Integrated Microbiota and Metabolite Changes following Rice Bran Intake during Murine Inflammatory Colitis-Associated Colon Cancer and in Colorectal Cancer Survivors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Bran and Control Diet Preparation
2.2. Ethics Statement
2.3. Animal Study Design and Sample Collection
2.4. Metataxonomics Sample Processing, Sequencing, and Analysis
2.5. Computation Detail for Microbiome Analysis
2.6. Non-Targeted Metabolomics Sample Process
2.7. Metabolomics Statistical Analysis
2.8. Human Study Design: Beans/Bran Enriching Nutritional Eating for Intestinal Health Trial (BENEFIT)
2.9. Availability of Data and Materials
3. Results
3.1. Rice Bran Mediated Changes to Fecal Microbiota Composition during Colon Carcinogenesis
3.2. Rice Bran Modulation of Fecal Metabolites for CRC Protection in Mice
3.3. Rice Bran Diet Mediated Metabolite Changes in Humans and AOM/DSS Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- A.C. Society; Cancer Facts and Figures: Atlanta, Georgia, 2012.
- Wang, K. Healthy lifestyle, endoscopic screening, and colorectal cancer incidence and mortality in the United States: A nationwide cohort study. PLoS Med. 2021, 18, e1003522. [Google Scholar] [CrossRef] [PubMed]
- Policy and Action for Cancer Prevention. Food, Nutrition, and Physical Activity: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2009.
- Zhao, J.; Zhu, Y.; Du, M.; Wang, Y.; Vallis, J.; Parfrey, P.S.; Mclaughlin, J.R.; Qi, X.; Wang, P.P. Association between Dietary Fiber Intake and Mortality among Colorectal Cancer Survivors: Results from the Newfoundland Familial Colorectal Cancer Cohort Study and a Meta-Analysis of Prospective Studies. Cancers 2022, 14, 3801. [Google Scholar] [CrossRef] [PubMed]
- Khil, H.; Kim, S.M.; Hong, S.; Gil, H.M.; Cheon, E.; Lee, D.H. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-De-Mesquita, B.; Skeie, G.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; et al. Dietary Fibre Intake and Risks of Cancers of the Colon and Rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2012, 7, e39361. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P. Epidemiology of cancer of the colon and rectum. Cancer 1971, 28, 3–13. [Google Scholar] [CrossRef]
- Kim, Y.S.; Milner, J.A. Dietary Modulation of Colon Cancer Risk. J. Nutr. 2007, 137, 2576S–2579S. [Google Scholar] [CrossRef]
- Xia, X.; Lin, Q.; Zhao, N.; Zeng, J.; Yang, J.; Liu, Z.; Huang, R. Anti-Colon Cancer Activity of Dietary Phytochemical Soyasaponin I and the Induction of Metabolic Shifts in HCT116. Molecules 2022, 27, 4382. [Google Scholar] [CrossRef]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 1–21. [Google Scholar] [CrossRef]
- Katyama, M.; Yoshimi, N.; Yamada, Y.; Sakata, K.; Kuno, T.; Yoshida, K.; Qiao, Z.; Vihn, P.Q.; Iwasaki, T.; Kobayashi, H.; et al. Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol. Rep. 2002, 9, 817–822. [Google Scholar] [CrossRef]
- Verschoyle, R.D.; Greaves, P.; Cai, H.; Edwards, R.E.; Steward, W.P.; Gescher, A.J. Evaluation of the cancer chemopreventive efficacy of rice bran in genetic mouse models of breast, prostate and intestinal carcinogenesis. Br. J. Cancer 2007, 96, 248–254. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Onuma, K.; Kanda, Y.; Ikeda, S.S.; Sakaki, R.; Nonomura, T.; Kobayashi, M.; Osaki, M.; Shikanai, M.; Kobayashi, H.; Okada, F. Fermented Brown Rice and Rice Bran with Aspergillus oryzae (FBRA) Prevents Inflammation-Related Carcinogenesis in Mice, through Inhibition of Inflammatory Cell Infiltration. Nutrients 2015, 7, 10237–10250. [Google Scholar] [CrossRef]
- Tantamango, Y.M.; Knutsen, S.F.; Beeson, W.L.; Fraser, G.; Sabate, J. Foods and Food Groups Associated with the Incidence of Colorectal Polyps: The Adventist Health Study. Nutr. Cancer 2011, 63, 565–572. [Google Scholar] [CrossRef]
- Parker, K.; Maurya, A.; Ibrahim, H.; Rao, S.; Hove, P.; Kumar, D.; Kant, R.; Raina, B.; Agarwal, R.; Kuhn, K.; et al. Dietary Rice Bran-Modified Human Gut Microbial Consortia Confers Protection against Colon Carcinogenesis Following Fecal Transfaunation. Biomedicines 2021, 9, 144. [Google Scholar] [CrossRef]
- Kumar, R.; Maurya, A.K.; Parker, K.D.; Kant, R.; Ibrahim, H.; Kabir, I.; Kumar, D.; Weber, A.M.; Agarwal, R.; Kuhn, K.A.; et al. Gender-based effect of absence of gut microbiota on the protective efficacy of Bifidobacterium longum-fermented rice bran diet against inflammation-associated colon tumorigenesis. Mol. Carcinog. 2022, 61, 941–957. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Li, S.-C.; Chou, T.-C.; Shih, C.-K. Effects of brown rice, rice bran, and polished rice on colon carcinogenesis in rats. Food Res. Int. 2011, 44, 209–216. [Google Scholar] [CrossRef]
- Sheflin, A.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.; Lu, S.; Brown, R.J.; Broeckling, C.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2016, 61, 1500905. [Google Scholar] [CrossRef]
- Saad, N.; Esa, N.M.; Ithnin, H. Suppression of β-catenin and Cyclooxygenase-2 Expression and Cell Proliferation in Azoxymethane-Induced Colonic Cancer in Rats by Rice Bran Phytic Acid (PA). Asian Pac. J. Cancer Prev. 2013, 14, 3093–3099. [Google Scholar] [CrossRef]
- Brown, D.G.; Borresen, E.C.; Brown, R.J.; Ryan, E.P. Heat-stabilised rice bran consumption by colorectal cancer survivors modulates stool metabolite profiles and metabolic networks: A randomised controlled trial. Br. J. Nutr. 2017, 117, 1244–1256. [Google Scholar] [CrossRef]
