What’s Next after Hypomethylating Agents Failure in Myeloid Neoplasms? A Rational Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Hypomethylating Agents Failure: Definition, Mechanisms, and Prognosis
3. HMA Resistant MDS/AML Assessment and Prognostication Tools
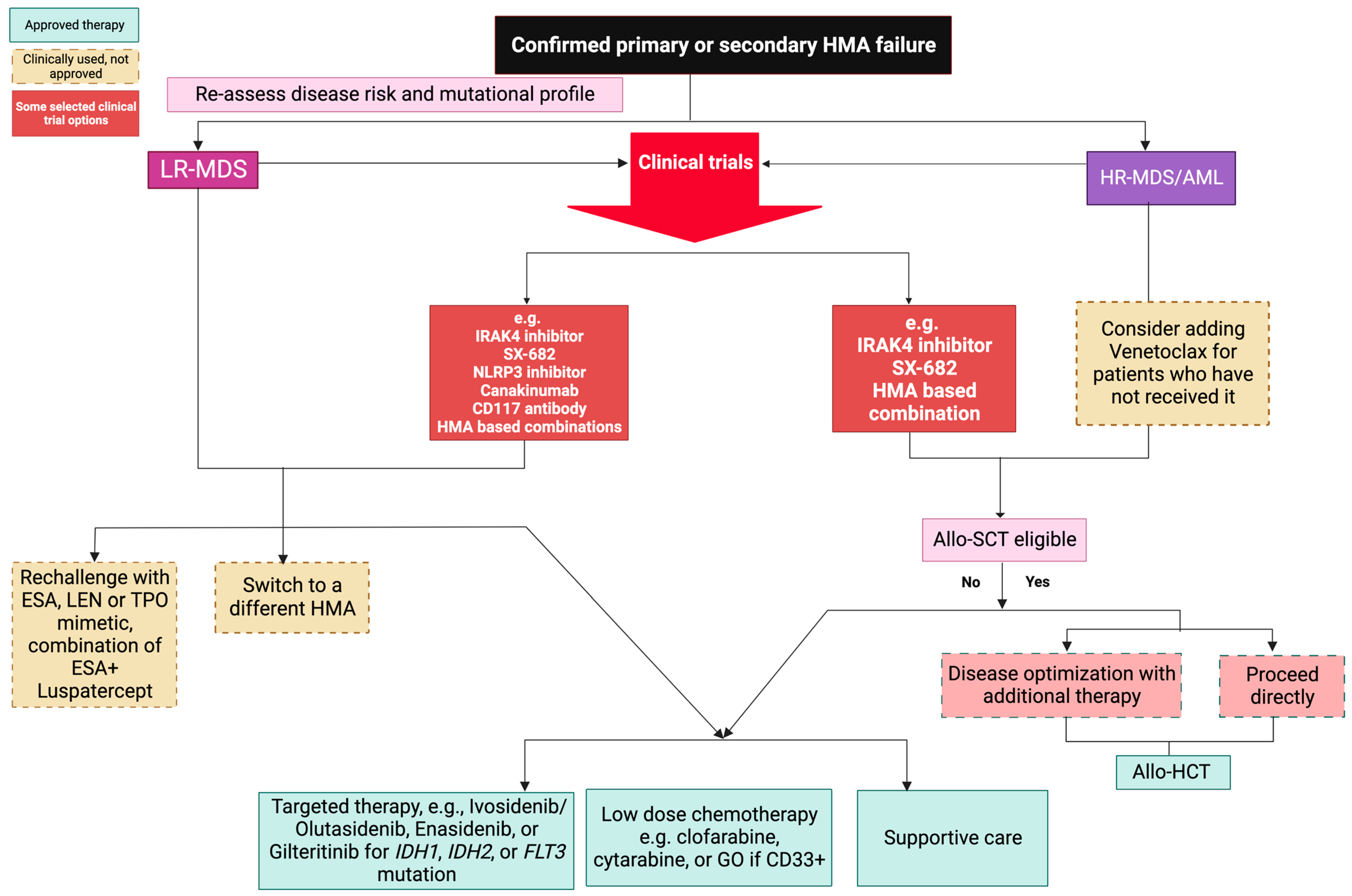
4. Management of Patients after HMA Failure
4.1. HMA Switch
4.2. Allogenic Hematopoietic Cell Transplantation (Allo-HCT)
4.3. Low-Dose vs. Intensive Chemotherapy
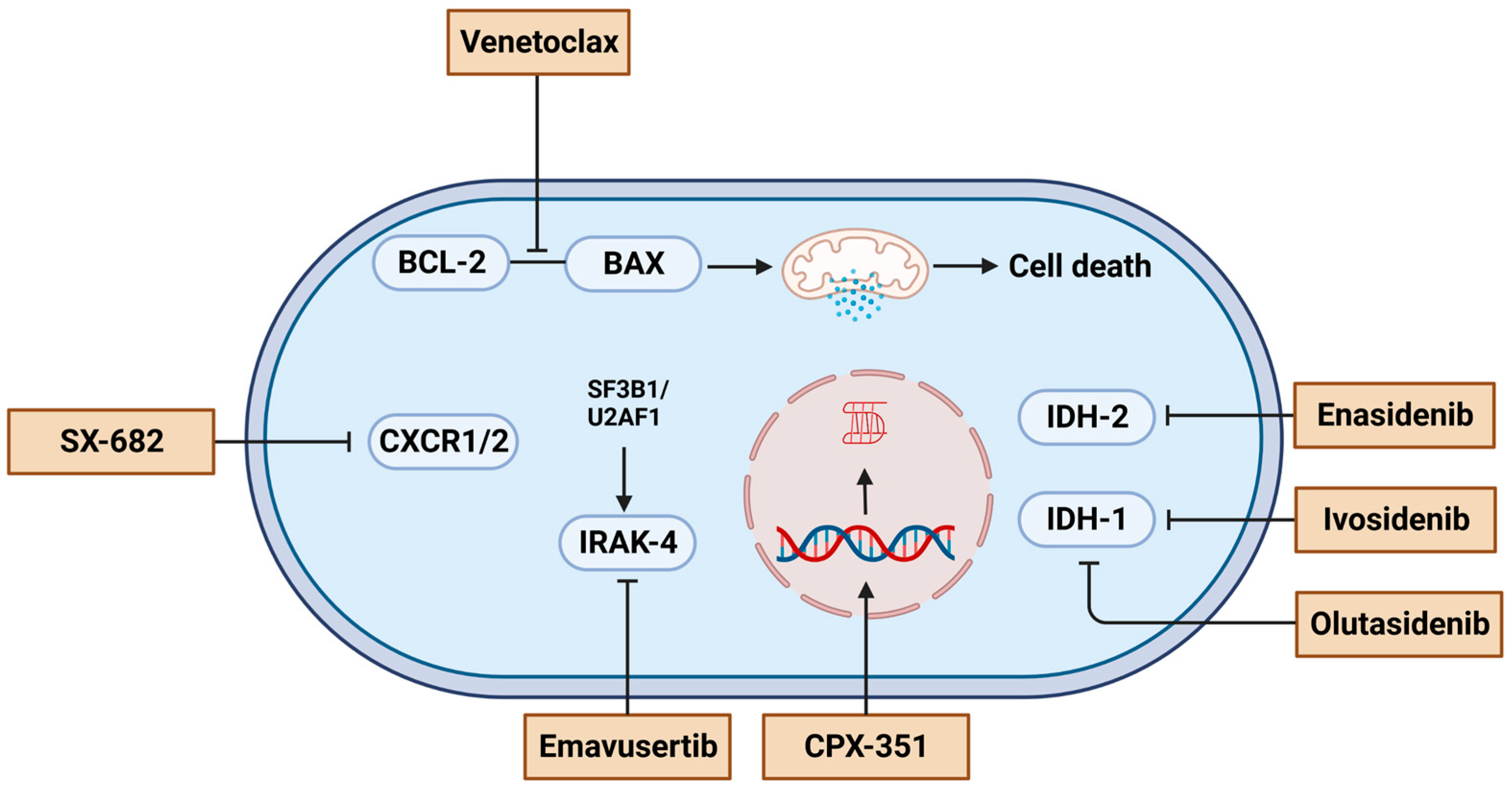
4.4. Targeted Therapy
4.5. Experimental Agents
4.6. Supportive Care
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Short, N.J.; Kantarjian, H. Hypomethylating agents for the treatment of myelodysplastic syndromes and acute myeloid leukemia: Past discoveries and future directions. Am. J. Hematol. 2022, 97, 1616–1626. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Taylor, J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review. JAMA 2022, 328, 872–880. [Google Scholar] [CrossRef]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J.; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood 2020, 136, 674–683. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X. Epidemiology of myelodysplastic syndromes: Why characterizing the beast is a prerequisite to taming it. Blood Rev. 2019, 34, 1–15. [Google Scholar] [CrossRef]
- Gurnari, C.; Xie, Z.; Zeidan, A.M. How I Manage Transplant Ineligible Patients with Myelodysplastic Neoplasms. Clin. Hematol. Int. 2022, 5, 8–20. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Itzykson, R.; Thépot, S.; Quesnel, B.; Dreyfus, F.; Beyne-Rauzy, O.; Turlure, P.; Vey, N.; Recher, C.; Dartigeas, C.; Legros, L.; et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 2011, 117, 403–411. [Google Scholar] [CrossRef]
- Itzykson, R.; Thépot, S.; Quesnel, B.; Dreyfus, F.; Recher, C.; Wattel, E.; Gardin, C.; Adès, L.; Fenaux, P. Long-term outcome of higher-risk MDS patients treated with azacitidine: An update of the GFM compassionate program cohort. Blood 2012, 119, 6172–6173. [Google Scholar] [CrossRef]
- Lübbert, M.; Suciu, S.; Baila, L.; Rüter, B.H.; Platzbecker, U.; Giagounidis, A.; Selleslag, D.; Labar, B.; Germing, U.; Salih, H.R.; et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: Final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J. Clin. Oncol. 2011, 29, 1987–1996. [Google Scholar] [CrossRef]
- Pfeilstöcker, M.; Tuechler, H.; Sanz, G.; Schanz, J.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Time-dependent changes in mortality and transformation risk in MDS. Blood 2016, 128, 902–910. [Google Scholar] [CrossRef]
- Musto, P.; Maurillo, L.; Spagnoli, A.; Gozzini, A.; Rivellini, F.; Lunghi, M.; Villani, O.; Aloe-Spiriti, M.A.; Venditti, A.; Santini, V. Azacitidine for the treatment of lower risk myelodysplastic syndromes: A retrospective study of 74 patients enrolled in an Italian named patient program. Cancer 2010, 116, 1485–1494. [Google Scholar] [CrossRef]
- Sanchez-Garcia, J.; Falantes, J.; Medina Perez, A.; Hernandez-Mohedo, F.; Hermosin, L.; Torres-Sabariego, A.; Bailen, A.; Hernandez-Sanchez, J.M.; Solé Rodriguez, M.; Casaño, F.J.; et al. Prospective randomized trial of 5 days azacitidine versus supportive care in patients with lower-risk myelodysplastic syndromes without 5q deletion and transfusion-dependent anemia. Leuk. Lymphoma 2018, 59, 1095–1104. [Google Scholar] [CrossRef]
- Kantarjian, H.; Issa, J.P.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; DiPersio, J.; Klimek, V.; Slack, J.; de Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study. Cancer 2006, 106, 1794–1803. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Jabbour, E.; Borthakur, G.; Faderl, S.; Estrov, Z.; Yang, H.; Maddipoti, S.; Godley, L.A.; Gabrail, N.; Berdeja, J.G.; et al. Randomized open-label phase II study of decitabine in patients with low- or intermediate-risk myelodysplastic syndromes. J. Clin. Oncol. 2013, 31, 2548–2553. [Google Scholar] [CrossRef]
