly, C.; Veeraraghavan, J.; Fisher, T.; Doherty, M.; Klimatcheva, E.; Mallow, C.; Cornelius, C.; Leonard, J.E.; et al. SEMA4D Compromises Blood-Brain Barrier, Activates Microglia, and Inhibits Remyelination in Neurodegenerative Disease. Neurobiol. Dis. 2015, 73, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Momeny, M.; Saunus, J.M.; Marturana, F.; McCart Reed, A.E.; Black, D.; Sala, G.; Iacobelli, S.; Holland, J.D.; Yu, D.; Da Silva, L.; et al. Heregulin-HER3-HER2 Signaling Promotes Matrix Metalloproteinase-Dependent Blood-Brain-Barrier Transendothelial Migration of Human Breast Cancer Cell Lines. Oncotarget 2015, 6, 3932–3946. [Google Scholar] [CrossRef]
- Palmieri, D.; Bronder, J.L.; Herring, J.M.; Yoneda, T.; Weil, R.J.; Stark, A.M.; Kurek, R.; Vega-Valle, E.; Feigenbaum, L.; Halverson, D.; et al. Her-2 Overexpression Increases the Metastatic Outgrowth of Breast Cancer Cells in the Brain. Cancer Res. 2007, 67, 4190–4198. [Google Scholar] [CrossRef] [PubMed]
- Oshima, A. Structure and Closure of Connexin Gap Junction Channels. FEBS Lett. 2014, 588, 1230–1237. [Google Scholar] [CrossRef]
- Stoletov, K.; Strnadel, J.; Zardouzian, E.; Momiyama, M.; Park, F.D.; Kelber, J.A.; Pizzo, D.P.; Hoffman, R.; VandenBerg, S.R.; Klemke, R.L. Role of Connexins in Metastatic Breast Cancer and Melanoma Brain Colonization. J. Cell Sci. 2013, 126, 904–913. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Gadalla, R.; El-Ghonaimy, E.A.; Samir, O.; Mohamed, H.T.; Hassan, H.; Greve, B.; El-Shinawi, M.; Mohamed, M.M.; Götte, M. Syndecan-1 Is a Novel Molecular Marker for Triple Negative Inflammatory Breast Cancer and Modulates the Cancer Stem Cell Phenotype via the IL-6/STAT3, Notch and EGFR Signaling Pathways. Mol. Cancer 2017, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Fleurot, E.; Goudin, C.; Hanoux, V.; Bonnamy, P.-J.; Levallet, J. Estrogen Receptor α Regulates the Expression of Syndecan-1 in Human Breast Carcinoma Cells. Endocr. Relat. Cancer 2019, 26, 615–628. [Google Scholar] [CrossRef]
- Sayyad, M.R.; Puchalapalli, M.; Vergara, N.G.; Wangensteen, S.M.; Moore, M.; Mu, L.; Edwards, C.; Anderson, A.; Kall, S.; Sullivan, M.; et al. Syndecan-1 Facilitates Breast Cancer Metastasis to the Brain. Breast Cancer Res. Treat. 2019, 178, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Cordero, A.; Kanojia, D.; Lesniak, M.S. The Network of Cytokines in Brain Metastases. Cancers 2021, 13, 142. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the Chemokine Network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Curtaz, C.J.; Schmitt, C.; Herbert, S.-L.; Feldheim, J.; Schlegel, N.; Gosselet, F.; Hagemann, C.; Roewer, N.; Meybohm, P.; Wöckel, A.; et al. Serum-Derived Factors of Breast Cancer Patients with Brain Metastases Alter Permeability of a Human Blood-Brain Barrier Model. Fluids Barriers CNS 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-C.; Lee, T.-H.; Avraham, S.; Avraham, H.K. Involvement of the Chemokine Receptor CXCR4 and Its Ligand Stromal Cell-Derived Factor 1alpha in Breast Cancer Cell Migration through Human Brain Microvascular Endothelial Cells. Mol. Cancer Res. 2004, 2, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Fazakas, C.; Haskó, J.; Sipos, O.; Nagy, K.; Nyúl-Tóth, Á.; Farkas, A.E.; Végh, A.G.; Váró, G.; Galajda, P.; et al. Transmigration Characteristics of Breast Cancer and Melanoma Cells through the Brain Endothelium: Role of Rac and PI3K. Cell Adh. Migr. 2016, 10, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Krizbai, I.A.; Gasparics, Á.; Nagyőszi, P.; Fazakas, C.; Molnár, J.; Wilhelm, I.; Bencs, R.; Rosivall, L.; Sebe, A. Endothelial-Mesenchymal Transition of Brain Endothelial Cells: Possible Role during Metastatic Extravasation. PLoS ONE 2015, 10, e0123845. [Google Scholar] [CrossRef]
- Clere, N.; Renault, S.; Corre, I. Endothelial-to-Mesenchymal Transition in Cancer. Front. Cell Dev. Biol. 2020, 8, 747. [Google Scholar] [CrossRef]
- Xiao, L.; Kim, D.J.; Davis, C.L.; McCann, J.V.; Dunleavey, J.M.; Vanderlinden, A.K.; Xu, N.; Pattenden, S.G.; Frye, S.V.; Xu, X.; et al. Tumor Endothelial Cells with Distinct Patterns of TGFβ-Driven Endothelial-to-Mesenchymal Transition. Cancer Res. 2015, 75, 1244–1254. [Google Scholar] [CrossRef]
- Dong, H.; Lei, J.; Ding, L.; Wen, Y.; Ju, H.; Zhang, X. MicroRNA: Function, Detection, and Bioanalysis. Chem. Rev. 2013, 113, 6207–6233. [Google Scholar] [CrossRef] [PubMed]
- Jansson, M.D.; Lund, A.H. MicroRNA and Cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef]