- Vilander, A.C.; Hess, A.; Abdo, Z.; Ibrahim, H.; Doumbia, L.; Douyon, S.; Koné, K.; Boré, A.; Zambrana, E.L.; Vilchez, S.; et al. A Randomized Controlled Trial of Dietary Rice Bran Intake on Microbiota Diversity, Enteric Dysfunction, and Fecal Secretory IgA in Malian and Nicaraguan Infants. J. Nutr. 2022, 152, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, L.E.; McKeen, S.; Ibrahim, H.; Zarei, I.; Borresen, E.C.; Doumbia, L.; Boré, A.; Cissoko, A.; Douyon, S.; Koné, K.; et al. Rice bran supplementation modulates growth, microbiota and metabolome in weaning infants: A clinical trial in Nicaragua and Mali. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive Properties of Dietary Rice Bran: Current Status and Future Prospects. Adv. Nutr. Int. Rev. J. 2012, 3, 643–653. [Google Scholar] [CrossRef]
- Norazalina, S.; Norhaizan, M.; Hairuszah, I.; Norashareena, M. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp. Toxicol. Pathol. 2010, 62, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.K.; Lam, W.; Chiu, L.C.; Ooi, V.E.; Sun, S.S.; Wong, Y.-S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharmacol. 2009, 77, 1487–1496. [Google Scholar] [CrossRef]
- Kim, S.P.; Kang, M.Y.; Nam, S.H.; Friedman, M. Dietary rice bran component γ-oryzanol inhibits tumor growth in tumor-bearing mice. Mol. Nutr. Food Res. 2012, 56, 935–944. [Google Scholar] [CrossRef]
- Komiyama, Y.; Andoh, A.; Fujiwara, D.; Ohmae, H.; Araki, Y.; Fujiyama, Y.; Mitsuyama, K.; Kanauchi, O. New prebiotics from rice bran ameliorate inflammation in murine colitis models through the modulation of intestinal homeostasis and the mucosal immune system. Scand. J. Gastroenterol. 2010, 46, 40–52. [Google Scholar] [CrossRef]
- Tajasuwan, L.; Kettawan, A.; Rungruang, T.; Wunjuntuk, K.; Prombutara, P.; Muangnoi, C.; Kettawan, A.K. Inhibitory Effect of Dietary Defatted Rice Bran in an AOM/DSS-Induced Colitis-Associated Colorectal Cancer Experimental Animal Model. Foods 2022, 11, 3488. [Google Scholar] [CrossRef]
- Yamada, Y.; Phutthaphadoong, S.; Hirata, A.; Tomita, H.; Hara, A.; Limtrakul, P.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemopreventive effect of fermented brown rice and rice bran (FBRA) on the inflammation-related colorectal carcinogenesis in ApcMin/+ mice. Oncol. Rep. 2009, 23, 53–59. [Google Scholar] [CrossRef]
- Henderson, A.J.; Kumar, A.; Barnett, B.; Dow, S.W.; Ryan, E.P. Consumption of Rice Bran Increases Mucosal Immunoglobulin A Concentrations and Numbers of Intestinal Lactobacillus spp. J. Med. Food 2012, 15, 469–475. [Google Scholar] [CrossRef]
- Abdo, Z.; LeCureux, J.; LaVoy, A.; Eklund, B.; Ryan, E.P.; Dean, G.A. Impact of oral probiotic Lactobacillus acidophilus vaccine strains on the immune response and gut microbiome of mice. PLoS ONE 2019, 14, e0225842. [Google Scholar] [CrossRef]
- Kukongviriyapan, V.; Phusrisom, S.; Senggunprai, L.; Prawan, A.; Kongpetch, S.; Kukongviriyapan, U.; Thawornchinsombut, S.; Siriamornpun, S.; Chumroenphat, T.; Changsri, R. Anti-tumor activity of rice bran hydrolysates on migration, invasion and angiogenesis. Asian Pac. J. Trop. Biomed. 2021, 11, 317. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Koh, A.; de Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the emerging role of the microbiome in cancer immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, S.R.G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Gut Microbiota, Inflammation, and Colorectal Cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zheng, S.; Li, M.; Xu, C.; Jia, D.; Qi, Y.; Hou, T.; Wang, L.; Wang, B.; et al. Fusobacterium nucleatum promotes colorectal cancer cells adhesion to endothelial cells and facilitates extravasation and metastasis by inducing ALPK1/NF-κB/ICAM1 axis. Gut Microbes 2022, 14, 2038852. [Google Scholar] [CrossRef]
- Okumura, S.; Konishi, Y.; Narukawa, M.; Sugiura, Y.; Yoshimoto, S.; Arai, Y.; Sato, S.; Yoshida, Y.; Tsuji, S.; Uemura, K.; et al. Gut bacteria identified in colorectal cancer patients promote tumourigenesis via butyrate secretion. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Huo, R.-X.; Wang, Y.-J.; Hou, S.-B.; Wang, W.; Zhang, C.-Z.; Wan, X.-H. Gut mucosal microbiota profiles linked to colorectal cancer recurrence. World J. Gastroenterol 2022, 28, 1946–1964. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef] [PubMed]
- Forster, G.M.; Raina, K.; Kumar, A.; Kumar, S.; Agarwal, R.; Chen, M.-H.; Bauer, J.E.; McClung, A.M.; Ryan, E.P. Rice varietal differences in bioactive bran components for inhibition of colorectal cancer cell growth. Food Chem. 2013, 141, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Henderson, A.; Forster, G.M.; Goodyear, A.W.; Weir, T.L.; Leach, J.E.; Dow, S.W.; Ryan, E.P. Dietary rice bran promotes resistance to Salmonella enterica serovar Typhimurium colonization in mice. BMC Microbiol. 2012, 12, 71. [Google Scholar] [CrossRef]
- Nealon, N.; Parker, K.; Lahaie, P.; Ibrahim, H.; Maurya, A.; Raina, K.; Ryan, E. Bifidobacterium longum-fermented rice bran and rice bran supplementation affects the gut microbiome and metabolome. Benef. Microbes 2019, 10, 823–839. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 28 March 2023).