- Jabbour, E.; Short, N.J.; Montalban-Bravo, G.; Huang, X.; Bueso-Ramos, C.; Qiao, W.; Yang, H.; Zhao, C.; Kadia, T.; Borthakur, G.; et al. Randomized phase 2 study of low-dose decitabine vs low-dose azacitidine in lower-risk MDS and MDS/MPN. Blood 2017, 130, 1514–1522. [Google Scholar] [CrossRef]
- Gurion, R.; Vidal, L.; Gafter-Gvili, A.; Belnik, Y.; Yeshurun, M.; Raanani, P.; Shpilberg, O. 5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome—A systematic review and meta-analysis. Haematologica 2010, 95, 303–310. [Google Scholar] [CrossRef]
- Budziszewska, B.K.; Pluta, A.; Sulek, K.; Wierzbowska, A.; Robak, T.; Giebel, S.; Holowiecka-Goral, A.; Sawicki, W.; Ejduk, A.; Patkowska, E.; et al. Treatment of elderly patients with acute myeloid leukemia adjusted for performance status and presence of comorbidities: A Polish Adult Leukemia Group study. Leuk. Lymphoma 2015, 56, 2331–2338. [Google Scholar] [CrossRef]
- Ferrara, F.; Barosi, G.; Venditti, A.; Angelucci, E.; Gobbi, M.; Pane, F.; Tosi, P.; Zinzani, P.; Tura, S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: A project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 2013, 27, 997–999. [Google Scholar] [CrossRef]
- Palmieri, R.; Othus, M.; Halpern, A.B.; Percival, M.M.; Godwin, C.D.; Becker, P.S.; Walter, R.B. Accuracy of SIE/SIES/GITMO Consensus Criteria for Unfitness to Predict Early Mortality After Intensive Chemotherapy in Adults With AML or Other High-Grade Myeloid Neoplasm. J. Clin. Oncol. 2020, 38, 4163–4174. [Google Scholar] [CrossRef]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Thomas, X.G.; Dmoszynska, A.; Wierzbowska, A.; Mazur, G.; Mayer, J.; Gau, J.P.; Chou, W.C.; Buckstein, R.; Cermak, J.; et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 2670–2677. [Google Scholar] [CrossRef]
- Maurillo, L.; Venditti, A.; Spagnoli, A.; Gaidano, G.; Ferrero, D.; Oliva, E.; Lunghi, M.; D’Arco, A.M.; Levis, A.; Pastore, D.; et al. Azacitidine for the treatment of patients with acute myeloid leukemia: Report of 82 patients enrolled in an Italian Compassionate Program. Cancer 2012, 118, 1014–1022. [Google Scholar] [CrossRef]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef]
- Bhatnagar, B.; Duong, V.H.; Gourdin, T.S.; Tidwell, M.L.; Chen, C.; Ning, Y.; Emadi, A.; Sausville, E.A.; Baer, M.R. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk. Lymphoma 2014, 55, 1533–1537. [Google Scholar] [CrossRef]
- Ritchie, E.K.; Feldman, E.J.; Christos, P.J.; Rohan, S.D.; Lagassa, C.B.; Ippoliti, C.; Scandura, J.M.; Carlson, K.; Roboz, G.J. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk. Lymphoma 2013, 54, 2003–2007. [Google Scholar] [CrossRef]
- Blum, W.; Garzon, R.; Klisovic, R.B.; Schwind, S.; Walker, A.; Geyer, S.; Liu, S.; Havelange, V.; Becker, H.; Schaaf, L.; et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc. Natl. Acad. Sci. USA 2010, 107, 7473–7478. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Fenaux, P.; Gobbi, M.; Mayer, J.; Roboz, G.J.; Krauter, J.; Robak, T.; Kantarjian, H.M.; Novák, J.; Jedrzejczak, W.W.; et al. Prospective comparison of outcomes with azacitidine and decitabine in patients with AML ineligible for intensive chemotherapy. Blood 2022, 140, 285–289. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Sekeres, M.A.; Garcia-Manero, G.; Steensma, D.P.; Zell, K.; Barnard, J.; Ali, N.A.; Zimmerman, C.; Roboz, G.; DeZern, A.; et al. Comparison of risk stratification tools in predicting outcomes of patients with higher-risk myelodysplastic syndromes treated with azanucleosides. Leukemia 2016, 30, 649–657. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Wang, R.; Wang, X.; Shallis, R.M.; Podoltsev, N.A.; Bewersdorf, J.P.; Huntington, S.F.; Neparidze, N.; Giri, S.; Gore, S.D.; et al. Clinical outcomes of older patients with AML receiving hypomethylating agents: A large population-based study in the United States. Blood Adv. 2020, 4, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Récher, C.; Röllig, C.; Bérard, E.; Bertoli, S.; Dumas, P.Y.; Tavitian, S.; Kramer, M.; Serve, H.; Bornhäuser, M.; Platzbecker, U.; et al. Long-term survival after intensive chemotherapy or hypomethylating agents in AML patients aged 70 years and older: A large patient data set study from European registries. Leukemia 2022, 36, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Davidoff, A.J.; Long, J.B.; Hu, X.; Wang, R.; Ma, X.; Gross, C.P.; Abel, G.A.; Huntington, S.F.; Podoltsev, N.A.; et al. Comparative clinical effectiveness of azacitidine versus decitabine in older patients with myelodysplastic syndromes. Br. J. Haematol. 2016, 175, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Stahl, M.; Hu, X.; Wang, R.; Huntington, S.F.; Podoltsev, N.A.; Gore, S.D.; Ma, X.; Davidoff, A.J. Long-term survival of older patients with MDS treated with HMA therapy without subsequent stem cell transplantation. Blood 2018, 131, 818–821. [Google Scholar] [CrossRef]
- Jabbour, E.J.; Garcia-Manero, G.; Strati, P.; Mishra, A.; Al Ali, N.H.; Padron, E.; Lancet, J.; Kadia, T.; Daver, N.; O’Brien, S.; et al. Outcome of patients with low-risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure: A report on behalf of the MDS Clinical Research Consortium. Cancer 2015, 121, 876–882. [Google Scholar] [CrossRef]
- Prébet, T.; Gore, S.D.; Esterni, B.; Gardin, C.; Itzykson, R.; Thepot, S.; Dreyfus, F.; Rauzy, O.B.; Recher, C.; Adès, L.; et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J. Clin. Oncol. 2011, 29, 3322–3327. [Google Scholar] [CrossRef]
- Nanah, R.; McCullough, K.; Hogan, W.; Begna, K.; Patnaik, M.; Elliott, M.; Litzow, M.; Al-Kali, A. Outcome of elderly patients after failure to hypomethylating agents given as frontline therapy for acute myeloid leukemia: Single institution experience. Am. J. Hematol. 2017, 92, 866–871. [Google Scholar] [CrossRef]
- Ilyas, R.; Johnson, I.M.; McCullough, K.; Al-Kali, A.; Alkhateeb, H.B.; Begna, K.; Mangaonkar, A.A.; Litzow, M.R.; Hogan, W.J.; Shah, M.V.; et al. Outcome of Patients with Acute Myeloid Leukemia Following Failure of Front-Line Venetoclax Plus Hypomethylating Agent Therapy. Blood 2022, 140, 1286–1287. [Google Scholar] [CrossRef]
- Silverman, L.R.; McKenzie, D.R.; Peterson, B.L.; Holland, J.F.; Backstrom, J.T.; Beach, C.L.; Larson, R.A. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: Studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J. Clin. Oncol. 2006, 24, 3895–3903. [Google Scholar] [CrossRef]
- Garcia, J.S.; Jain, N.; Godley, L.A. An update on the safety and efficacy of decitabine in the treatment of myelodysplastic syndromes. OncoTargets Ther. 2010, 3, 1–13. [Google Scholar] [CrossRef]
- Šimoničová, K.; Janotka, Ľ.; Kavcová, H.; Sulová, Z.; Breier, A.; Messingerova, L. Different mechanisms of drug resistance to hypomethylating agents in the treatment of myelodysplastic syndromes and acute myeloid leukemia. Drug Resist. Updates 2022, 61, 100805. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Jelinek, J.; Si, J.; Shu, J.; Issa, J.P. Mechanisms of resistance to 5-aza-2’-deoxycytidine in human cancer cell lines. Blood 2009, 113, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Gruber, E.; Franich, R.L.; Shortt, J.; Johnstone, R.W.; Kats, L.M. Distinct and overlapping mechanisms of resistance to azacytidine and guadecitabine in acute myeloid leukemia. Leukemia 2020, 34, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Valencia, A.; Masala, E.; Rossi, A.; Martino, A.; Sanna, A.; Buchi, F.; Canzian, F.; Cilloni, D.; Gaidano, V.; Voso, M.T.; et al. Expression of nucleoside-metabolizing enzymes in myelodysplastic syndromes and modulation of response to azacitidine. Leukemia 2014, 28, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Geng, S.; Weng, J.; Deng, C.; Lu, Z.; Luo, C.; Du, X. The hENT1 and DCK genes underlie the decitabine response in patients with myelodysplastic syndrome. Leuk. Res. 2015, 39, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Choy, G.; Redkar, S.; Taverna, P.; Azab, M.; Karpf, A.R. SGI-110: DNA Methyltransferase Inhibitor Oncolytic. Drugs Future 2013, 38, 535–543. [Google Scholar] [PubMed]