- Di Leva, G.; Croce, C.M. Roles of Small RNAs in Tumor Formation. Trends Mol. Med. 2010, 16, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Kanchan, R.K.; Siddiqui, J.A.; Mahapatra, S.; Batra, S.K.; Nasser, M.W. MicroRNAs Orchestrate Pathophysiology of Breast Cancer Brain Metastasis: Advances in Therapy. Mol. Cancer 2020, 19, 29. [Google Scholar] [CrossRef]
- Hammad, S.; Mabondzo, A.; Hamoudi, R.; Harati, R. Regulation of P-Glycoprotein by MiR-27a-3p at the Brain Endothelial Barrier. J. Pharm. Sci. 2022, 111, 1470–1479. [Google Scholar] [CrossRef]
- Harati, R.; Hammad, S.; Tlili, A.; Mahfood, M.; Mabondzo, A.; Hamoudi, R. MiR-27a-3p Regulates Expression of Intercellular Junctions at the Brain Endothelium and Controls the Endothelial Barrier Permeability. PLoS ONE 2022, 17, e0262152. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Sharma, S.; Liu, Y.; Mo, Y.-Y.; Wu, K.; Zhang, Y.-Y.; Pochampally, R.; Martinez, L.A.; Lo, H.-W.; Watabe, K. MiR-509 Suppresses Brain Metastasis of Breast Cancer Cells by Modulating RhoC and TNF-α. Oncogene 2015, 34, 4890–4900. [Google Scholar] [CrossRef]
- Harati, R.; Mohammad, M.G.; Tlili, A.; El-Awady, R.A.; Hamoudi, R. Loss of MiR-101-3p Promotes Transmigration of Metastatic Breast Cancer Cells through the Brain Endothelium by Inducing COX-2/MMP1 Signaling. Pharmaceuticals 2020, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Harati, R.; Mabondzo, A.; Tlili, A.; Khoder, G.; Mahfood, M.; Hamoudi, R. Combinatorial Targeting of MicroRNA-26b and MicroRNA-101 Exerts a Synergistic Inhibition on Cyclooxygenase-2 in Brain Metastatic Triple-Negative Breast Cancer Cells. Breast Cancer Res. Treat. 2021, 187, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Harati, R.; Hafezi, S.; Mabondzo, A.; Tlili, A. Silencing MiR-202-3p Increases MMP-1 and Promotes a Brain Invasive Phenotype in Metastatic Breast Cancer Cells. PLoS ONE 2020, 15, e0239292. [Google Scholar] [CrossRef]
- Hammash, D.; Mahfood, M.; Khoder, G.; Ahmed, M.; Tlili, A.; Hamoudi, R.; Harati, R. MiR-623 Targets Metalloproteinase-1 and Attenuates Extravasation of Brain Metastatic Triple-Negative Breast Cancer Cells. Breast Cancer (Dove Med. Press) 2022, 14, 187–198. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain Metastatic Cancer Cells Release MicroRNA-181c-Containing Extracellular Vesicles Capable of Destructing Blood-Brain Barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.-J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Prim. 2015, 1, 15003. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Modesto, A.; Chira, C.; Sol, J.-C.; Lubrano, V.; Boulinguez, S.; Pagès, C.; Sibaud, V.; Gomez-Roca, C.; Moyal, É.; Meyer, N. [Treatment of patients with brain metastases from a melanoma]. Cancer Radiother. 2019, 23, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Samlowski, W.E.; Moon, J.; Witter, M.; Atkins, M.B.; Kirkwood, J.M.; Othus, M.; Ribas, A.; Sondak, V.K.; Flaherty, L.E. High Frequency of Brain Metastases after Adjuvant Therapy for High-Risk Melanoma. Cancer Med. 2017, 6, 2576–2585. [Google Scholar] [CrossRef] [PubMed]
- McAleer, M.F.; Kim, D.W.; Trinh, V.A.; Hwu, W.-J. Management of Melanoma Brain Metastases. Melanoma Manag. 2015, 2, 225–239. [Google Scholar] [CrossRef]
- Vosoughi, E.; Lee, J.M.; Miller, J.R.; Nosrati, M.; Minor, D.R.; Abendroth, R.; Lee, J.W.; Andrews, B.T.; Leng, L.Z.; Wu, M.; et al. Survival and Clinical Outcomes of Patients with Melanoma Brain Metastasis in the Era of Checkpoint Inhibitors and Targeted Therapies. BMC Cancer 2018, 18, 490. [Google Scholar] [CrossRef]
- Phadke, M.; Ozgun, A.; Eroglu, Z.; Smalley, K.S.M. Melanoma Brain Metastases: Biological Basis and Novel Therapeutic Strategies. Exp. Dermatol. 2022, 31, 31–42. [Google Scholar] [CrossRef]
- Bedikian, A.Y.; Wei, C.; Detry, M.; Kim, K.B.; Papadopoulos, N.E.; Hwu, W.-J.; Homsi, J.; Davies, M.; McIntyre, S.; Hwu, P. Predictive Factors for the Development of Brain Metastasis in Advanced Unresectable Metastatic Melanoma. Am. J. Clin. Oncol. 2011, 34, 603–610. [Google Scholar] [CrossRef]
- Eroglu, Z.; Topcu, T.O.; Yu, H.M.; Margolin, K.A. How I Treat Brain Metastases of Melanoma. ESMO Open 2022, 7, 100598. [Google Scholar] [CrossRef]
- Stallcup, W.B.; You, W.-K.; Kucharova, K.; Cejudo-Martin, P.; Yotsumoto, F. NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression. Microcirculation 2016, 23, 122–133. [Google Scholar] [CrossRef]
- Xie, T.; Huang, F.-J.; Aldape, K.D.; Kang, S.-H.; Liu, M.; Gershenwald, J.E.; Xie, K.; Sawaya, R.; Huang, S. Activation of Stat3 in Human Melanoma Promotes Brain Metastasis. Cancer Res. 2006, 66, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Vasco, C.; Girgenti, V.; Fugnanesi, V.; Calatozzolo, C.; Canazza, A.; Salmaggi, A.; Rivoltini, L.; Morbin, M.; Ciusani, E. Melanoma Cells Homing to the Brain: An in Vitro Model. Biomed. Res. Int. 2015, 2015, 476069. [Google Scholar] [CrossRef]