- Borresen, E.C.; Brown, D.G.; Harbison, G.; Taylor, L.; Fairbanks, A.; O’Malia, J.; Bazan, M.; Rao, S.; Bailey, S.M.; Wdowik, M.; et al. A Randomized Controlled Trial to Increase Navy Bean or Rice Bran Consumption in Colorectal Cancer Survivors. Nutr. Cancer 2016, 68, 1269–1280. [Google Scholar] [CrossRef]
- Zarei, R.C.I.; Oppel, E.C.; Borresen, R.J.B.; Ryan, E.P. Modulation of plasma and urine metabolome in colorectal cancer survivors consuming rice bran. Integr. Food Nutr. Metab. 2019, 6, 10. [Google Scholar] [CrossRef]
- Bishehsari, F.; Engen, P.A.; Preite, N.Z.; Tuncil, Y.E.; Naqib, A.; Shaikh, M.; Rossi, M.; Wilber, S.; Green, S.J.; Hamaker, B.R.; et al. Dietary Fiber Treatment Corrects the Composition of Gut Microbiota, Promotes SCFA Production, and Suppresses Colon Carcinogenesis. Genes 2018, 9, 102. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Panala, V.; Field, R.; Williams, D.; Shackelford, L. Chemopreventive potential of selected cereals on chemically induced colon cancer in a Fisher 344 rat model. Cancer Epidemiol. Biomark. Prev. 2014, 16, B12. [Google Scholar]
- Slavin, J.L. Mechanisms for the Impact of Whole Grain Foods on Cancer Risk. J. Am. Coll. Nutr. 2000, 19, 300S–307S. [Google Scholar] [CrossRef]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat bran promotes enrichment within the human colonic microbiota of butyrate-producing bacteria that release ferulic acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Tao, S.; Zhou, X.; Pi, Y.; Gerrits, W.J.; Johnston, L.J.; Zhang, S.; Yang, H.; Liu, L.; et al. Effect of dietary fiber fermentation on short-chain fatty acid production and microbial composition in vitro. J. Sci. Food Agric. 2020, 100, 4282–4291. [Google Scholar] [CrossRef]
- Gylswyk, N.V.; Toorn, J.D. Eubacterium uniforme sp. nov. and Eubacterium xylanophilum sp. nov., Fiber-Digesting Bacteria from the Rumina of Sheep Fed Corn Stover. Int. J. Syst. Evol. Microbiol. 1985, 35, 323–326. [Google Scholar] [CrossRef]
- Meehan, C.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef]
- Shih, C.-K.; Ho, C.-J.; Li, S.-C.; Yang, S.-H.; Hou, W.-C.; Cheng, H.-H. Preventive effects of rice bran oil on 1,2-dimethylhydrazine/dextran sodium sulphate-induced colon carcinogenesis in rats. Food Chem. 2011, 126, 562–567. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Chriett, S.; Dąbek, A.; Wojtala, M.; Vidal, H.; Balcerczyk, A.; Pirola, L. Prominent action of butyrate over β-hydroxybutyrate as histone deacetylase inhibitor, transcriptional modulator and anti-inflammatory molecule. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. Msystems 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Uchiyama, K.; Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Wang, X.; Yang, R.; Zhu, X.; Zhang, Y.; Chen, C.; Yuan, H.; Yang, Z.; Sun, L. Gut bacteria Akkermansia is associated with reduced risk of obesity: Evidence from the American Gut Project. Nutr. Metab. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- Earley, H.; Lennon, G.; Balfe, J.C.; Coffey, D.C.W.; O’Connell, P.R. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; de Vos, W.M.; Satokari, R. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Acuña, I.; Ruiz, A.; Cerdó, T.; Cantarero, S.; López-Moreno, A.; Aguilera, M.; Campoy, C.; Suárez, A. Rapid and simultaneous determination of histidine metabolism intermediates in human and mouse microbiota and biomatrices. Biofactors 2021, 48, 315–328. [Google Scholar] [CrossRef]