- Mahfouz, R.Z.; Jankowska, A.; Ebrahem, Q.; Gu, X.; Visconte, V.; Tabarroki, A.; Terse, P.; Covey, J.; Chan, K.; Ling, Y.; et al. Increased CDA expression/activity in males contributes to decreased cytidine analog half-life and likely contributes to worse outcomes with 5-azacytidine or decitabine therapy. Clin. Cancer Res. 2013, 19, 938–948. [Google Scholar] [CrossRef]
- Wu, L.; Shi, W.; Li, X.; Chang, C.; Xu, F.; He, Q.; Wu, D.; Su, J.; Zhou, L.; Song, L.; et al. High expression of the human equilibrative nucleoside transporter 1 gene predicts a good response to decitabine in patients with myelodysplastic syndrome. J. Transl. Med. 2016, 14, 66. [Google Scholar] [CrossRef]
- Qin, T.; Castoro, R.; El Ahdab, S.; Jelinek, J.; Wang, X.; Si, J.; Shu, J.; He, R.; Zhang, N.; Chung, W.; et al. Mechanisms of resistance to decitabine in the myelodysplastic syndrome. PLoS ONE 2011, 6, e23372. [Google Scholar] [CrossRef]
- Hopper-Borge, E.; Xu, X.; Shen, T.; Shi, Z.; Chen, Z.S.; Kruh, G.D. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009, 69, 178–184. [Google Scholar] [CrossRef]
- Platzbecker, U. Treatment of MDS. Blood 2019, 133, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Stomper, J.; Ihorst, G.; Suciu, S.; Sander, P.N.; Becker, H.; Wijermans, P.W.; Plass, C.; Weichenhan, D.; Bissé, E.; Claus, R.; et al. Fetal hemoglobin induction during decitabine treatment of elderly patients with high-risk myelodysplastic syndrome or acute myeloid leukemia: A potential dynamic biomarker of outcome. Haematologica 2019, 104, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, H.; Hong, M.; Zhu, H.; Zhu, Y.; Lian, Y.; Li, S.; Li, J.; Qian, S. Early recovery of the platelet count after decitabine-based induction chemotherapy is a prognostic marker of superior response in elderly patients with newly diagnosed acute myeloid leukaemia. BMC Cancer 2018, 18, 1269. [Google Scholar] [CrossRef] [PubMed]
- Itzykson, R.; Crouch, S.; Travaglino, E.; Smith, A.; Symeonidis, A.; Hellström-Lindberg, E.; Sanz, G.; Čermák, J.; Stauder, R.; Elena, C.; et al. Early platelet count kinetics has prognostic value in lower-risk myelodysplastic syndromes. Blood Adv. 2018, 2, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Falconi, G.; Fabiani, E.; Criscuolo, M.; Fianchi, L.; Finelli, C.; Cerqui, E.; Pelosi, E.; Screnci, M.; Gurnari, C.; Zangrilli, I.; et al. Transcription factors implicated in late megakaryopoiesis as markers of outcome after azacitidine and allogeneic stem cell transplantation in myelodysplastic syndrome. Leuk. Res. 2019, 84, 106191. [Google Scholar] [CrossRef]
- Craddock, C.F.; Houlton, A.E.; Quek, L.S.; Ferguson, P.; Gbandi, E.; Roberts, C.; Metzner, M.; Garcia-Martin, N.; Kennedy, A.; Hamblin, A.; et al. Outcome of Azacitidine Therapy in Acute Myeloid Leukemia Is not Improved by Concurrent Vorinostat Therapy but Is Predicted by a Diagnostic Molecular Signature. Clin. Cancer Res. 2017, 23, 6430–6440. [Google Scholar] [CrossRef]
- Nazha, A.; Sekeres, M.A.; Bejar, R.; Rauh, M.J.; Othus, M.; Komrokji, R.S.; Barnard, J.; Hilton, C.B.; Kerr, C.M.; Steensma, D.P.; et al. Genomic Biomarkers to Predict Resistance to Hypomethylating Agents in Patients With Myelodysplastic Syndromes Using Artificial Intelligence. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Schimmer, R.R.; Kovtonyuk, L.V.; Klemm, N.; Fullin, J.; Stolz, S.M.; Mueller, J.; Caiado, F.; Kurppa, K.J.; Ebert, B.L.; Manz, M.G.; et al. TP53 mutations confer resistance to hypomethylating agents and BCL-2 inhibition in myeloid neoplasms. Blood Adv. 2022, 6, 3201–3206. [Google Scholar] [CrossRef]