- Herwig, N.; Belter, B.; Wolf, S.; Haase-Kohn, C.; Pietzsch, J. Interaction of Extracellular S100A4 with RAGE Prompts Prometastatic Activation of A375 Melanoma Cells. J. Cell. Mol. Med. 2016, 20, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Herwig, N.; Belter, B.; Pietzsch, J. Extracellular S100A4 Affects Endothelial Cell Integrity and Stimulates Transmigration of A375 Melanoma Cells. Biochem. Biophys. Res. Commun. 2016, 477, 963–969. [Google Scholar] [CrossRef]
- Fazakas, C.; Wilhelm, I.; Nagyoszi, P.; Farkas, A.E.; Haskó, J.; Molnár, J.; Bauer, H.; Bauer, H.-C.; Ayaydin, F.; Dung, N.T.K.; et al. Transmigration of Melanoma Cells through the Blood-Brain Barrier: Role of Endothelial Tight Junctions and Melanoma-Released Serine Proteases. PLoS ONE 2011, 6, e20758. [Google Scholar] [CrossRef]
- Artym, V.V.; Kindzelskii, A.L.; Chen, W.-T.; Petty, H.R. Molecular Proximity of Seprase and the Urokinase-Type Plasminogen Activator Receptor on Malignant Melanoma Cell Membranes: Dependence on Beta1 Integrins and the Cytoskeleton. Carcinogenesis 2002, 23, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Murry, B.P.; Blust, B.E.; Singh, A.; Foster, T.P.; Marchetti, D. Heparanase Mechanisms of Melanoma Metastasis to the Brain: Development and Use of a Brain Slice Model. J. Cell. Biochem. 2006, 97, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, H.; Chen, H.; Jiang, X.; Fang, D.; Wang, Y.; Zhu, D. An Artificial MiRNA against HPSE Suppresses Melanoma Invasion Properties, Correlating with a down-Regulation of Chemokines and MAPK Phosphorylation. PLoS ONE 2012, 7, e38659. [Google Scholar] [CrossRef] [PubMed]
- Jilaveanu, L.B.; Parisi, F.; Barr, M.L.; Zito, C.R.; Cruz-Munoz, W.; Kerbel, R.S.; Rimm, D.L.; Bosenberg, M.W.; Halaban, R.; Kluger, Y.; et al. PLEKHA5 as a Biomarker and Potential Mediator of Melanoma Brain Metastasis. Clin. Cancer Res. 2015, 21, 2138–2147. [Google Scholar] [CrossRef]
- Perides, G.; Zhuge, Y.; Lin, T.; Stins, M.F.; Bronson, R.T.; Wu, J.K. The Fibrinolytic System Facilitates Tumor Cell Migration across the Blood-Brain Barrier in Experimental Melanoma Brain Metastasis. BMC Cancer 2006, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Bertrand, Y.; Michaud-Levesque, J.; Jodoin, J.; Rolland, Y.; Gabathuler, R.; Béliveau, R. Regulation of Plasminogen Activation: A Role for Melanotransferrin (P97) in Cell Migration. Blood 2003, 102, 1723–1731. [Google Scholar] [CrossRef]
- Michaud-Levesque, J.; Demeule, M.; Béliveau, R. Stimulation of Cell Surface Plasminogen Activation by Membrane-Bound Melanotransferrin: A Key Phenomenon for Cell Invasion. Exp. Cell Res. 2005, 308, 479–490. [Google Scholar] [CrossRef]
- Rolland, Y.; Demeule, M.; Fenart, L.; Béliveau, R. Inhibition of Melanoma Brain Metastasis by Targeting Melanotransferrin at the Cell Surface. Pigment. Cell Melanoma Res. 2009, 22, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Schwartz, H.; Sagi-Assif, O.; Meshel, T.; Izraely, S.; Ben Menachem, S.; Bengaiev, R.; Ben-Shmuel, A.; Nahmias, C.; Couraud, P.-O.; et al. Astrocytes Facilitate Melanoma Brain Metastasis via Secretion of IL-23. J. Pathol. 2015, 236, 116–127. [Google Scholar] [CrossRef]
- Doron, H.; Amer, M.; Ershaid, N.; Blazquez, R.; Shani, O.; Lahav, T.G.; Cohen, N.; Adler, O.; Hakim, Z.; Pozzi, S.; et al. Inflammatory Activation of Astrocytes Facilitates Melanoma Brain Tropism via the CXCL10-CXCR3 Signaling Axis. Cell Rep. 2019, 28, 1785–1798.e6. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Sagi-Assif, O.; Meshel, T.; Telerman, A.; Izraely, S.; Ben-Menachem, S.; Bayry, J.; Marzese, D.M.; Ohe, S.; Hoon, D.S.B.; et al. CCR4 Is a Determinant of Melanoma Brain Metastasis. Oncotarget 2017, 8, 31079–31091. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-Β1-Mediated Exosomal Lnc-MMP2-2 Increases Blood-Brain Barrier Permeability via the MiRNA-1207-5p/EPB41L5 Axis to Promote Non-Small Cell Lung Cancer Brain Metastasis. Cell Death Dis. 2021, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- García-Martín, A.B.; Zwicky, P.; Gruber, T.; Matti, C.; Moalli, F.; Stein, J.V.; Francisco, D.; Enzmann, G.; Levesque, M.P.; Hewer, E.; et al. VLA-4 Mediated Adhesion of Melanoma Cells on the Blood-Brain Barrier Is the Critical Cue for Melanoma Cell Intercalation and Barrier Disruption. J. Cereb. Blood Flow Metab. 2019, 39, 1995–2010. [Google Scholar] [CrossRef]
- Gajos-Michniewicz, A.; Czyz, M. Role of MiRNAs in Melanoma Metastasis. Cancers 2019, 11, 326. [Google Scholar] [CrossRef]