- Wang, Y.; Jacobs, E.J.; Carter, B.D.; Gapstur, S.M.; Stevens, V.L. Plasma Metabolomic Profiles and Risk of Advanced and Fatal Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 56–65. [Google Scholar] [CrossRef]
- Silvério, R.; Laviano, A.; Fanelli, F.R.; Seelaender, M. l-carnitine and cancer cachexia: Clinical and experimental aspects. J. Cachex-Sarcopenia Muscle 2011, 2, 37–44. [Google Scholar] [CrossRef]
- Nealon, N.J.; Ryan, E.P. Chapter 4: Nutrition and Health Properties of Whole Grain Rice. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T.C., Eds.; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Zarei, I.; Ryan, E.P. Chapter 18: Lignans: Dietary Bioactive Food Components for Health. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T.C., Eds.; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Li, Y.; Wang, F.; Li, J.; Ivey, K.L.; Wilkinson, J.E.; Wang, D.D.; Li, R.; Liu, G.; Eliassen, H.A.; Chan, A.T.; et al. Dietary lignans, plasma enterolactone levels, and metabolic risk in men: Exploring the role of the gut microbiome. BMC Microbiol. 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Hullar, M.A.; Lancaster, S.M.; Li, F.; Tseng, E.; Beer, K.; Atkinson, C.; Wähälä, K.; Copeland, W.K.; Randolph, T.W.; Newton, K.M.; et al. Enterolignan-Producing Phenotypes Are Associated with Increased Gut Microbial Diversity and Altered Composition in Premenopausal Women in the United States Enterolignans Associated with Increased Bacterial Diversity. Cancer Epidemiol. Biomark. Prev. 2015, 24, 546–554. [Google Scholar] [CrossRef]
- Kyrø, C.; Frederiksen, K.; Holm, M.; Nørskov, N.P.; Knudsen, K.E.B.; Overvad, K.; Tjønneland, A.; Olsen, A. Prediagnosis plasma concentrations of enterolactone and survival after colorectal cancer: The Danish Diet, Cancer and Health cohort. Br. J. Nutr. 2018, 122, 552–563. [Google Scholar] [CrossRef]
- Mali, A.V.; Padhye, S.B.; Anant, S.; Hegde, M.V.; Kadam, S.S. Anticancer and antimetastatic potential of enterolactone: Clinical, preclinical and mechanistic perspectives. Eur. J. Pharmacol. 2019, 852, 107–124. [Google Scholar] [CrossRef]
- Wolf, B. Biotinidase deficiency. In GeneReviews®[Internet]; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallis, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1322/ (accessed on 17 July 2022).
- Zarei, I.; Luna, E.; Leach, J.E.; McClung, A.; Vilchez, S.; Koita, O.; Ryan, E.P. Comparative Rice Bran Metabolomics across Diverse Cultivars and Functional Rice Gene–Bran Metabolite Relationships. Metabolites 2018, 8, 63. [Google Scholar] [CrossRef]