- Radakovich, N.; Sallman, D.A.; Buckstein, R.; Brunner, A.; Dezern, A.; Mukerjee, S.; Komrokji, R.; Al-Ali, N.; Shreve, J.; Rouphail, Y.; et al. A machine learning model of response to hypomethylating agents in myelodysplastic syndromes. iScience 2022, 25, 104931. [Google Scholar] [CrossRef]
- Nazha, A.; Komrokji, R.S.; Garcia-Manero, G.; Barnard, J.; Roboz, G.J.; Steensma, D.P.; DeZern, A.E.; Zell, K.; Zimmerman, C.; Ali, N.A.; et al. The efficacy of current prognostic models in predicting outcome of patients with myelodysplastic syndromes at the time of hypomethylating agent failure. Haematologica 2016, 101, e224–e227. [Google Scholar] [CrossRef]
- Prebet, T.; Fenaux, P.; Vey, N. Predicting outcome of patients with myelodysplastic syndromes after failure of azacitidine: Validation of the North American MDS consortium scoring system. Haematologica 2016, 101, e427–e428. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Ossa, J.E.A.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef]
- Kewan, T.; Bahaj, W.; Durmaz, A.; Aly, M.M.; Ogbue, O.D.; Carraway, H.E.; Sekeres, M.A.; Visconte, V.; Gurnari, C.; Maciejewski, J.P. Validation of the Molecular International Prognostic Scoring System in patients with myelodysplastic syndromes. Blood 2023, 141, 1768–1772. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627, quiz 3699. [Google Scholar] [CrossRef]
- Voso, M.T.; Gurnari, C. Have we reached a molecular era in myelodysplastic syndromes? Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 418–427. [Google Scholar] [CrossRef]
- Gurnari, C.; Visconte, V. ‘We cannot paint them all with the same brush’: The need for a better definition of patients with myelodysplastic syndromes for clinical trial design. Br. J. Haematol. 2022, 196, 268–269. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Zeidan, A.M. Following in the footsteps of acute myeloid leukemia: Are we witnessing the start of a therapeutic revolution for higher-risk myelodysplastic syndromes? Leuk. Lymphoma 2020, 61, 2295–2312. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Fenaux, P.; Al-Kali, A.; Baer, M.R.; Sekeres, M.A.; Roboz, G.J.; Gaidano, G.; Scott, B.L.; Greenberg, P.; Platzbecker, U.; et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): A randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 496–508. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Winer, E.S.; DeAngelo, D.J.; Tarantolo, S.; Sallman, D.A.; Dugan, J.; Groepper, S.; Giagounidis, A.; Götze, K.; Metzeler, K.H.; et al. S129: Takeaim Leukemia-A Phase 1/2a Study of the Irak4 Inhibitor Emavusertib (Ca-4948) as Monotherapy or in Combination With Azacitidine or Venetoclax in Relapsed/Refractory AML or MDS. HemaSphere 2022, 6, 30–31. [Google Scholar] [CrossRef]
- Apuri, S.; Al Ali, N.; Padron, E.; Lancet, J.E.; List, A.F.; Komrokji, R.S. Evidence for Selective Benefit of Sequential Treatment With Hypomethylating Agents in Patients With Myelodysplastic Syndrome. Clin. Lymphoma Myeloma Leuk. 2017, 17, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.H.; Bhatnagar, B.; Zandberg, D.P.; Vannorsdall, E.J.; Tidwell, M.L.; Chen, Q.; Baer, M.R. Lack of objective response of myelodysplastic syndromes and acute myeloid leukemia to decitabine after failure of azacitidine. Leuk. Lymphoma 2015, 56, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Harel, S.; Cherait, A.; Berthon, C.; Willekens, C.; Park, S.; Rigal, M.; Brechignac, S.; Thépot, S.; Quesnel, B.; Gardin, C.; et al. Outcome of patients with high risk Myelodysplastic Syndrome (MDS) and advanced Chronic Myelomonocytic Leukemia (CMML) treated with decitabine after azacitidine failure. Leuk. Res. 2015, 39, 501–504. [Google Scholar] [CrossRef]