- Hanniford, D.; Zhong, J.; Koetz, L.; Gaziel-Sovran, A.; Lackaye, D.J.; Shang, S.; Pavlick, A.; Shapiro, R.; Berman, R.; Darvishian, F.; et al. A MiRNA-Based Signature Detected in Primary Melanoma Tissue Predicts Development of Brain Metastasis. Clin. Cancer Res. 2015, 21, 4903–4912. [Google Scholar] [CrossRef]
- Hwang, S.J.; Seol, H.J.; Park, Y.M.; Kim, K.H.; Gorospe, M.; Nam, D.-H.; Kim, H.H. MicroRNA-146a Suppresses Metastatic Activity in Brain Metastasis. Mol. Cells 2012, 34, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Moubarak, R.S.; Koetz-Ploch, L.; Mullokandov, G.; Gaziel, A.; de Pablos-Aragoneses, A.; Argibay, D.; Kleffman, K.; Sokolova, E.; Berwick, M.; Thomas, N.E.; et al. In Vivo MiRNA Decoy Screen Reveals MiR-124a as a Suppressor of Melanoma Metastasis. Front. Oncol. 2022, 12, 852952. [Google Scholar] [CrossRef]
- Lu, S.; Xu, Q. MicroRNA-23a Inhibits Melanoma Cell Proliferation, Migration, and Invasion in Mice through a Negative Feedback Regulation of Sdcbp and the MAPK/ERK Signaling Pathway. IUBMB Life 2019, 71, 587–600. [Google Scholar] [CrossRef]
- Xu, D.; Chen, X.; He, Q.; Luo, C. MicroRNA-9 Suppresses the Growth, Migration, and Invasion of Malignant Melanoma Cells via Targeting NRP1. Onco Targets Ther. 2016, 9, 7047–7057. [Google Scholar] [CrossRef]
- Fawzy, M.S.; Ibrahiem, A.T.; Bayomy, N.A.; Makhdoom, A.K.; Alanazi, K.S.; Alanazi, A.M.; Mukhlef, A.M.; Toraih, E.A. MicroRNA-155 and Disease-Related Immunohistochemical Parameters in Cutaneous Melanoma. Diagnostics 2023, 13, 1205. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Kang, Y.; Jin, Y.; Li, Q.; Yuan, X. Advances in Lung Cancer Driver Genes Associated With Brain Metastasis. Front. Oncol. 2020, 10, 606300. [Google Scholar] [CrossRef]
- Steeg, P.S.; Camphausen, K.A.; Smith, Q.R. Brain Metastases as Preventive and Therapeutic Targets. Nat. Rev. Cancer 2011, 11, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Münsterberg, J.; Loreth, D.; Brylka, L.; Werner, S.; Karbanova, J.; Gandrass, M.; Schneegans, S.; Besler, K.; Hamester, F.; Robador, J.R.; et al. ALCAM Contributes to Brain Metastasis Formation in Non-Small-Cell Lung Cancer through Interaction with the Vascular Endothelium. Neuro Oncol. 2020, 22, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Jassam, S.A.; Maherally, Z.; Smith, J.R.; Ashkan, K.; Roncaroli, F.; Fillmore, H.L.; Pilkington, G.J. TNF-α Enhancement of CD62E Mediates Adhesion of Non-Small Cell Lung Cancer Cells to Brain Endothelium via CD15 in Lung-Brain Metastasis. Neuro Oncol. 2016, 18, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Jassam, S.A.; Maherally, Z.; Smith, J.R.; Ashkan, K.; Roncaroli, F.; Fillmore, H.L.; Pilkington, G.J. CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis. Int. J. Mol. Sci. 2017, 18, 1474. [Google Scholar] [CrossRef]
- Jassam, S.A.; Maherally, Z.; Ashkan, K.; Pilkington, G.J.; Fillmore, H.L. Fucosyltransferase 4 and 7 Mediates Adhesion of Non-Small Cell Lung Cancer Cells to Brain-Derived Endothelial Cells and Results in Modification of the Blood-Brain-Barrier: In Vitro Investigation of CD15 and CD15s in Lung-to-Brain Metastasis. J. Neurooncol. 2019, 143, 405–415. [Google Scholar] [CrossRef]
- Shintani, Y.; Higashiyama, S.; Ohta, M.; Hirabayashi, H.; Yamamoto, S.; Yoshimasu, T.; Matsuda, H.; Matsuura, N. Overexpression of ADAM9 in Non-Small Cell Lung Cancer Correlates with Brain Metastasis. Cancer Res. 2004, 64, 4190–4196. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-C.; Li, Q.; Peng, J.-Y.; Zhouwen, J.-L.; Diao, J.-F.; Niu, J.-X.; Wang, X.; Guan, X.-D.; Jia, W.; Jiang, W.-G. Claudin-5 Regulates Blood-Brain Barrier Permeability by Modifying Brain Microvascular Endothelial Cell Proliferation, Migration, and Adhesion to Prevent Lung Cancer Metastasis. CNS Neurosci. Ther. 2017, 23, 947–960. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Zhou, C.; Chen, X.; Cao, Y. Adenosine A2A Receptor Activation Reduces Brain Metastasis via SDF-1/CXCR4 Axis and Protecting Blood-Brain Barrier. Mol. Carcinog. 2020, 59, 390–398. [Google Scholar] [CrossRef]
- Liu, W.; Song, J.; Du, X.; Zhou, Y.; Li, Y.; Li, R.; Lyu, L.; He, Y.; Hao, J.; Ben, J.; et al. AKR1B10 (Aldo-Keto Reductase Family 1 B10) Promotes Brain Metastasis of Lung Cancer Cells in a Multi-Organ Microfluidic Chip Model. Acta Biomater. 2019, 91, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.-S.; Wu, P.-F.; Li, Q.; Dai, W.-M.; Yuan, S.; Xu, Z.-H.; Liu, T.-T.; Miao, Z.-W.; Fang, W.-G.; et al. Brain Microvascular Endothelium Induced-Annexin A1 Secretion Contributes to Small Cell Lung Cancer Brain Metastasis. Int. J. Biochem. Cell Biol. 2015, 66, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, W.-D.; Tan, Z.-M.; Fang, W.-G.; Zhu, L.; Chen, Y.-H. Involvement of Rho/ROCK Signalling in Small Cell Lung Cancer Migration through Human Brain Microvascular Endothelial Cells. FEBS Lett. 2006, 580, 4252–4260. [Google Scholar] [CrossRef]