- Ren, J.-G.; Seth, P.; Ye, H.; Guo, K.; Hanai, J.-I.; Husain, Z.; Sukhatme, V.P. Citrate Suppresses Tumor Growth in Multiple Models through Inhibition of Glycolysis, the Tricarboxylic Acid Cycle and the IGF-1R Pathway. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Falck, P.; Precha-Atsawanan, S.; Grey, C.; Immerzeel, P.; Stålbrand, H.; Adlercreutz, P.; Nordberg Karlsson, E. Xylooligosaccharides from hardwood and cereal xylans produced by a thermostable xylanase as carbon sources for Lactobacillus brevis and Bifidobacterium adolescentis. J. Agric. Food Chem. 2013, 61, 7333–7340. [Google Scholar] [CrossRef]
- Li, Y.; Pan, H.; Liu, J.-X.; Li, T.; Liu, S.; Shi, W.; Sun, C.; Fan, M.; Xue, L.; Wang, Y.; et al. l-Arabinose Inhibits Colitis by Modulating Gut Microbiota in Mice. J. Agric. Food Chem. 2019, 67, 13299–13306. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Lima, T.F.O.; Arcaro, C.A.; Inacio, M.D.; Batista-Duharte, A.; Carlos, I.Z.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Trigonelline and curcumin alone, but not in combination, counteract oxidative stress and inflammation and increase glycation product detoxification in the liver and kidney of mice with high-fat diet-induced obesity. J. Nutr. Biochem. 2019, 76, 108303. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.-F.; Krishnamachary, B.; Wildes, F.; Mironchik, Y.; Mezzanzanica, D.; Podo, F.; De Reggi, M.; Gharib, B.; Bhujwalla, Z.M. Effect of Pantethine on Ovarian Tumor Progression and Choline Metabolism. Front. Oncol. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Amirani, E.; Aghadavod, E.; Shafabakhsh, R.; Asemi, Z.; Tabassi, Z.; Panahandeh, I.; Naderi, F.; Abed, A. Anti-inflammatory and antioxidative effects of thiamin supplements in patients with gestational diabetes mellitus. J. Matern. Neonatal Med. 2020, 35, 2085–2090. [Google Scholar] [CrossRef]
- Sun, W.; Xu, W.; Liu, H.; Liu, J.; Wang, Q.; Zhou, J.; Dong, F.; Chen, B. γ-Tocotrienol induces mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Nutr. Biochem. 2009, 20, 276–284. [Google Scholar] [CrossRef]
- Constantinou, C.; Charalambous, C.; Kanakis, D. Vitamin E and cancer: An update on the emerging role of γ and δ tocotrienols. Eur. J. Nutr. 2020, 59, 845–857. [Google Scholar] [CrossRef]
- Hunsu, V.O.; Facey, C.O.; Fields, J.Z.; Boman, B.M. Retinoids as chemo-preventive and molecular-targeted anti-cancer therapies. Int. J. Mol. Sci. vol. 2021, 22, 7731. [Google Scholar] [CrossRef]
- Holowatyj, A.N.; Ose, J.; Gigic, B.; Lin, T.; Ulvik, A.; Geijsen, A.J.M.R.; Brezina, S.; Kiblawi, R.; van Roekel, E.H.; Baierl, A.; et al. Higher vitamin B6 status is associated with improved survival among patients with stage I–III colorectal cancer. Am. J. Clin. Nutr. 2022, 116, 303–313. [Google Scholar] [CrossRef]
- Gylling, B.; Myte, R.; Schneede, J.; Hallmans, G.; Häggström, J.; Johansson, I.; Ulvik, A.; Ueland, P.M.; Van Guelpen, B.; Palmqvist, R. Vitamin B-6 and colorectal cancer risk: A prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am. J. Clin. Nutr. 2017, 105, 897–904. [Google Scholar] [CrossRef]
- Zhang, P.; Suda, T.; Suidasari, S.; Kumrungsee, T.; Yanaka, N.; Kato, N. Novel Preventive Mechanisms of Vitamin B6 against Inflammation, Inflammasome, and Chronic Diseases. In Molecular Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 283–299. [Google Scholar]