- Stomper, J.; Rotondo, J.C.; Greve, G.; Lübbert, M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: Mechanisms of resistance and novel HMA-based therapies. Leukemia 2021, 35, 1873–1889. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Ahdab, S.E.; Ravandi, F.; Faderl, S.; Ferrajoli, A.; Newman, B.; Issa, J.P.; Kantarjian, H. Activity of decitabine in patients with myelodysplastic syndrome previously treated with azacitidine. Leuk. Lymphoma 2008, 49, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, Q.; Li, S.; Wang, X. Resistance to Hypomethylating Agents in Myelodysplastic Syndrome and Acute Myeloid Leukemia From Clinical Data and Molecular Mechanism. Front. Oncol. 2021, 11, 706030. [Google Scholar] [CrossRef]
- Runde, V.; de Witte, T.; Arnold, R.; Gratwohl, A.; Hermans, J.; van Biezen, A.; Niederwieser, D.; Labopin, M.; Walter-Noel, M.P.; Bacigalupo, A.; et al. Bone marrow transplantation from HLA-identical siblings as first-line treatment in patients with myelodysplastic syndromes: Early transplantation is associated with improved outcome. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998, 21, 255–261. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the intensity of conditioning regimens: Working definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef]
- Nakamura, R.; Saber, W.; Martens, M.J.; Ramirez, A.; Scott, B.; Oran, B.; Leifer, E.; Tamari, R.; Mishra, A.; Maziarz, R.T.; et al. Biologic Assignment Trial of Reduced-Intensity Hematopoietic Cell Transplantation Based on Donor Availability in Patients 50-75 Years of Age With Advanced Myelodysplastic Syndrome. J. Clin. Oncol. 2021, 39, 3328–3339. [Google Scholar] [CrossRef]
- Field, T.; Perkins, J.; Huang, Y.; Kharfan-Dabaja, M.A.; Alsina, M.; Ayala, E.; Fernandez, H.F.; Janssen, W.; Lancet, J.; Perez, L.; et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2010, 45, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Gerds, A.T.; Gooley, T.A.; Estey, E.H.; Appelbaum, F.R.; Deeg, H.J.; Scott, B.L. Pretransplantation therapy with azacitidine vs. induction chemotherapy and posttransplantation outcome in patients with MDS. Biol. Blood Marrow Transplant. 2012, 18, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Kharfan-Dabaja, M.A.; Komrokji, R.S. Beyond hypomethylating agents failure in patients with myelodysplastic syndromes. Curr. Opin. Hematol. 2014, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.; Kim, S.; Singh, V.; Ayash, L.; Alavi, A.; Ratanatharathorn, V.; Uberti, J.P.; Deol, A. Pre-transplant hypomethylating agents do not influence post-transplant survival in myelodysplastic syndrome. Leuk. Lymphoma 2019, 60, 2762–2770. [Google Scholar] [CrossRef]
- Qin, Y.; Kuang, P.; Zeng, Q.; Wu, Y.; Liu, T. Hypomethylating agents for patients with myelodysplastic syndromes prior to hematopoietic stem cell transplantation: A systematic review and meta-analysis. Ann. Hematol. 2019, 98, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Leone, G.; Piciocchi, A.; Fianchi, L.; Santarone, S.; Candoni, A.; Criscuolo, M.; Masciulli, A.; Cerqui, E.; Molteni, A.; et al. Feasibility of allogeneic stem-cell transplantation after azacitidine bridge in higher-risk myelodysplastic syndromes and low blast count acute myeloid leukemia: Results of the BMT-AZA prospective study. Ann. Oncol. 2017, 28, 1547–1553. [Google Scholar] [CrossRef]
- Gurnari, C.; Gagelmann, N.; Badbaran, A.; Awada, H.; Dima, D.; Pagliuca, S.; D’Aveni-Piney, M.; Attardi, E.; Voso, M.T.; Cerretti, R.; et al. Outcome prediction in myelodysplastic neoplasm undergoing hematopoietic cell transplant in the molecular era of IPSS-M. Leukemia 2023, 37, 717–719. [Google Scholar] [CrossRef]
- Festuccia, M.; Baker, K.; Gooley, T.A.; Sandmaier, B.M.; Deeg, H.J.; Scott, B.L. Hematopoietic Cell Transplantation in Myelodysplastic Syndromes after Treatment with Hypomethylating Agents. Biol. Blood Marrow Transpl. 2017, 23, 1509–1514. [Google Scholar] [CrossRef]
- Shimoni, A.; Robin, M.; Iacobelli, S.; Beelen, D.; Mufti, G.J.; Ciceri, F.; Bethge, W.; Volin, L.; Blaise, D.; Ganser, A.; et al. Allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome using treosulfan based compared to other reduced-intensity or myeloablative conditioning regimens. A report of the chronic malignancies working party of the EBMT. Br. J. Haematol. 2021, 195, 417–428. [Google Scholar] [CrossRef]
