Thyroglobulin Value Predict Iodine-123 Imaging Result in Differentiated Thyroid Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Methods
- (i)
- Neck-ultrasonography carried out by expert ultrasonographers using high-resolution ultrasound systems (Logiq3 Expert, GE Healthcare, Little Chalfont, United Kingdom or ACUSON 3000 Siemens, Erlangen, Germany) equipped with a high energy linear probe (14 MHz or more);
- (ii)
- Laboratory test. Basal Tg and TgAb values were measured on day 1 (basal), before the first rhTSH administration. Stimulated Tg values were measured on day 3 (early stimulated Tg) and 5 (late stimulated Tg) after the last rhTSH-administration. Serum TSH and TgAb were measured at Core Laboratory “G. Martino” University Hospital in Messina (Italy) by fully automated Access® TSH immunochemiluminescent assay (Beckman Coulter, US; reference range 0.4–4.2 mUI/L) and Elecsys® TgAb chemiluminescence immunoassay (Roche, Switzerland; functional sensitivity 40 IU/mL), respectively. For the purpose of the present study, patients’ sera were frozen at −80 °C and centralized at the Laboratory of Clinical Chemistry and Immunology, Department of Laboratory Medicine, Ente Ospedaliero Cantonale, Bellinzona (Switzerland), and serum Tg was measured on the Kryptor® Compact Plus instrument (BRAHMS Thermo Fisher Scientific, Waltham, MA, USA). The Kryptor® hTg-sensitive assay is calibrated against the BCR® 457 international reference standard and the FS (corresponding to inter-assay imprecision of 20%) has been specified as 0.15 µg/L by the manufacturer (Instructions for Use, ThermoFisher). To conform to the 2015 ATA response criteria, Tg values below 0.20 were transformed in 0.20 ng/mL for statistical analysis.
- (iii)
- 123I-Dx-WBS-SPECT/CT was obtained using a double-headed gamma camera (Xeleris, GE Medical System. Chicago, IL, USA; Symens Symbia T-series, Erlangen, Germany) equipped with low-energy high-resolution parallel-hole collimators (HEHRPAR). Whole-body images were obtained from head to proximal thighs (anterior and posterior views, matrix 1024 · 256, magnification: 1, acquisition time: 10 cm/min). The study was integrated by static images of the neck and thorax (anterior and posterior views, magnification: 1; matrix: 256 · 256; frame time: 900 s). SPECT/CT images were obtained in step-and-shoot acquisition mode (40 s/step, 6° angle, 30 steps/detector) while CT images were acquired under the following conditions: tube voltage of 120 kVp, tube current of 80–210 mA, helical thickness of 2.5 mm, table speed of 37 mm/s, table feed per rotation of 18.75 mm/rot, tube rotation time of 0.8 s and pitch of 0.938:1. Using the aforementioned parameters, the overall efficacy doses released to the body linked to the use of 123I and CT imaging were 3.145 and <3 mSv, respectively. Finally, all patients were required to drink at least 1.5 L of water and take laxative drugs 1 day before the examination in order to achieve a better target/background ratio.
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campenni, A.; Giovanella, L. Nuclear medicine therapy of thyroid cancer post-thyroidectomy. In Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://seer.cancer.gov/archive/csr/1975_2014/ (accessed on 5 April 2017).