- Li, B.; Wang, C.; Zhang, Y.; Zhao, X.Y.; Huang, B.; Wu, P.F.; Li, Q.; Li, H.; Liu, Y.S.; Cao, L.Y.; et al. Elevated PLGF Contributes to Small-Cell Lung Cancer Brain Metastasis. Oncogene 2013, 32, 2952–2962. [Google Scholar] [CrossRef]
- Adeghate, E. Visfatin: Structure, Function and Relation to Diabetes Mellitus and Other Dysfunctions. Curr. Med. Chem. 2008, 15, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Miao, Z.; Jiang, J.; Yuan, S.; Fang, W.; Li, B.; Chen, Y. Visfatin Mediates SCLC Cells Migration across Brain Endothelial Cells through Upregulation of CCL2. Int. J. Mol. Sci. 2015, 16, 11439–11451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Karschnia, P.; von Mücke-Heim, I.-A.; Mulazzani, M.; Zhou, X.; Blobner, J.; Mueller, N.; Teske, N.; Dede, S.; Xu, T.; et al. In Vivo Two-Photon Characterization of Tumor-Associated Macrophages and Microglia (TAM/M) and CX3CR1 during Different Steps of Brain Metastasis Formation from Lung Cancer. Neoplasia 2021, 23, 1089–1100. [Google Scholar] [CrossRef]
- Seike, T.; Fujita, K.; Yamakawa, Y.; Kido, M.A.; Takiguchi, S.; Teramoto, N.; Iguchi, H.; Noda, M. Interaction between Lung Cancer Cells and Astrocytes via Specific Inflammatory Cytokines in the Microenvironment of Brain Metastasis. Clin. Exp. Metastasis 2011, 28, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, S. CXCR4/CXCL12 in Non-Small-Cell Lung Cancer Metastasis to the Brain. Int. J. Mol. Sci. 2013, 14, 1713–1727. [Google Scholar] [CrossRef]
- Ciccone, V.; Filippelli, A.; Bacchella, C.; Monzani, E.; Morbidelli, L. The Nitric Oxide Donor [Zn(PipNONO)Cl] Exhibits Antitumor Activity through Inhibition of Epithelial and Endothelial Mesenchymal Transitions. Cancers 2022, 14, 4240. [Google Scholar] [CrossRef]
- Wu, D.-M.; Liu, T.; Deng, S.-H.; Han, R.; Zhang, T.; Li, J.; Xu, Y. Alpha-1 Antitrypsin Induces Epithelial-to-Mesenchymal Transition, Endothelial-to-Mesenchymal Transition, and Drug Resistance in Lung Cancer Cells. Onco Targets Ther. 2020, 13, 3751–3763. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Q.; Lv, Z.; Ling, Y.; Hou, X.; Chen, Z.; Dinglin, X.; Ma, S.; Li, D.; Wu, Y.; et al. N6-Methyladenosine Induced MiR-143-3p Promotes the Brain Metastasis of Lung Cancer via Regulation of VASH1. Mol. Cancer 2019, 18, 181. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Li, X.-Y.; Zhao, Y.-Q.; Liu, W.-J.; Wu, H.-J.; Liu, J.; Mu, X.-Q.; Wu, H.-B. Down-Regulated MicroRNA-375 Expression as a Predictive Biomarker in Non-Small Cell Lung Cancer Brain Metastasis and Its Prognostic Significance. Pathol. Res. Pract. 2017, 213, 882–888. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Ma, M.; Liu, J.; Xu, Y.; Ye, L.; Hou, H.; Wang, C.; Li, X.; Jiang, Y. The Predictive Value and Potential Mechanisms of MiRNA-328 and MiRNA-378 for Brain Metastases in Operable and Advanced Non-Small-Cell Lung Cancer. Jpn. J. Clin. Oncol. 2015, 45, 464–473. [Google Scholar] [CrossRef]
- Vogetseder, A.; Thies, S.; Ingold, B.; Roth, P.; Weller, M.; Schraml, P.; Goodman, S.L.; Moch, H. Av-Integrin Isoform Expression in Primary Human Tumors and Brain Metastases. Int. J. Cancer 2013, 133, 2362–2371. [Google Scholar] [CrossRef]
- Wu, Y.J.; Pagel, M.A.; Muldoon, L.L.; Fu, R.; Neuwelt, E.A. High Av Integrin Level of Cancer Cells Is Associated with Development of Brain Metastasis in Athymic Rats. Anticancer Res. 2017, 37, 4029–4040. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Morad, G.; Jedinak, A.; Moses, M.A. Metalloproteinases and Their Roles in Human Cancer. Anat. Rec. (Hoboken) 2020, 303, 1557–1572. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Mercurio, A.M. VEGF Targets the Tumour Cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Nakayama, J.; Han, Y.; Kuroiwa, Y.; Azuma, K.; Yamamoto, Y.; Semba, K. The In Vivo Selection Method in Breast Cancer Metastasis. Int. J. Mol. Sci. 2021, 22, 1886. [Google Scholar] [CrossRef]
- Niessner, H.; Schmitz, J.; Tabatabai, G.; Schmid, A.M.; Calaminus, C.; Sinnberg, T.; Weide, B.; Eigentler, T.K.; Garbe, C.; Schittek, B.; et al. PI3K Pathway Inhibition Achieves Potent Antitumor Activity in Melanoma Brain Metastases In Vitro and In Vivo. Clin. Cancer Res. 2016, 22, 5818–5828. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Dong, X.; Lu, H.; Tong, F.; Chen, L.; Zhang, R.; Dong, J.; Hu, Y.; Wu, G.; Dong, X. LPCAT1 Promotes Brain Metastasis of Lung Adenocarcinoma by Up-Regulating PI3K/AKT/MYC Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 95. [Google Scholar] [CrossRef] [PubMed]