- Aggarwal, V.; Kashyap, D.; Sak, K.; Tuli, H.S.; Jain, A.; Chaudhary, A.; Garg, V.K.; Sethi, G.; Yerer, M.B. Molecular Mechanisms of Action of Tocotrienols in Cancer: Recent Trends and Advancements. Int. J. Mol. Sci. 2019, 20, 656. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Li, D.-M.; Ma, Y.; He, N.; Gu, Q.; Wang, F.-S.; Jiang, S.-Q.; Chen, B.-Q.; Liu, J.-R. γ-Tocotrienol Induces Paraptosis-Like Cell Death in Human Colon Carcinoma SW620 Cells. PLoS ONE 2013, 8, e57779. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Im, S.; Nakatsu, C.; Jones-Hall, Y.; Jiang, Q. Vitamin E delta-tocotrienol and metabolite 13’-carboxychromanol inhibit colitis-associated colon tumorigenesis and modulate gut microbiota in mice. J. Nutr. Biochem. 2021, 89, 108567. [Google Scholar] [CrossRef]
- Nakashima, K.; Virgona, N.; Miyazawa, M.; Watanabe, T.; Yano, T. The tocotrienol-rich fraction from rice bran enhances cisplatin-induced cytotoxicity in human mesothelioma H28 cells. Phytotherapy Res. 2010, 24, 1317–1321. [Google Scholar] [CrossRef]
- Barnett, M.; Young, W.; Armstrong, K.; Brewster, D.; Cooney, J.; Ellett, S.; Espley, R.; Laing, W.; Maclean, P.; McGhie, T.; et al. A Polyphenol Enriched Variety of Apple Alters Circulating Immune Cell Gene Expression and Faecal Microbiota Composition in Healthy Adults: A Randomized Controlled Trial. Nutrients 2021, 13, 1092. [Google Scholar] [CrossRef]
- Yadav, M.; Lomash, A.; Kapoor, S.; Pandey, R.; Chauhan, N.S. Mapping of the benzoate metabolism by human gut microbiome indicates food-derived metagenome evolution. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Janicke, B.; Hegardt, C.; Krogh, M.; Önning, G.; Åkesson, B.; Cirenajwis, H.M.; Oredsson, S.M. The Antiproliferative Effect of Dietary Fiber Phenolic Compounds Ferulic Acid and p-Coumaric Acid on the Cell Cycle of Caco-2 Cells. Nutr. Cancer 2011, 63, 611–622. [Google Scholar] [CrossRef]

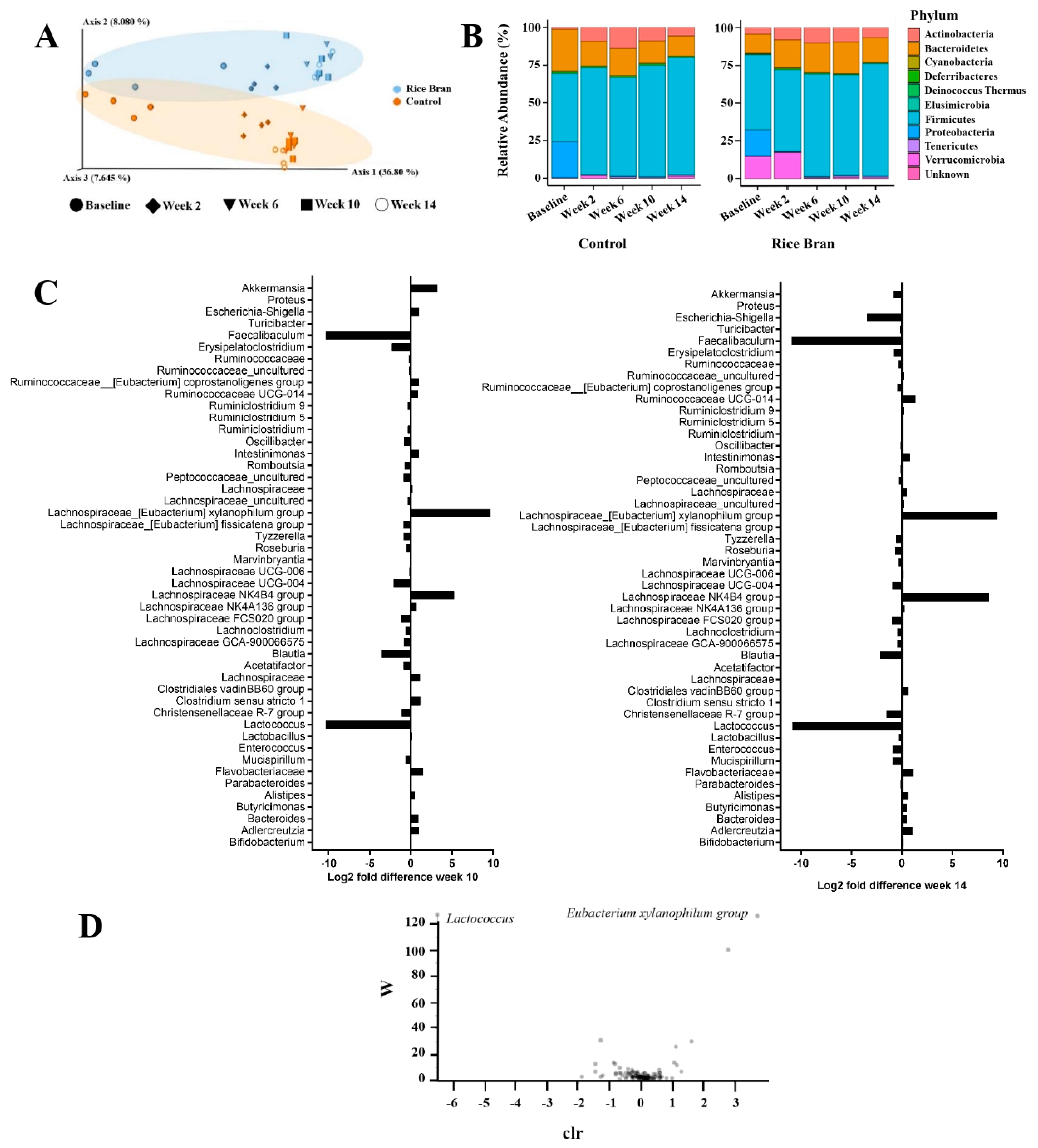
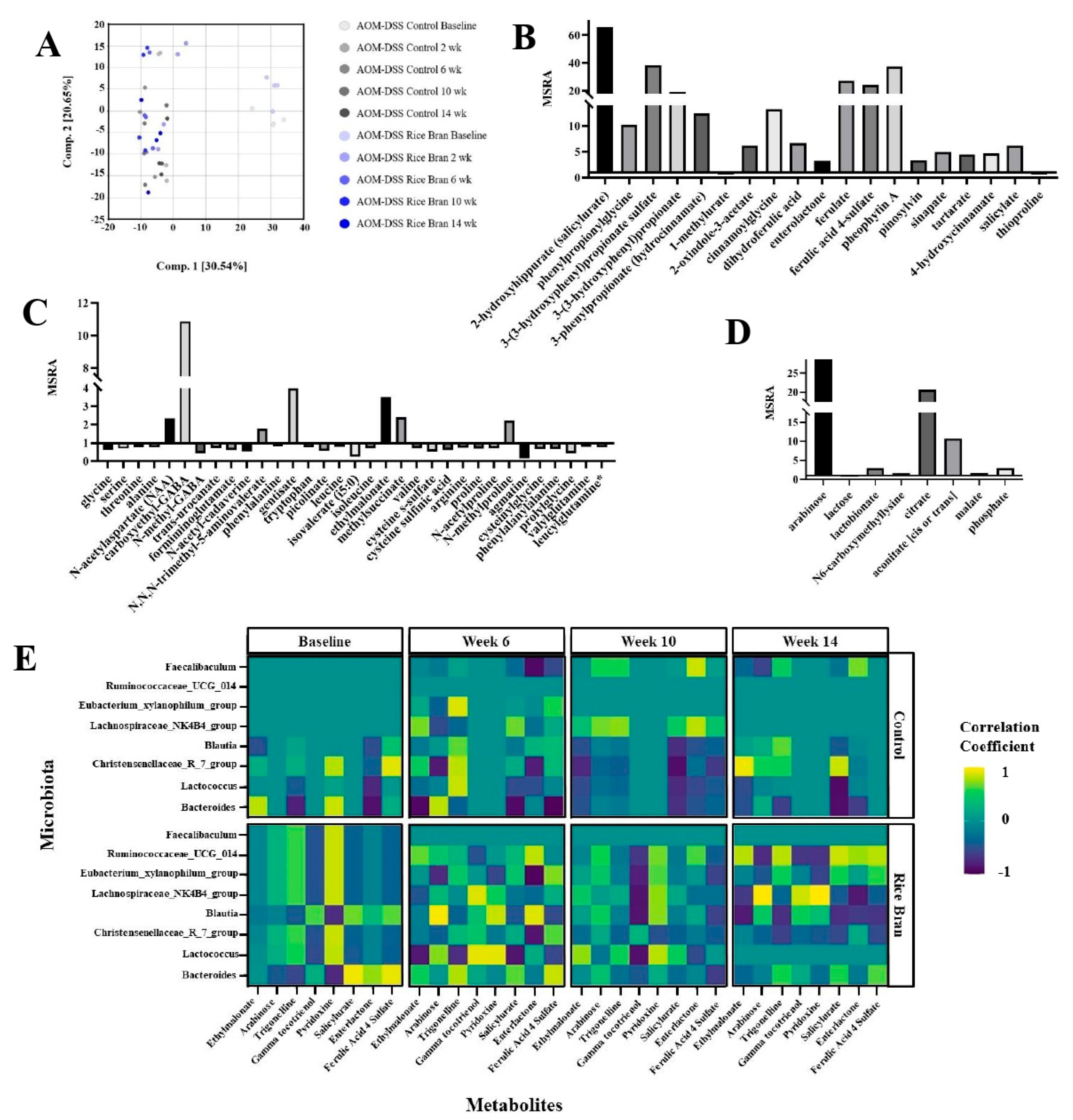

| Chemical Class | Metabolic Pathway | Metabolite | 10 Weeks | 14 Weeks |
|---|---|---|---|---|
| Amino Acids | Alanine and Aspartate Metabolism | N-acetylaspartate (NAA) | 1.62↑ | 2.34↑* |
| Glutamate Metabolism | Carboxyethyl-GABA | 6.97↑* | 10.85↑* | |
| Polyamine Metabolism | Agmatine | 0.18↓* | 0.16↓* | |
| Spermidine | 0.38↓* | 0.63↓ | ||
| Diacetylspermidine | 0.37↓* | 1.12↓ | ||
| Carbohydrates | Pentose Metabolism | Arabinose | 9.61↑* | 34.58↑* |
| Energy | TCA Cycle | Malate | 1.54↑* | 1.74↑* |
| Citrate | 6.70↑* | 20.56↑* | ||
| Aconitate | 2.54↑ | 10.72↑* | ||
| Oxidative Phosphorylation | Phosphate | 1.13↑ | 2.98↑* | |
| Lipids | Fatty Acid Synthesis | Malonate | 1.82↑* | 2.59↑* |
| Short-Chain Fatty Acid | Valerate (5:0) | 0.44↓ | 0.29↓* | |
| Medium-Chain Fatty Acid | Caproate (6:0) | 0.40↓* | 0.46↓* | |
| Caprylate (8:0) | 0.81↓ | 0.31↓* | ||
| Long-Chain Fatty Acid | Trans-nonadecenoate (tr 19:1) | 0.35↓* | 0.34↓* | |
| Eicosenoate (20:1) | 0.47↓* | 0.39↓* | ||
| Erucate (22:1n9) | 0.49↓* | 0.40↓* | ||
| Polyunsaturated Fatty Acid (n3 and n6) | Linoleate (18:2n6) | 1.45↑* | 1.84↑* | |
| Linolenate (alpha or gamma; (18:3n3 or 6)) | 1.7↑* | 2.22↑* | ||