- Campennì, A.; Giovanella, L.; Pignata, S.A.; Violi, M.A.; Siracusa, M.; Alibrandi, A.; Moleti, M.; Amato, E.; Ruggeri, R.M.; Vermiglio, F.; et al. Thyroid remnant ablation in differentiated thyroid cancer: Searching for the most effective radioiodine activity and stimulation strategy in a real-life scenario. Nucl. Med. Commun. 2015, 36, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Campennì, A.; Ruggeri, R.M.; Siracusa, M.; Comis, A.D.; Romano, D.; Vento, A.; Lanzafame, H.; Capoccetti, F.; Alibrandi, A.; Baldari, S.; et al. Early preablation rhTSH-stimulated thyroglobulin predicts outcome of differentiated thyroid cancer (DTC) patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef]
- Horn-Ross, P.L.; Lichtensztajn, D.Y.; Clarke, C.A.; Dosiou, C.; Oakley-Girvan, I.; Reynolds, P.; Gomez, S.L.; Nelson, D.O. Continued rapid increase in thyroid cancer incidence in california: Trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 1067–1079. [Google Scholar] [CrossRef]
- Cancer Statistics Review, 1975–2013; Based on November 2015 SEER Data Submission; National Cancer Institute: Bethesda, MD, USA, 2016. Available online: https://seer.cancer.gov/archive/csr/1975_2013/ (accessed on 5 April 2017).
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2018; National Cancer Institute: Bethesda, MD, USA, 2021. Available online: https://seer.cancer.gov/csr/1975_2018/ (accessed on 5 April 2017).
- Campennì, A.; Barbaro, D.; Guzzo, M.; Capoccetti, F.; Giovanella, L. Personalized management of differentiated thyroid cancer in real life-practical guidance from a multidisciplinary panel of experts. Endocrine 2020, 70, 280–291. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Ahuja, S.; Avram, A.M.; Bernet, V.J.; Bourguet, P.; Daniels, G.H.; Dillehay, G.; Draganescu, C.; Flux, G.; Führer, D.; et al. Controversies, Consensus, and Collaboration in the Use of 131I Therapy in Differentiated Thyroid Cancer: A Joint Statement from the American Thyroid Association, the European Association of Nuclear Medicine, the Society of Nuclear Medicine and Molecular Imaging, and the European Thyroid Association. Thyroid. Off. J. Am. Thyroid. Assoc. 2019, 29, 461–470. [Google Scholar] [CrossRef]