- Potenta, S.; Zeisberg, E.; Kalluri, R. The Role of Endothelial-to-Mesenchymal Transition in Cancer Progression. Br. J. Cancer 2008, 99, 1375–1379. [Google Scholar] [CrossRef]
- Lopez-Rincon, A.; Martinez-Archundia, M.; Martinez-Ruiz, G.U.; Schoenhuth, A.; Tonda, A. Automatic Discovery of 100-MiRNA Signature for Cancer Classification Using Ensemble Feature Selection. BMC Bioinform. 2019, 20, 480. [Google Scholar] [CrossRef]
- Wang, S.; Liu, N.; Tang, Q.; Sheng, H.; Long, S.; Wu, W. MicroRNA-24 in Cancer: A Double Side Medal With Opposite Properties. Front. Oncol. 2020, 10, 553714. [Google Scholar] [CrossRef]
- Strell, C.; Entschladen, F. Extravasation of Leukocytes in Comparison to Tumor Cells. Cell Commun. Signal 2008, 6, 10. [Google Scholar] [CrossRef]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment Strategies for Breast Cancer Brain Metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Sarmey, N.; Kaisman-Elbaz, T.; Mohammadi, A.M. Management Strategies for Large Brain Metastases. Front. Oncol. 2022, 12, 827304. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Cheng, H.; Wang, X.; Vishnoi, M.; Teh, B.S.; Rostomily, R.; Chang, J.; Wong, S.T.; Zhao, H. Emerging Treatment Strategies for Breast Cancer Brain Metastasis: From Translational Therapeutics to Real-World Experience. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936151. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-L.; Le, A.; Lam, F.C.; Scherrer, E.; Kerr, R.G.; Lau, A.C.; Han, J.; Jiang, R.; Diede, S.J.; Shui, I.M. Current Treatment Approaches and Global Consensus Guidelines for Brain Metastases in Melanoma. Front. Oncol. 2022, 12, 885472. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Hernanz, R.; Vallejo, C.; Guerrero, L.; Mielgo, X.; Lopez, A.; Trujillo-Reyes, J.C.; Couñago, F. Brain Metastases from Non-Small Cell Lung Carcinoma: An Overview of Classical and Novel Treatment Strategies. Rep. Pract. Oncol. Radiother 2022, 27, 527–544. [Google Scholar] [CrossRef]
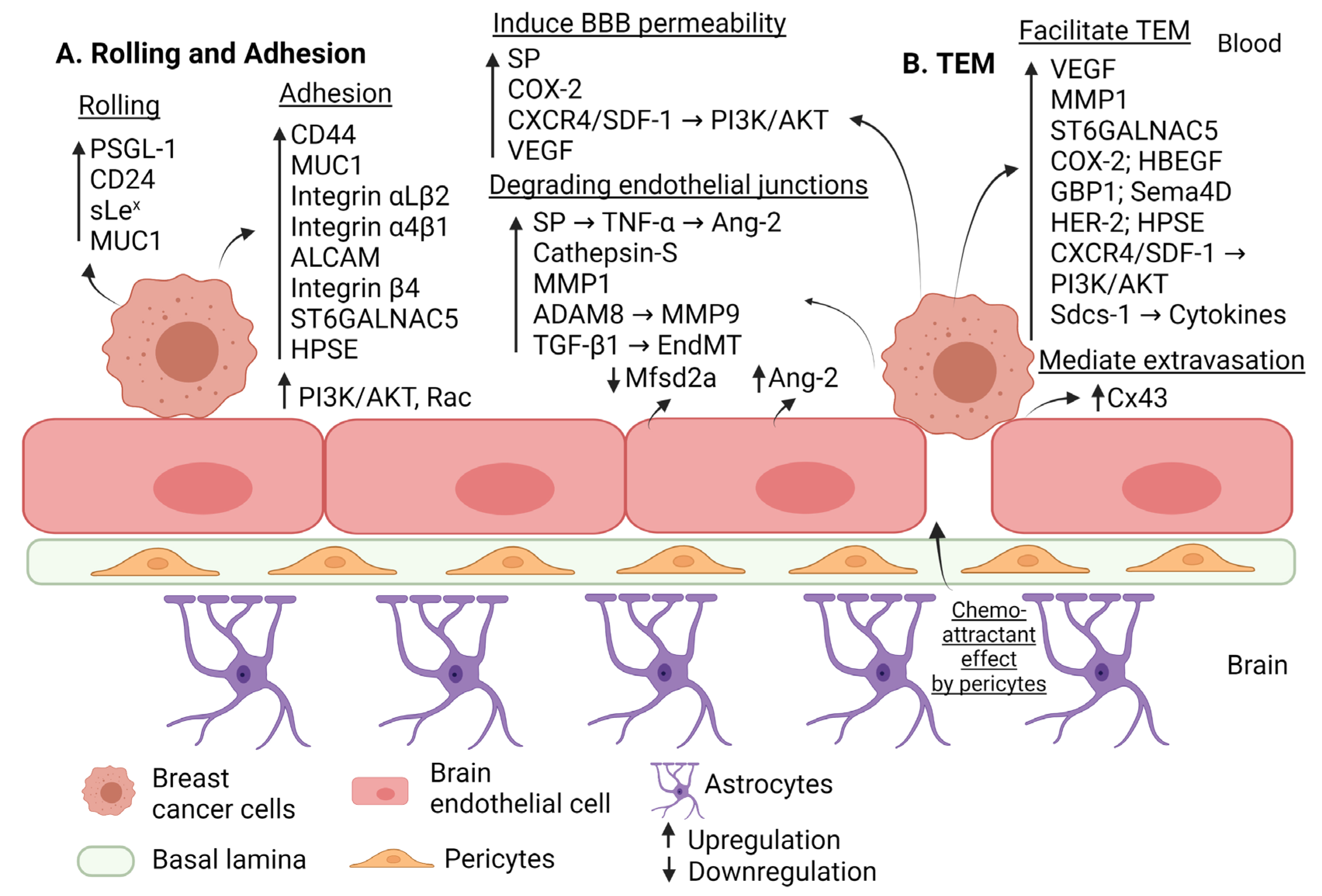
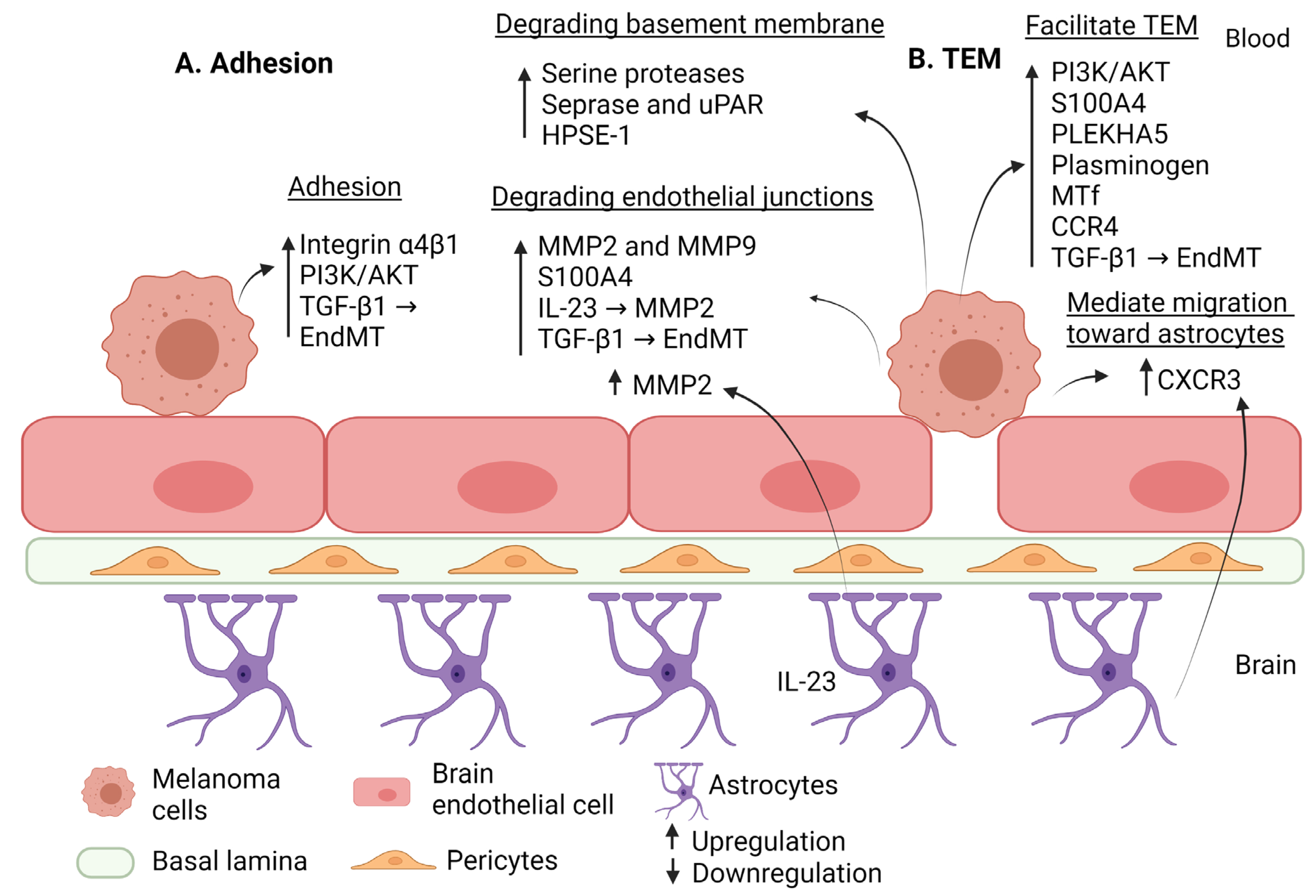
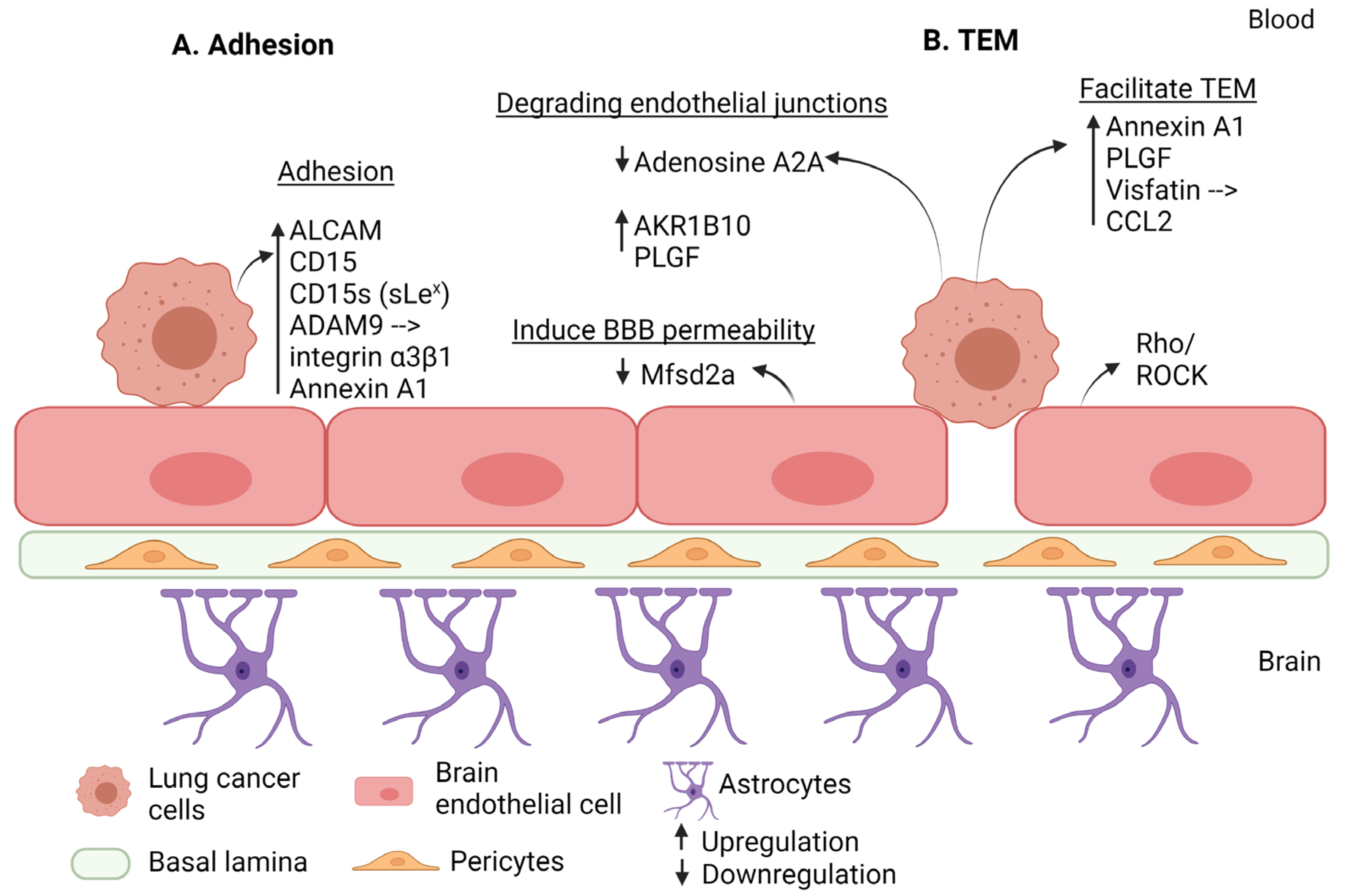
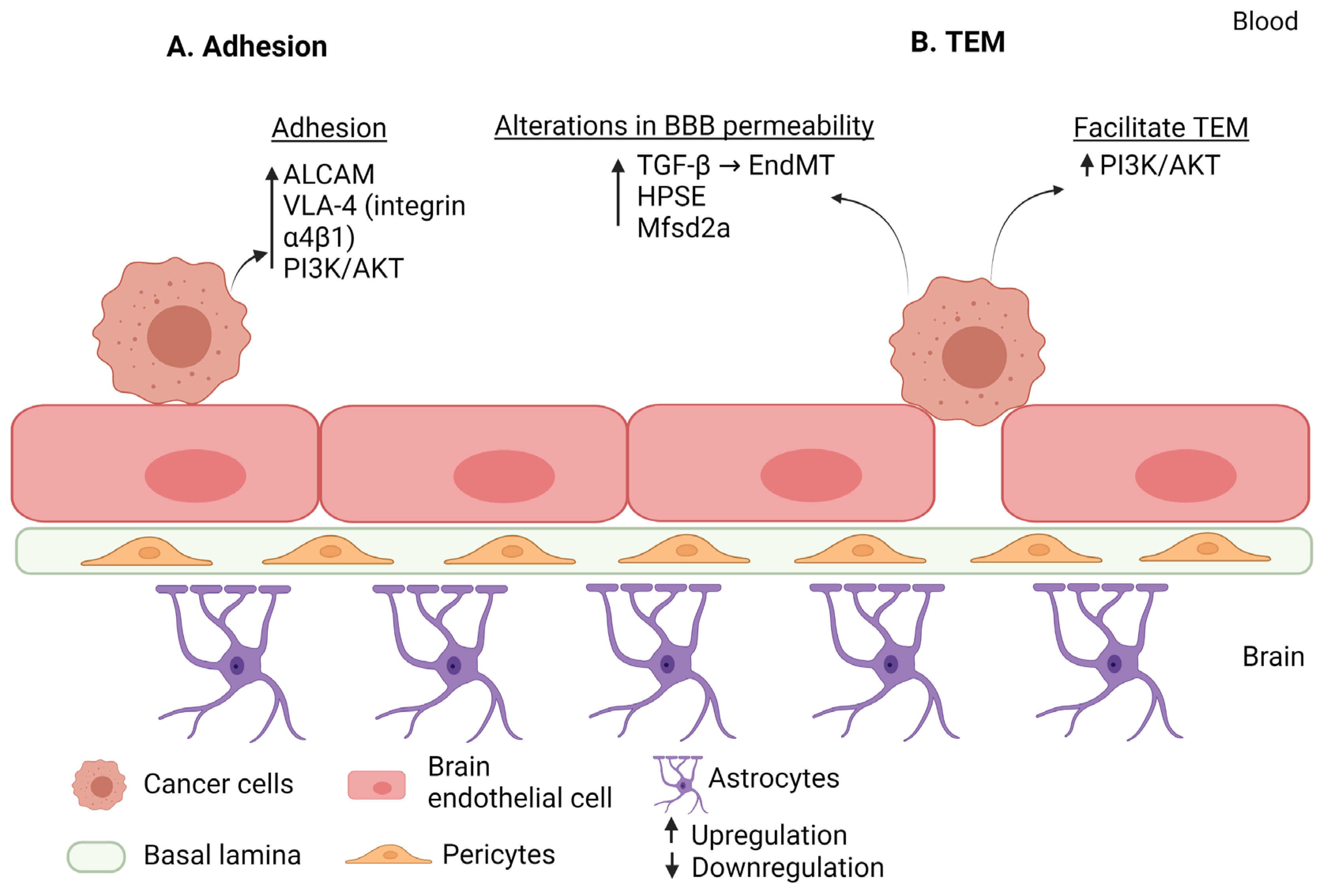
| Step of Extravasation | Factor | Effect | Regulation in BM | Reference |
|---|---|---|---|---|
| Rolling | PSGL-1 CD24 sLex MUC1 | Interacts with endothelial E-selectin to induce rolling. | Upregulation | [28] [29] [29] [33] |
| Adhesion | ALCAM | Interacts with endothelial ALCAM to induce adhesion. | Upregulation | [28] |
| VLA-4 (integrin α4β1) | Interacts with endothelial VCAM-1 to induce adhesion. | Upregulation | [28] | |
| CD 44 | Interacts with endothelial E-selectin to induce adhesion. | Upregulation | [30] | |
| Hyaluronan | Interacts with CD44 to induce adhesion, BE disruption and migration of triple negative breast cancer. | Upregulation | [32] | |
| LFA-1 (integrin αLβ2) MUC1 | Interacts with endothelial ICAM-1 to induce adhesion. | Upregulation | [28] [33] | |
| Integrin β4 | Mediates indirect adhesion to BE through inducing VEGF that disrupt (ZO-1, VE-cadherin). | Upregulation | [35] | |
| Pericytes | Secrete high amounts of extracellular matrix proteins and enhance adhesion. | Activation | [38] | |
| HPSE | Induces adhesion through driving the expression of integrin β1 by brain metastatic triple negative breast cancer cells and the expression of VCAM-1 in BECs. | Upregulation | [53] | |
| ST6GALNAC5 | Induces adhesion to BE through cell-surface sialylation. | Upregulation | [58] | |
| PI3K/AKT | Induces adhesion. | Upregulation | [74] | |
| Alteration of the BBB permeability | MMP1 | Degrades claudin-5 and occludin. | Upregulation | [43] |
| COX2 | Increases permeability of BBB through prostaglandin generation and upregulating MMP1. | Upregulation | [43] | |
| ADAM8 | Upregulates MMP9 and shed of PSGL-1 from breast cancer cells. | Upregulation | [45] | |
| Substance P (SP) | Increases BBB permeability and disrupts ZO-1, claudin-5 (through inducing TNF-a that in turn augment the secretion of Ang-2). | Upregulation | [46] | |
| Ang-2 | Secreted by endothelial cells to cause disruption of ZO-1, claudin-5; partially dependent on VEGF expression. | Upregulation | [47] | |
| VEGF | Increases permeability of BBB through redistributing actin fiber and disrupting VE-cadherin. | Upregulation | [48] | |
| Cathepsin S | Proteolytic cleavage of JAM-B. | Upregulation | [49] | |
| Mfsd2a | Its downregulation in BE will promote permeability of BBB. | Downregulation | [54] | |
| BCRP | Induces BBB permeability and preserves BBB integrity. | Downregulation | [57] | |