- Ball, B.; Komrokji, R.S.; Ades, L.; Sekeres, M.A.; DeZern, A.E.; Pleyer, L.; Vey, N.; Almeida, A.; Germing, U.; Cluzeau, T.; et al. Evaluation of induction chemotherapies after hypomethylating agent failure in myelodysplastic syndromes and acute myeloid leukemia. Blood Adv. 2018, 2, 2063–2071. [Google Scholar] [CrossRef]
- Jabbour, E.; Faderl, S.; Sasaki, K.; Kadia, T.; Daver, N.; Pemmaraju, N.; Patel, K.; Khoury, J.D.; Bueso-Ramos, C.; Bohannan, Z.; et al. Phase 2 study of low-dose clofarabine plus cytarabine for patients with higher-risk myelodysplastic syndrome who have relapsed or are refractory to hypomethylating agents. Cancer 2017, 123, 629–637. [Google Scholar] [CrossRef]
- Feldman, E.J.; Lancet, J.E.; Kolitz, J.E.; Ritchie, E.K.; Roboz, G.J.; List, A.F.; Allen, S.L.; Asatiani, E.; Mayer, L.D.; Swenson, C.; et al. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.; Kadia, T.M.; Montalban-Bravo, G.; Faderl, S.; Sasaki, K.; Short, N.J.; Daver, N.; DiNardo, C.D.; Masarova, L.; Ferrajoli, A.; et al. Liposomal Cytarabine and Daunorubicin (CPX-351) in Combination with Gemtuzumab Ozogamicin (GO) in Relapsed Refractory (R/R) Acute Myeloid Leukemia (AML) and Post-Hypomethylating Agent (Post-HMA) Failure High-Risk Myelodysplastic Syndrome (HR-MDS). Blood 2021, 138, 2323. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Aldoss, I.; Pullarkat, V.; Stein, A.S. Venetoclax-containing regimens in acute myeloid leukemia. Ther. Adv. Hematol. 2021, 12, 2040620720986646. [Google Scholar] [CrossRef] [PubMed]
- Tenold, M.E.; Moskoff, B.N.; Benjamin, D.J.; Hoeg, R.T.; Rosenberg, A.S.; Abedi, M.; Tuscano, J.M.; Jonas, B.A. Outcomes of Adults With Relapsed/Refractory Acute Myeloid Leukemia Treated With Venetoclax Plus Hypomethylating Agents at a Comprehensive Cancer Center. Front. Oncol. 2021, 11, 649209. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Borate, U.; Pollyea, D.A.; Brunner, A.M.; Roncolato, F.; Garcia, J.S.; Filshie, R.; Odenike, O.; Watson, A.M.; Krishnadasan, R.; et al. A phase 1b study of venetoclax and azacitidine combination in patients with relapsed or refractory myelodysplastic syndromes. Am. J. Hematol. 2023, 98, 272–281. [Google Scholar] [CrossRef]
- Chan, S.M.; Thomas, D.; Corces-Zimmerman, M.R.; Xavy, S.; Rastogi, S.; Hong, W.J.; Zhao, F.; Medeiros, B.C.; Tyvoll, D.A.; Majeti, R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 2015, 21, 178–184. [Google Scholar] [CrossRef]
- Ball, B.J.; Famulare, C.A.; Stein, E.M.; Tallman, M.S.; Derkach, A.; Roshal, M.; Gill, S.I.; Manning, B.M.; Koprivnikar, J.; McCloskey, J.; et al. Venetoclax and hypomethylating agents (HMAs) induce high response rates in MDS, including patients after HMA therapy failure. Blood Adv. 2020, 4, 2866–2870. [Google Scholar] [CrossRef]
- Stein, E.M.; Fathi, A.T.; DiNardo, C.D.; Pollyea, D.A.; Roboz, G.J.; Collins, R.; Sekeres, M.A.; Stone, R.M.; Attar, E.C.; Frattini, M.G.; et al. Enasidenib in patients with mutant IDH2 myelodysplastic syndromes: A phase 1 subgroup analysis of the multicentre, AG221-C-001 trial. Lancet Haematol. 2020, 7, e309–e319. [Google Scholar] [CrossRef]
- Ades, L.; Dimicoli-Salazar, S.; Sebert, M.; Cluzeau, T.; Stamatoulas Bastard, A.; Laribi, K.; Fossard, G.; Itzykson, R.; Beyne Rauzy, O.; Garnier, A.; et al. Enasidenib (ENA) Is Effective in Patients with IDH2 Mutated Myelodysplastic Syndrome (MDS): The Ideal Phase 2 Study By the GFM Group. Blood 2021, 138, 63. [Google Scholar] [CrossRef]