- Giovanella, L.; Duntas, L.H. Management of Endocrine Disease: The role of rhTSH in the management of differentiated thyroid cancer: Pros and cons. Eur. J. Endocrinol. 2019, 181, R133–R145. [Google Scholar] [CrossRef]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E.; European Association of Nuclear Medicine (EANM). Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. Off. J. Am. Thyroid. Assoc. 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Campennì, A.; Vrachimis, A.; Siracusa, M.; Baldari, S.; Giovanella, L. Usefulness of 123I-spect/ct to assess the response to initial therapy in differentiated thyroid cancer patients. Endocrine 2021, 74, 193–196. [Google Scholar] [CrossRef]
- Spanu, A.; Nuvoli, S.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Role of Diagnostic 131I SPECT/CT in Long-Term Follow-up of Patients with Papillary Thyroid Microcarcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 1510–1515. [Google Scholar] [CrossRef]
- Spanu, A.; Nuvoli, S.; Marongiu, A.; Gelo, I.; Mele, L.; Piras, B.; Madeddu, G. Neck lymph node metastasis detection in patients with differentiated thyroid carcinoma (DTC) in long-term follow-up: A 131I-SPECT/CT study. BMC Cancer 2020, 20, 239. [Google Scholar] [CrossRef]
- Campennì, A.; Giovanella, L.; Siracusa, M.; Stipo, M.E.; Alibrandi, A.; Cucinotta, M.; Ruggeri, R.M.; Baldari, S. Is malignant nodule topography an additional risk factor for metastatic disease in low-risk differentiated thyroid cancer? Thyroid. Off. J. Am. Thyroid. Assoc. 2014, 24, 1607–1611. [Google Scholar] [CrossRef]
- Campennì, A.; Giovanella, L.; Pignata, S.A.; Vento, A.; Alibrandi, A.; Sturiale, L.; Laudicella, R.; Comis, A.D.; Filice, R.; Giuffrida, G.; et al. Undetectable or low (<1 ng/ml) postsurgical thyroglobulin values do not rule out metastases in early stage differentiated thyroid cancer patients. Oncotarget 2018, 9, 17491–17500. [Google Scholar] [CrossRef]
- Campennì, A.; Ruggeri, R.M.; Siracusa, M.; Giacoppo, G.; La Torre, F.; Saccomanno, A.; Alibrandi, A.; Dionigi, G.; Tuccari, G.; Baldari, S.; et al. Isthmus topography is a risk factor for persistent disease in patients with differentiated thyroid cancer. Eur. J. Endocrinol. 2021, 185, 397–404. [Google Scholar] [CrossRef]