| Glut1 | Preserves BBB integrity. | Upregulation | [57] | |
| Glut1 | Induces BBB permeability. | Downregulation | [57] | |
| CXCR4/SDF1 | Increases vascular permeability through activating PI3K/AKT pathway and calcium signaling. | Upregulation | [73] | |
| EndMT (TGF-β dependent manner) | Decreases VE-cadherin levels. | Upregulation | [75] | |
| Trans-endothelial Migration | MMP1 VEGF HPSE COX2 HB-EGF ST6GALNAC5 HER2 PI3K/AKT | Increases TEM. | Upregulation | [43] [48] [53] [58] [58] [58] [64] [74] |
| GBP1 | Triggered by T lymphocytes to induce transmigration. | Upregulation | [59] | |
| Sema4D | Interacts with its receptor Plexin-B1 to induce transmigration. | Upregulation | [60] | |
| HRG-HER3-HER2 | Induces adhesion and TEM; increases expression of MMP2, MMP9. | Upregulation | [63] | |
| Cx43 | Mediates extravasation. | Upregulation | [66] | |
| Sdcs-1 | Facilitates transmigration through inducing cytokines. | Upregulation | [69] | |
| CXCR4/SDF1 | Induces TEM by activating PI3K/AKT and calcium signaling. | Upregulation | [73] |
| MiR | Target | Mechanism | Regulation in Breast Cancer BM | Reference |
|---|---|---|---|---|
| MiR-1258 | HPSE | Increases HPSE expression and activity, mediating BM. | Downregulation | [52] |
| MiR-509 | TNF-α and RhoC-induced MMP9 | Induces BBB permeability and TEM. | Downregulation | [84] |
| MiR-101-3p | COX2-MMP1 | Degrades BE protein junctions (VE-cadherin and claudin-5). | Downregulation | [85] |
| MiR-101-3p and miR-26-5b | COX2-MMP1 | Degrades BE protein junctions (claudin-5, VE-cadherin, ZO-1, and β-catenin). | Downregulation | [86] |
| MiR-202-3p | MMP1 | Degrades BE protein junction (ZO-1, claudin-5, and β-catenin). | Downregulation | [87] |
| MiR-623 | MMP1 | Degrades BE protein junction (VE-cadherin and claudin-5). | Downregulation | [88] |
| MiR-105 | ZO-1 | Disrupts BE. | Upregulation | [89] |
| MiR-181c | PDPK1 | Delocalizes actin fiber, inducing the destruction of BBB. | Upregulation | [90] |
| Step of Extravasation | Factor | Effect | Regulation in BM | Reference |
|---|---|---|---|---|
| Adhesion | Pericytes | Secrete high amounts of extracellular matrix proteins and enhance adhesion. | Activation | [38] |
| PI3K/AKT EndMT (TGF-β-dependent manner) | Enhance adhesion and transmigration. | Upregulation | [74] [75] | |
| VLA-4 (integrin α4β1) | Interacts with endothelial VCAM-1 to facilitate adhesion of melanoma cells to the BBB. | Upregulation | [119] | |
| Alteration in the BBB permeability | EndMT (TGF-β-dependent manner) | Reduces expression of claudin-5 and VE-cadherin. | Upregulation | [75] |
| STAT3 | Upregulates MMP2 expression and subsequently enhances BM. | Upregulation | [102] | |
| MMP2 and MMP9 | Disrupts BBB. | Upregulation | [103] | |
| S100A4 | Interacts with RAGE to induce degradation of inter-endothelial junctions, occludin, and VE-cadherin. | Upregulation | [105] | |
| IL-23 | Produced by astrocytes to induce TEM; upregulates MMP2 expression in melanoma cells. | Upregulation | [115] | |
| Degradation of the basement membrane | Serine proteases | Degrades basement membrane components, mediating extravasation. | Upregulation | [106] |
| Seprase and uPAR | Forms a membrane complex to degrade ECM. | Upregulation | [107] | |
| HPSE-1 | Degrades ECM of BBB. | Upregulation | [108] | |
| Trans-endothelial migration | PI3K/AKT | Induces adhesion and transmigration. | Upregulation | [74] |
| EndMT (TGF-β dependent manner) | Enhances adhesion and transmigration. | Upregulation | [75] | |
| S100A4 | Promotes transmigration of melanoma cells. | Upregulation | [105] | |
| PLEKHA5 | Induces transmigration through mediating PI3K/AKT pathway. | Upregulation | [110] | |
| Plasminogen | Promotes TEM. | Upregulation | [111] | |
| MTf | Promotes TEM. | Upregulation | [114] | |
| IL-23 | Produced by astrocytes to induce TEM. Upregulates MMP2 expression in melanoma cells. | Upregulation | [115] | |
| CXCL10 | Produced by astrocytes to enhance migration of melanoma cells toward astrocytes via secretion of CXC3R. | Upregulation | [116] | |
| CCR4 | Interaction of CCR4 with its ligand CCL17 promotes TEM of melanoma cells. | Upregulation | [117] |
| Step of Extravasation | Factor | Commonality | Reference |
|---|---|---|---|
| Adhesion | PI3K/AKT | Breast cancer Melanoma | [74] [74] |
| VLA-4 (integrin α4β1) | Breast cancer Melanoma | [28] [119] | |
| ALCAM | Breast cancer Lung cancer | [28] [130] | |
| Alteration in the BBB permeability | HPSE | Melanoma Breast cancer | [53] [108] |
| Mfsd2a | Breast cancer Lung cancer | [54] [54] | |
| EndMT (TGF-β dependent manner) | Breast cancer Melanoma | [75] [75] | |