| Fatty Acid, Dicarboxylate | 2-hydroxyglutarate | 2.05↑* | 3.65↑* | |
| Pimelate (C7-DC) | 0.84↓ | 3.72↑* | ||
| Suberate (C8-DC) | 1.31↑* | 1.69↑* | ||
| Azelate (C9-DC) | 1.48↑* | 2.30↑* | ||
| Hexadecanedioate (C16-DC) | 1.75↑* | 1.63↑* | ||
| Octadecanedioate (C18-DC) | 1.54↑* | 1.76↑* | ||
| Octadecenedioate (C18:1-DC) | 11.24↑* | 12.04↑* | ||
| Octadecadienedioate (C18:2-DC) | 0.77↓ | 0.38↓* | ||
| Eicosanodioate (C20-DC) | 1.55↑* | 1.52↑* | ||
| Fatty Acid Metabolism (Acyl Carnitine) | Palmitoylcarnitine (C16) | 0.72↓ | 0.35↓* | |
| Eicosenoylcarnitine (C20:1) | 0.75↓ | 0.41↓* | ||
| Margaroylcarnitine (C17) | 0.75↓ | 0.40↓* | ||
| Stearoylcarnitine (C18) | 0.84↓ | 0.46↓* | ||
| Fatty Acid Monohydroxyl | 16-hydroxypalmitate | 7.09↑* | 12.32↑* | |
| 2-hydroxybehenate | 0.44↓ | 0.46↓* | ||
| Fatty Acid, Dihydroxy | 9,10-DiHOME | 1.15↑ | 1.61↑* | |
| Choline phosphate | 1.97↑ | 2.49↑* | ||
| Lysophospholipid | 1-palmitoyl-GPI (16:0) | 2.04↑ | 2.07↑* | |
| 2-stearoyl-GPE (18:0) | 0.38↓* | 0.29↓* | ||
| Glycerolipid Metabolism | Glycerol 3-phosphate | 2.66↑* | 2.64↑* | |
| Monoacylglycerol | 1-oleoylglycerol (18:1) | 2.70↑* | 3.89↑* | |
| 1-linoleoylglycerol (18:2) | 2.83↑* | 4.48↑* | ||
| 2-oleoylglycerol (18:1) | 3.15↑* | 5.12↑* | ||
| 2-linoleoylglycerol (18:2) | 2.84↑* | 4.53↑* | ||
| Diacylglycerol | Oleoyl-linoleoyl-glycerol (18:1/18:2) [2] | 6.18↑* | 2.55↑* | |
| Sphingolipid Synthesis | Sphingadienine | 0.96↓ | 0.45↓* | |
| Dihydroceramides | N-stearoyl-spinganine (d18:0/18:0) | 0.79↓ | 0.45↓* | |
| Ceramides | N-stearoyl-sphingosine (d18:1/18:0) | 0.68↓ | 0.49↓* | |
| Mevalonate Metabolism | 3-hydroxy-3-mehtylglutarate | 1.38↑ | 3.00↑* | |
| Sterol | Lanosterol | 9.92↑* | 4.95↑* | |
| 4-cholesten-3-one | 1.98↑* | 1.73↑* | ||
| Primary Bile Acid Metabolism | Glycocholate sulfate | 0.19↓* | 0.22↓* | |
| Secondary Bile Acid Metabolism | Taurochenodeoxycholate sulfate | 0.12↓ | 0.05↓* | |
| Isohyodeoxycholate | 1.47↑* | 1.92↑* | ||
| Cofactors and Vitamins | Nicotinate and Nicotinamide Metabolism | Quinolinate | 1.93↑* | 3.92↑* |
| Nicotinate ribonucleoside | 3.30↑* | 10.24↑* | ||
| 1-methylnicotinamide | 0.93↓ | 4.03↑* | ||
| Trigonelline (N’-methylnicotinate) | 2.74↑* | 4.00↑* | ||
| Pantothenate and CoA Metabolism | Pantetheine | 2.53↑* | 2.50↑* | |
| Tocopherol Metabolism | Delta-tocopherol | 0.50* | 0.37↓* | |
| Alpha-tocotrienol | 1.70↑* | 1.59↑* | ||
| Gamma-tocotrienol | 1.64↑* | 1.46↑* | ||
| Gamma-tocopherol/Beta-tocopherol | 0.44↓* | 0.33↓* | ||
| Hemoglobin and Porphyrin Metabolism | Protoporphyrin IX | 1.74↑* | 1.74↑* | |
| Bilirubin (Z,Z) | 0.23↓* | 0.20↓* | ||
| Bilirubin (E,E)* | 0.18↓* | 0.20↓* | ||
| Biliverdin | 0.34↓* | 0.36↓* | ||
| Thiamin Metabolism | Thiamin (Vitamin B1) | 1.29↑ | 1.77↑* | |
| Vitamin A Metabolism | Retinol (Vitamin A) | 1.25↑ | 2.42↑* | |
| Carotene diol (1) | 3.76↑* | 3.04↑* | ||
| Vitamin B6 Metabolism | Pyridoxine (Vitamin B6) | 16.86↑* | 41.89↑* | |
| Pyridoxate | 1.40↑ | 2.07↑* | ||
| Pyridoxamine | 1.60↑* | 1.67↑* | ||
| Xenobiotics | Benzoate Metabolism | 2-hydroxyhippurate (salicylurate) | 11.69↑* | 65.75↑* |
| Phenylpropionylglycine | 3.14↑ | 10.2↑* | ||