- Pacini, F.; Schlumberger, M.; Dralle, H.; Elisei, R.; Smit, J.W.; Wiersinga, W.; European Thyroid Cancer Taskforce. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur. J. Endocrinol. 2006, 154, 787–803. [Google Scholar] [CrossRef]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer; Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. Off. J. Am. Thyroid. Assoc. 2009, 19, 1167–1214. [Google Scholar] [CrossRef]
- Sisson, J.C.; Avram, A.M.; Lawson, S.A.; Gauger, P.G.; Doherty, G.M. The so-called stunning of thyroid tissue. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006, 47, 1406–1412. [Google Scholar]
- Lamartina, L.; Deandreis, D.; Durante, C.; Filetti, S. Endocrine Tumours: Imaging in the follow-up of differentiated thyroid cancer: Current evidence and future perspectives for a risk-adapted approach. Eur. J. Endocrinol. 2016, 175, R185–R202. [Google Scholar] [CrossRef] [PubMed]
- Pirich, C.; Schweighofer-Zwink, G. Less is more: Reconsidering the need for regular use of diagnostic whole body radioiodine scintigraphy in the follow-up of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, M.; Dressler, J.; Eschner, W.; Grünwald, F.; Lassmann, M.; Leisner, B.; Luster, M.; Reiners, C.; Schicha, H.; Schober, O. Procedure guideline for iodine-131 whole-body scintigraphy for differentiated thyroid cancer (version 3). Nucl. Med. 2007, g46, 206–212. [Google Scholar]
- Gonzalez Carvalho, J.M.; Görlich, D.; Schober, O.; Wenning, C.; Riemann, B.; Verburg, F.A.; Vrachimis, A. Evaluation of 131I scintigraphy and stimulated thyroglobulin levels in the follow up of patients with DTC: A retrospective analysis of 1420 patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Barwick, T.; Murray, I.; Megadmi, H.; Drake, W.M.; Plowman, P.N.; Akker, S.A.; Chew, S.L.; Grossman, A.B.; Avril, N. Single photon emission computed tomography (SPECT)/computed tomography using Iodine-123 in patients with differentiated thyroid cancer: Additional value over whole body planar imaging and SPECT. Eur. J. Endocrinol. 2010, 162, 1131–1139. [Google Scholar] [CrossRef]