- Sebert, M.; Cluzeau, T.; Beyne Rauzy, O.; Stamatoulas Bastard, A.; Dimicoli-Salazar, S.; Thepot, S.; Peterlin, P.; Park, S.; Gourin, M.-P.; Brehar, O.; et al. Ivosidenib Monotherapy Is Effective in Patients with IDH1 Mutated Myelodysplastic Syndrome (MDS): The Idiome Phase 2 Study By the GFM Group. Blood 2021, 138, 62. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Wang, E.S.; Watts, J.M.; Lee, S.; Baer, M.R.; Dao, K.-H.; Dinner, S.; Yang, J.; Donnellan, W.B.; Schwarer, A.P.; et al. Olutasidenib (FT-2102) Induces Rapid Remissions in Patients with IDH1-Mutant Myelodysplastic Syndrome: Results of Phase 1/2 Single Agent Treatment and Combination with Azacitidine. Blood 2019, 134, 674. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Al Ali, N.H.; Padron, E.; Lancet, J.; List, A.; Komrokji, R.S. Lenalidomide Treatment for Lower Risk Nondeletion 5q Myelodysplastic Syndromes Patients Yields Higher Response Rates When Used Before Azacitidine. Clin. Lymphoma Myeloma Leuk. 2015, 15, 705–710. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Smith, B.D.; Carraway, H.E.; Gojo, I.; DeZern, A.; Gore, S.D. A phase 2 trial of high dose lenalidomide in patients with relapsed/refractory higher-risk myelodysplastic syndromes and acute myeloid leukaemia with trilineage dysplasia. Br. J. Haematol. 2017, 176, 241–247. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Al Ali, N.; Ball, S.; Chan, O.; Kuykendall, A.; Sweet, K.; Lancet, J.E.; Padron, E.; Sallman, D.A. Luspatercept for Treatment of Lower Risk Myelodysplastic Syndromes: Real World Data Replicates Medalist Study Results and Confirms Activity Among Hypomethylating Agents and Lenalidomide Treated Patients. Blood 2022, 140, 4039–4041. [Google Scholar] [CrossRef]
- Komrokji, R.; Garcia-Manero, G.; Ades, L.; Prebet, T.; Steensma, D.P.; Jurcic, J.G.; Sekeres, M.A.; Berdeja, J.; Savona, M.R.; Beyne-Rauzy, O.; et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: A phase 2, dose-ranging trial. Lancet Haematol. 2018, 5, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.; Starczynowski, D.T. IRAK1 and IRAK4 as emerging therapeutic targets in hematologic malignancies. Curr. Opin. Hematol. 2022, 29, 8–19. [Google Scholar] [CrossRef]
- Smith, M.A.; Choudhary, G.S.; Pellagatti, A.; Choi, K.; Bolanos, L.C.; Bhagat, T.D.; Gordon-Mitchell, S.; Von Ahrens, D.; Pradhan, K.; Steeples, V.; et al. U2AF1 mutations induce oncogenic IRAK4 isoforms and activate innate immune pathways in myeloid malignancies. Nat. Cell Biol. 2019, 21, 640–650. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Pellagatti, A.; Agianian, B.; Smith, M.A.; Bhagat, T.D.; Gordon-Mitchell, S.; Sahu, S.; Pandey, S.; Shah, N.; Aluri, S.; et al. Activation of targetable inflammatory immune signaling is seen in myelodysplastic syndromes with SF3B1 mutations. eLife 2022, 11, e78136. [Google Scholar] [CrossRef] [PubMed]
- Rhyasen, G.W.; Starczynowski, D.T. IRAK signalling in cancer. Br. J. Cancer 2015, 112, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; DeZern, A.E.; Gayle, A.A.; Kahn, S.J.; Padron, E.; Lancet, J.E.; Kuykendall, A.; Sweet, K.; Chan, O.; Maeda, D.Y.; et al. Phase 1 Results of the First-in-Class CXCR1/2 Inhibitor SX-682 in Patients with Hypomethylating Agent Failure Myelodysplastic Syndromes. Blood 2022, 140, 2070–2072. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awada, H.; Gurnari, C.; Xie, Z.; Bewersdorf, J.P.; Zeidan, A.M. What’s Next after Hypomethylating Agents Failure in Myeloid Neoplasms? A Rational Approach. Cancers 2023, 15, 2248. https://doi.org/10.3390/cancers15082248
Awada H, Gurnari C, Xie Z, Bewersdorf JP, Zeidan AM. What’s Next after Hypomethylating Agents Failure in Myeloid Neoplasms? A Rational Approach. Cancers. 2023; 15(8):2248. https://doi.org/10.3390/cancers15082248
Chicago/Turabian StyleAwada, Hussein, Carmelo Gurnari, Zhuoer Xie, Jan Philipp Bewersdorf, and Amer M. Zeidan. 2023. "What’s Next after Hypomethylating Agents Failure in Myeloid Neoplasms? A Rational Approach" Cancers 15, no. 8: 2248. https://doi.org/10.3390/cancers15082248
APA StyleAwada, H., Gurnari, C., Xie, Z., Bewersdorf, J. P., & Zeidan, A. M. (2023). What’s Next after Hypomethylating Agents Failure in Myeloid Neoplasms? A Rational Approach. Cancers, 15(8), 2248. https://doi.org/10.3390/cancers15082248