| 3-(3-hydroxyphenyl)propionate sulfate | 1.30↑ | 38.24↑* | ||
| 3-(3-hydroxyphenyl)propionate | 7.03↑* | 19.12↑* | ||
| 3-phenylpropionate (hydrocinnamate) | 6.31↑* | 12.38↑* | ||
| Food Component/Plant | Cinnamoylglycine | 3.24↑ | 13.21↑* | |
| 2-oxindole-3-acetate | 7.51↑* | 7.51↑* | ||
| Dihydroferulic acid | 3.42↑* | 6.66↑* | ||
| Enterolactone | 3.37↑* | 3.27↑* | ||
| Ferulate | 7.38↑* | 27.13↑* | ||
| Ferulic Acid 4-sulfate | 0.69↓ | 24.21↑* | ||
| Pheophytin A | 38.38↑* | 37.25↑* | ||
| Pinosylvin | 3.46↑* | 3.36↑* | ||
| Sinapate | 3.75↑* | 4.99↑* | ||
| 4-hydroxycinnamate | 2.01↑ | 4.66↑* | ||
| Drug–Tropical Agent | Salicylate | 2.13↑* | 6.23↑* |
| Metabolic Pathway | Metabolite | BENEFIT Fold Change (4 Weeks/Baseline) | Mice Fold Change (2 Weeks/Baseline) | Mice Fold Change (6 Weeks/2 Weeks) | Mice Fold Change (10 Weeks/6 Weeks) | Mice Fold Change (14 Weeks/10 Weeks) |
|---|---|---|---|---|---|---|
| Histidine Metabolism | N-acetylhistamine | 0.52↓ | 0. 13↓ | 0.18↓ | 0.98↓# | 2.48↑ |
| Leucine, Isoleucine, and Valine Metabolism | Beta-hydroxyisovalerate | 17.9↑ | 0.28↓ | 0.60↓ | 0.98↓# | 1.55↑ |
| Ethylmalonate | 0.70↓ | 0.20↓ | 0.73↓# | 1.12↓# | 2.46↑ | |
| Methionine, Cysteine, SAM, and Taurine Metabolism | N-acetylmethionine sulfoxide | 0.47↓ | 2.73↑ | 0.72↓# | 1.15↑# | 1.61↑ |
| Gamma-glutamyl Amino Acid | Gamma-glutamylphenylalanine | 1.02↑ | 0.29↓ | 0.76↓# | 0.65↓# | 2.36↑ |
| Benzoate Metabolism | p-cresol sulfate | 0.69↓ | 70.2↑ | 1.48↑ | 0.60↑# | 1.69↑# |
| Food Component/Plant | Apigenin | 15.32↑ | 3.15↑ | 3.86↑ | 0.43↓# | 0.01↓ |
| Enterolactone | 3.55↑ | 7.77↑ | 1.70↑ | 0.79↑# | 0.90↓# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, A.M.; Ibrahim, H.; Baxter, B.A.; Kumar, R.; Maurya, A.K.; Kumar, D.; Agarwal, R.; Raina, K.; Ryan, E.P. Integrated Microbiota and Metabolite Changes following Rice Bran Intake during Murine Inflammatory Colitis-Associated Colon Cancer and in Colorectal Cancer Survivors. Cancers 2023, 15, 2231. https://doi.org/10.3390/cancers15082231
Weber AM, Ibrahim H, Baxter BA, Kumar R, Maurya AK, Kumar D, Agarwal R, Raina K, Ryan EP. Integrated Microbiota and Metabolite Changes following Rice Bran Intake during Murine Inflammatory Colitis-Associated Colon Cancer and in Colorectal Cancer Survivors. Cancers. 2023; 15(8):2231. https://doi.org/10.3390/cancers15082231
Chicago/Turabian StyleWeber, Annika M., Hend Ibrahim, Bridget A. Baxter, Robin Kumar, Akhilendra K. Maurya, Dileep Kumar, Rajesh Agarwal, Komal Raina, and Elizabeth P. Ryan. 2023. "Integrated Microbiota and Metabolite Changes following Rice Bran Intake during Murine Inflammatory Colitis-Associated Colon Cancer and in Colorectal Cancer Survivors" Cancers 15, no. 8: 2231. https://doi.org/10.3390/cancers15082231
APA StyleWeber, A. M., Ibrahim, H., Baxter, B. A., Kumar, R., Maurya, A. K., Kumar, D., Agarwal, R., Raina, K., & Ryan, E. P. (2023). Integrated Microbiota and Metabolite Changes following Rice Bran Intake during Murine Inflammatory Colitis-Associated Colon Cancer and in Colorectal Cancer Survivors. Cancers, 15(8), 2231. https://doi.org/10.3390/cancers15082231