- Spanu, A.; Solinas, M.E.; Chessa, F.; Sanna, D.; Nuvoli, S.; Madeddu, G. 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: Incremental value versus planar imaging. J. Nucl. Med Off. Publ. Soc. Nucl. Med. 2009, 50, 184–190. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; AlShaikh, O.; Tuli, M.; Al-Sugair, A.; Alamawi, R.; Al-Rasheed, M.M. Diagnostic value of recombinant human thyrotropin-stimulated ¹²³I whole-body scintigraphy in the follow-up of patients with differentiated thyroid cancer. Clin. Nucl. Med. 2012, 37, 229–234. [Google Scholar] [CrossRef]
- Siddiqi, A.; Foley, R.R.; Britton, K.E.; Sibtain, A.; Plowman, P.N.; Grossman, A.B.; Monson, J.P.; Besser, G.M. The role of 123I-diagnostic imaging in the follow-up of patients with differentiated thyroid carcinoma as compared to 131I-scanning: Avoidance of negative therapeutic uptake due to stunning. Clin. Endocrinol. 2001, 55, 515–521. [Google Scholar] [CrossRef]
- Trimboli, P.; Imperiali, M.; Piccardo, A.; CampennÌ, A.; Giordani, I.; Ruggeri, R.M.; Baldari, S.; Orlandi, F.; Giovanella, L. Multicentre clinical evaluation of the new highly sensitive Elecsys® thyroglobulin II assay in patients with differentiated thyroid carcinoma. Clin. Endocrinol. 2018, 88, 295–302. [Google Scholar] [CrossRef]
- Trimboli, P.; Zilioli, V.; Imperiali, M.; Ceriani, L.; Giovanella, L. High-sensitive basal serum thyroglobulin 6–12 months after thyroid ablation is strongly associated with early response to therapy and event-free survival in patients with low-to-intermediate risk differentiated thyroid carcinomas. Eur. J. Endocrinol. 2017, 176, 497–504. [Google Scholar] [CrossRef]

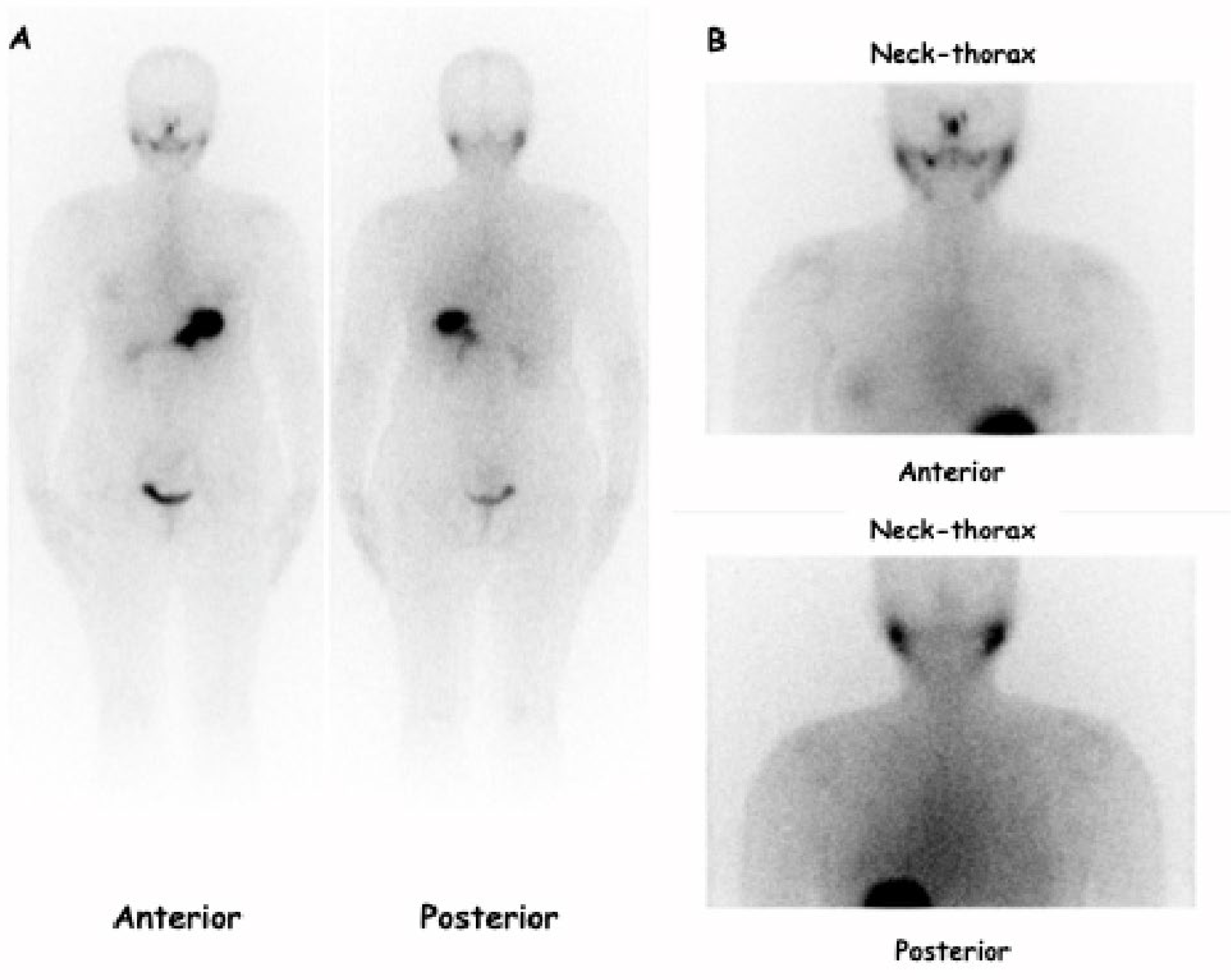
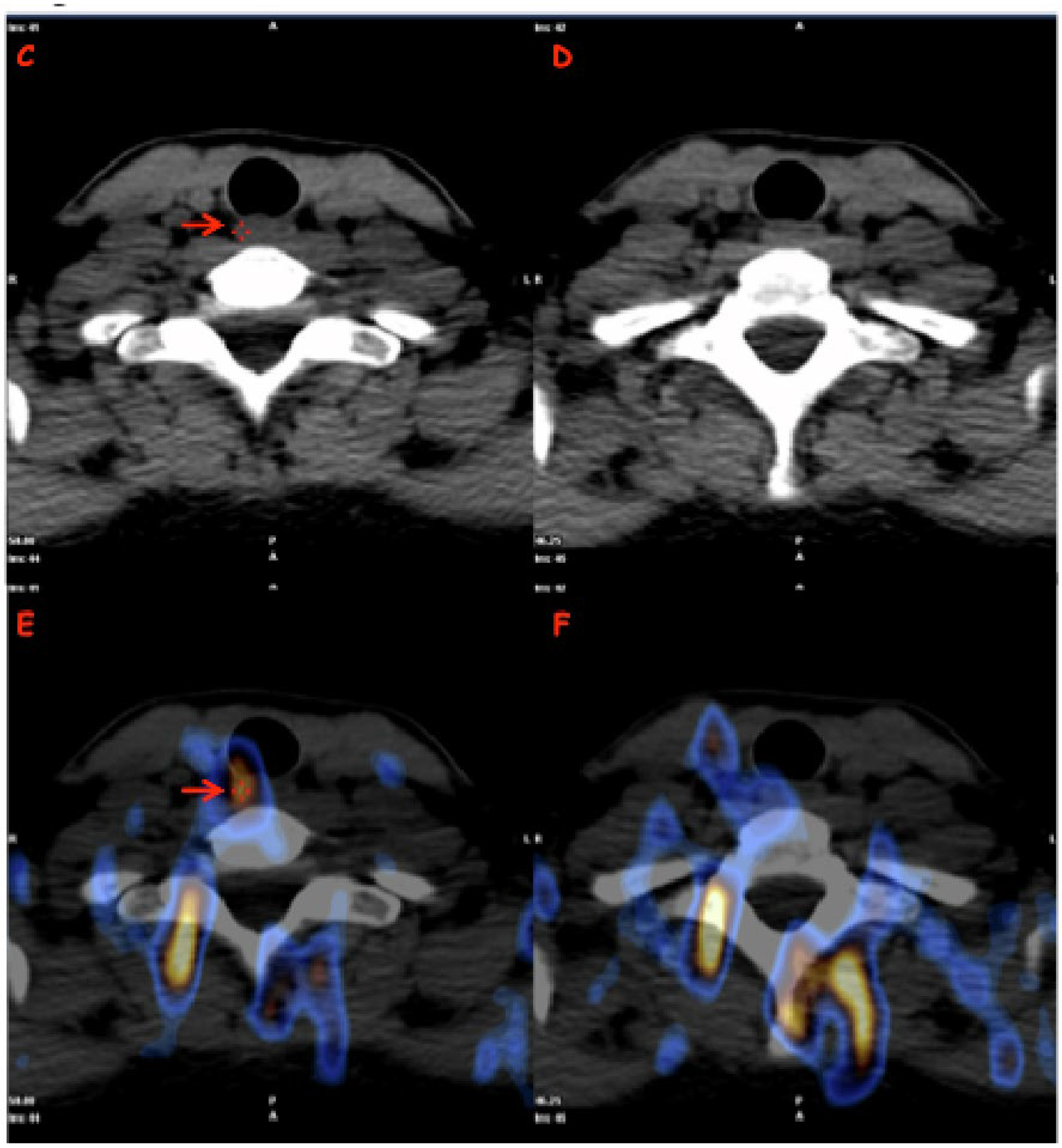
| Patients Number (%) | Female (%) | Male (%) | Median Age (Range) | Median Tumor Size (mm) [Range] | Histological Variant (No Aggressive Variant) [e.g.: * CV; § Minimally Invasive] (%) | Histological Variant (Aggressive Variant) [e.g.: ** FV, # TC, ∞ HV, §§ SV, ¶ HC] (%) | Low-Risk Class (2015 ATA) (%) | Intermediate Risk Class (2015 ATA) (%) | |
|---|---|---|---|---|---|---|---|---|---|
| PTC | 112 (90.3) | 85 (75.9) | 27 (24.1) | 44.5 (19–81) | 18.1 (3–39) | 71 (63.4) | 41 (36.6) | 52 (46.4) | 60 (53.6) |