| Trans-endothelial migration | PI3K/AKT | Breast cancer Melanoma | [74] [74] |
| Step of Extravasation Targeted | Strategy | Effect | Type of Cancer | Reference |
|---|---|---|---|---|
| Adhesion | VLA-4 -1 and ALCAM blocking | Reduction of adhesion and extravasation across the BE | Breast cancer | [28] |
| Inhibition of VCAM-1, and/or its specific ligand VLA-4/α4β1 integrin | Reduction of invasion and metastasis | Breast cancer | [28] | |
| CD44 knockdown | Reduction of the pericellular HA coat on cancer cells, ands consequently, cancer cells adhesion and invasion through the BE | Breast | [32] | |
| Inhibition of PI3K/AKT or Rac signaling pathways | Prevention of tumor cell adhesion and migration through the BE and inhibition of BM | Melanoma | [74] | |
| Inhibition of VLA-4 | Reduction of cell adhesion and preserving barrier integrity | Melanoma | [105] | |
| Suppression of HPSE by miR-155 | Block adhesion, invasion, and metastasis | Melanoma | [109] | |
| Deleting ALCAM expression through CRISPR/Cas9 technology | Reduction of adhesion and extravasation across the BE | Lung cancer | [130] | |
| Inhibition of CD15 and CD15s by targeting their encoding genes (FUT4 and FUT7) | Reduction of adhesion and maintenance of BBB integrity | Lung cancer | [131,132,133] | |
| Alteration in the BBB permeability | Knocking down MMP1 expression | Decreased BM | Breast cancer | [44] |
| Combination treatment of an Ang-2 inhibitor with a VEGF inhibitor | Preservation of BBB integrity | Breast cancer | [47] | |
| miR-509 | Suppression of BM by targeting TNF- and RhoC-induced MMP9, which limits BBB permeability and TEM | Breast cancer | [84] | |
| Combinatorial restoration of miR-101-3p and miR-26b-5p | Preserving the BBB integrity and suppression of TEM by inhibition of COX2/MMP1 | Breast cancer | [86] | |
| miR-202-3p miR-623 | Preserving the BBB integrity and suppression of TEM by inhibition of MMP1 | Breast cancer | [87] [88] | |
| Inhibition of serine proteases | Reduction of extravasation by preserving BBB integrity | Melanoma | [106] | |
| Activation of adenosine A2A receptors. | Inhibition of BM by interfering with SDF-1/CXCR4 signaling and preserving the integrity of BBB | Lung cancer | [136] | |
| Trans-endothelial migration | Inhibition of cathepsin S | Reduction of TEM | Breast cancer | [49] |
| Inhibition of COX2 | Reduction of TEM due to inhibition of MMP1 | Breast cancer | [58] | |
| Disruption of the interaction of Sema4D with Plexin-B1 receptor | Reduction of TEM | Breast cancer | [60] | |
| In a HRG-rich brain microenvironment, combined blockade of HER2 and HER3 via monoclonal antibodies | Reduction of TEM | Breast cancer | [63] | |
| Knocking out Cx43 | Reduction of TEM | Breast cancer | [66] | |
| Blocking CXCR4/SDF-1 pathway | Reduction of TEM | Breast cancer | [73] | |
| Inhibition of PI3K/AKT or Rac signaling pathways | Reduction of adhesion and TEM | Breast cancer | [74] | |
| Silencing PLEKHA5 | Reduction of TEM and invasion | Melanoma | [110] | |
| Plasmin inhibitor | Reduction of TEM | Melanoma | [111] | |
| Blocking Mtf | Attenuates TEM and reduced BM formation | Melanoma | [114] | |
| Silencing CXCR3 or CXCL10 | Reduced migration toward astrocytes | Melanoma | [116] | |
| Blocking CCR4 | Reduction of TEM and BM formation | Melanoma | [117] | |
| Inhibition of MEK/ERK signaling | Suppression of TEM in an MMP-dependent manner | Lung cancer | [137] | |
| Silencing annexin A1 | Inhibition of TEM and attenuation of BM formation | Lung cancer | [138] | |
| Inhibition of endothelial Rho/ROCK signaling pathway | Inhibition of TEM and maintaining the assembly of tight junction proteins | Lung cancer | [139] | |
| Endothelial-to-mesenchymal transition | Zn(PipNONO)Cl | Inhibition of EndMT | Lung cancer | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsabbagh, R.; Ahmed, M.; Alqudah, M.A.Y.; Hamoudi, R.; Harati, R. Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer. Cancers 2023, 15, 2258. https://doi.org/10.3390/cancers15082258
Alsabbagh R, Ahmed M, Alqudah MAY, Hamoudi R, Harati R. Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer. Cancers. 2023; 15(8):2258. https://doi.org/10.3390/cancers15082258
Chicago/Turabian StyleAlsabbagh, Rama, Munazza Ahmed, Mohammad A. Y. Alqudah, Rifat Hamoudi, and Rania Harati. 2023. "Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer" Cancers 15, no. 8: 2258. https://doi.org/10.3390/cancers15082258
APA StyleAlsabbagh, R., Ahmed, M., Alqudah, M. A. Y., Hamoudi, R., & Harati, R. (2023). Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer. Cancers, 15(8), 2258. https://doi.org/10.3390/cancers15082258