| FTC | 7 (5.6) | 5 (71.4) | 2 (28.6) | 56 (39–74) | 26 (10–38) | 7 (100) | 0 (0.0) | 6 (85.7) | 1 (14.3) |
| HC | 5 (4.1) | 4 (80) | 1 (20) | 54 (28–75) | 33 (25–40) | 0 (0.0) | 5 (100) | 3 (60) | 2 (40) |
| TOTAL | 124 | 94 (75.8) | 30 (24.2) | 46 (19–81) | 19.2 (3–40) | 78 (62.9) | 46 (37.1) | 61 (49.2) | 63 (50.8) |
| Patients Number (%) | Female (%) | Male (%) | Median Age (Range) | Median Tumor Size (mm) [Range] | Histological Variant: (No Aggressive Variant) [e.g.: * CV; § Minimally Invasive] (%) | Histological Variant (Aggressive Variant) [e.g.: ** FV, # TC, ∞ HV, §§ SV, ¶ HC] (%) | Low-Risk Class (2015 ATA) (%) | Intermediate Risk Class (2015 ATA) (%) | nUS n = Performed Studies [n = Positive studies] | 123I-Dx-Imaging n = Performed Studies [n = Positive Studies] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTC | 77 (88.6) | 58 (75.3) | 19 (24.7) | 48.9 (23–81) | 20.5 (3–39) | 49 (63.6) | 28 (36.4) | 36 (46.8) | 41 (53.2) | n = 77 [n = 0] | n = 77 [n = 0] |
| FTC | 5 (5.7) | 4 (80) | 1 (20) | 49 (39–61) | 26 (18–38) | 5 (100) | 0 (0.0) | 4 (80) | 1 (20) | n = 5 [n = 0] | n = 5 [n = 0] |
| HC | 5 (5.7) | 4 (20) | 1 (20) | 54 (28–75) | 33 (25–40) | 0 (0.0) | 5 (100) | 3 (60) | 2 (40) | n = 5 [n = 0] | n = 5 [n = 0] |
| TOTAL | 87 | 66 (75.9) | 21 (24.1) | 49.1 (23–81) | 22 (3–40) | 54 (62.1) | 33 (42.9) | 43 (49.4) | 44 (50.6) | n = 87 [n = 0] | n = 87 [n = 0] |
| Patients Number (%) | Female (%) | Male (%) | Median Age (Range) | Median Tumor Size (mm) [Range] | Histological Variant: (No Aggressive Variant) [e.g.: * CV; § Minimally Invasive] (%) | Histological Variant: (Aggressive Variant) [e.g.: ** FV, # TC, ∞ HV, §§ SV, ¶ HC] (%) | Low-Risk Class (2015 ATA) (%) | Intermediate Risk Class (2015 ATA) (%) | nUS n = Performed Studies [n = Positive Studies] | 123I-Dx-Imaging n = Performed Studies [n = Positive Studies] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTC | 18 (94.7) | 15 (83.3) | 3 (16.7) | 38 (18–50) | 15 (5–38) | 10 (55.6) | 8 (44.4) | 6 | 12 | n = 18 [n = 0] | n = 18 [n = 0] |
| FTC | 1 (5.3) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | n = 1 [n = 0] | n = 1 [n = 0] | ||
| TOTAL | 19 | 15 (78.9) | 4 (21.1) | 38 (18–50) | 15 (5–38) | 11 (57.9) | 8 (42.1) | 7 (36.8) | 12 (63.2) | n = 19 [n = 0] | n = 19 [n = 0] |
| Patients Number (%) | Female (%) | Male (%) | Median Age (Range) | Median Tumor Size (mm) [Range] | Histological Variant: (No Aggressive Variant) [e.g.: * CV; § Minimally Invasive] (%) | Histological Variant (Aggressive Variant) [e.g.: ** FV, # TC, ∞ HV, §§ SV, ¶ HC] (%) | Low-Risk Class (2015 ATA) (%) | Intermediate Risk Class (2015 ATA) (%) | nUS n = Performed Studies [n = Positive Studies] | 123I-Dx-Imaging n = Performed Studies [n = Positive Studies] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PTC | 17 (94.4) | 12 (70.6) | 5 (29.4) | 42 (24–72) | 12 (6–24) | 11 (64.7) | 6 (35.3) | 10 (58.8) | 7 (41.2) | n = 17 [n = 4] | n = 17 [n = 17] |
| FTC | 1 (5.6) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | 1 (100) | 0 (0) | n = 1 [n = 0] | n = 1 [n = 1] | ||
| TOTAL | 18 | 13 (72.2) | 5 (27.8) | 42 (24–72) | 12 (6–24) | 12 (66.7) | 6 (33.3) | 11 (61.1) | 7 (38.9) | n = 18 [n = 4] | n = 18 [n = 18] |
| Patients Number (%) | Female (%) | Male (%) | Median Age (year) | PTC (%) | FTC (%) | Median Tumor Size (mm) | Histological Variant: (No Aggressive Variant) [e.g.: * CV; § Minimally Invasive] (%) | Histological Variant (Aggressive Variant) [e.g.: ** FV, # TC, ∞ HV, §§ SV, ¶ HC] (%) | Low-Risk Class (2015 ATA) (%) | Intermediate Risk Class (2015 ATA) (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | 87 (70.2) | 66 (75.9) | 21 (24.1) | 49.1 | 77 (88.5) | 10 (11.5) | 22 | 54 (62.1) | 33 (42.9) | 43 (49.4) | 44 (50.6) |
| Group B | 19 (15.3) | 15 (78.9) | 4 (21.1) | 38 | 18 (94.7) | 1 (5.3) | 15 | 11 (57.9) | 8 (42.1) | 7 (36.8) | 12 (63.2) |
| Group C | 18 (14.5) | 13 (72.2) | 5 (27.8) | 42 | 17 (94.4) | 1 (5.6) | 12 | 12 (66.7) | 6 (33.3) | 11 (61.1) | 7 (38.9) |
| Basal-Tg (Day 1) | Early-Stimulated-Tg (Day 3) | Late-Stimulated-Tg (Day 5) | ||||
|---|---|---|---|---|---|---|
| Median (ng/mL) | Range (ng/mL) | Median (ng/mL) | Range (ng/mL) | Median (ng/mL) | Range (ng/mL) | |
| Group A (n = 87) | 0.15 | 0.15–0.51 | 0.15 | 0.15–0.83 | 0.15 | 0.15–0.67 |
| Group B (n = 19) | 0.70 | 0.15–2.1 | 2.15 | 0.15–16.9 | 3.6 | 0.9–19.8 |
| Group C (n = 18) | 0.97 | 0.15–11.4 | 3.50 | 0.42–47.3 | 7.6 | 0.76–41 |
| All patients (n = 124) | 0.15 | 0.15–11.4 | 0.15 | 0.15–47.3 | 0.15 | 0.15–41 |
| Independent Variables | Univariate Models | Multivariate Model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age | 0.997 | 0.962–1.033 | 0.866 | 1.000 | 0.953–1.049 | 0.994 |
| Gender | 0.802 | 0.261–2.471 | 0.701 | 1.045 | 0.223–4.902 | 0.955 |
| Histotype | 0.508 | 0.062–4.195 | 0.530 | 0.212 | 0.008–5.338 | 0.346 |
| Histological variant * | 0.830 | 0.299–2.308 | 0.721 | 1.594 | 0.362–7.019 | 0.538 |
| Basal-Tg (≥0.39 ng/mL) | 30.227 | 8.450–108.123 | <0.001 | 34.625 | 8.359–143.421 | <0.001 |
| Tumor size | 0.964 | 0.917–1.012 | 0.142 | 0.979 | 0.925–1.036 | 0.460 |
| Risk classification # (2015 ATA) | 0.568 | 0.205–1.578 | 0.278 | 0.406 | 0.105–1.570 | 0.192 |
| Malignant nodule topography | 0.980 | 0.111–8.660 | 0.986 | 0.634 | 0.035–11.401 | 0.757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campennì, A.; Ruggeri, R.M.; Siracusa, M.; Romano, D.; Giacoppo, G.; Crocè, L.; Rosarno, H.; Russo, S.; Cardile, D.; Capoccetti, F.; et al. Thyroglobulin Value Predict Iodine-123 Imaging Result in Differentiated Thyroid Cancer Patients. Cancers 2023, 15, 2242. https://doi.org/10.3390/cancers15082242
Campennì A, Ruggeri RM, Siracusa M, Romano D, Giacoppo G, Crocè L, Rosarno H, Russo S, Cardile D, Capoccetti F, et al. Thyroglobulin Value Predict Iodine-123 Imaging Result in Differentiated Thyroid Cancer Patients. Cancers. 2023; 15(8):2242. https://doi.org/10.3390/cancers15082242
Chicago/Turabian StyleCampennì, Alfredo, Rosaria Maddalena Ruggeri, Massimiliano Siracusa, Davide Romano, Giulia Giacoppo, Ludovica Crocè, Helena Rosarno, Simona Russo, Davide Cardile, Francesca Capoccetti, and et al. 2023. "Thyroglobulin Value Predict Iodine-123 Imaging Result in Differentiated Thyroid Cancer Patients" Cancers 15, no. 8: 2242. https://doi.org/10.3390/cancers15082242
APA StyleCampennì, A., Ruggeri, R. M., Siracusa, M., Romano, D., Giacoppo, G., Crocè, L., Rosarno, H., Russo, S., Cardile, D., Capoccetti, F., Alibrandi, A., Baldari, S., & Giovanella, L. (2023). Thyroglobulin Value Predict Iodine-123 Imaging Result in Differentiated Thyroid Cancer Patients. Cancers, 15(8), 2242. https://doi.org/10.3390/cancers15082242






