The Emerging Role of Histone Deacetylase Inhibitors in Cervical Cancer Therapy
Simple Summary
Abstract
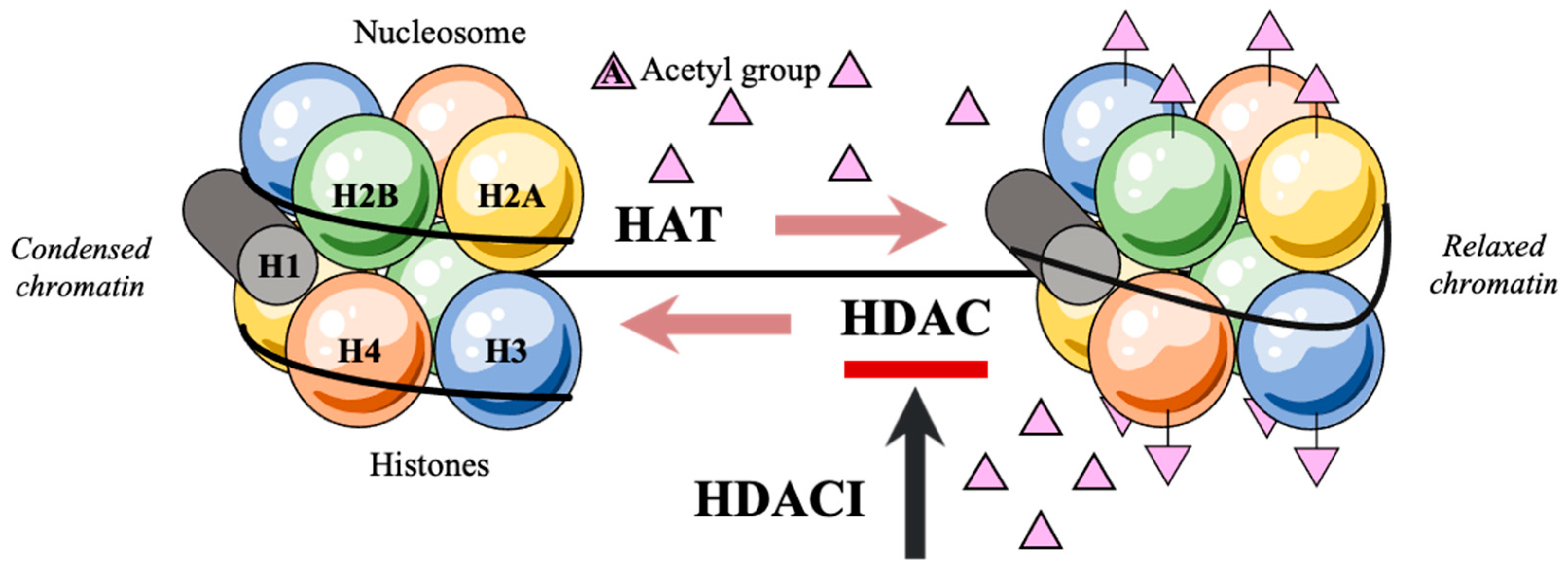
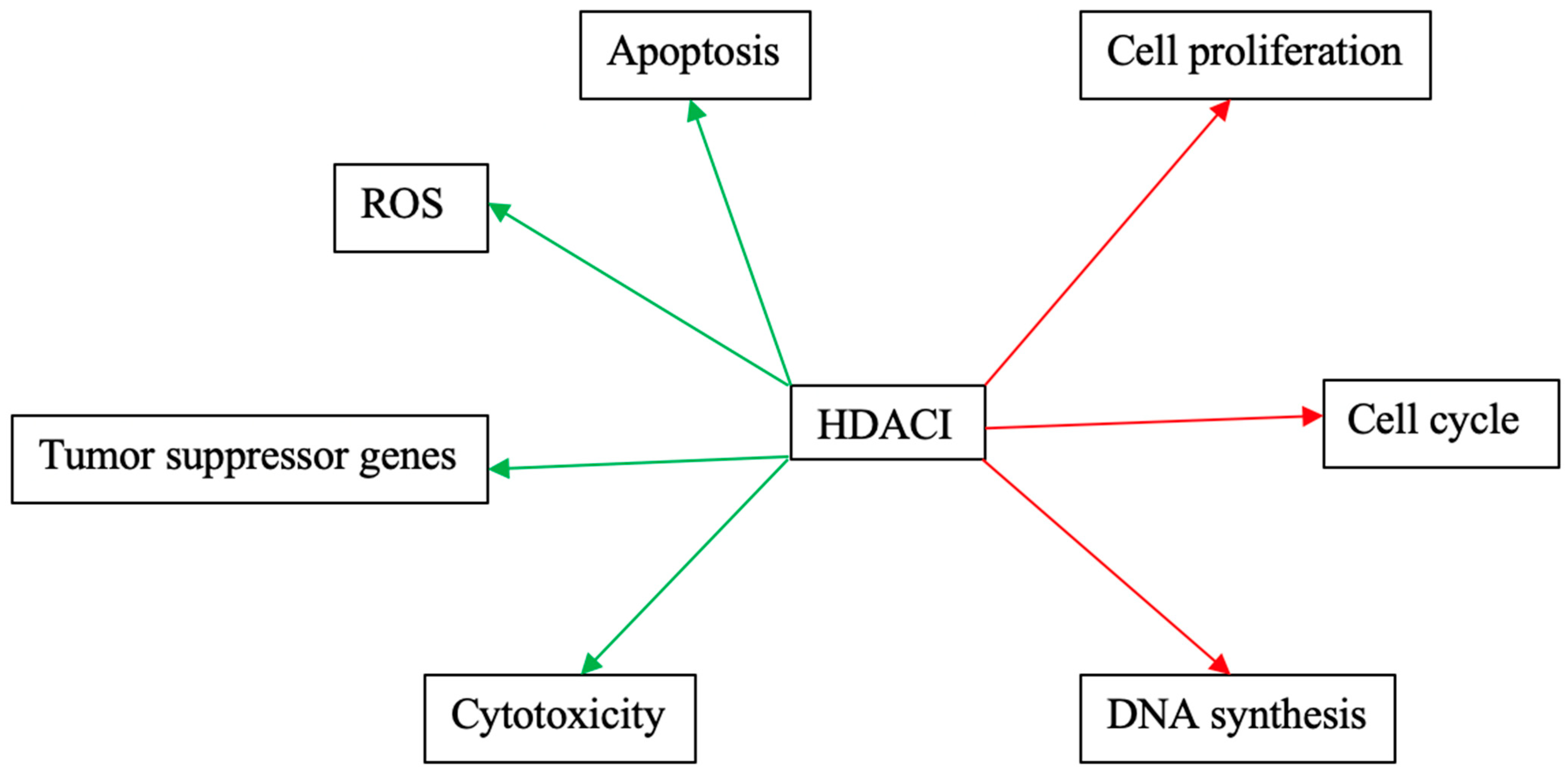
1. Introduction
2. Hydroxamic Acids
2.1. Trichostatin A
2.2. Suberoylanilide Hydroxamic Acid
2.3. Panobinostat
2.4. Abexinostat
2.5. Thiazole-5-hydroxamic Acid
2.6. Amino Benzohydroxamic Acid
2.7. Tubastatin A
3. Short Chain Fatty Acids
3.1. Valproic Acid
3.2. Sodium Butyrate
3.3. Phenylbutyrate
4. Benzamides
Domatinostat
5. Cyclic Tetrapeptides
5.1. Romidepsin
5.2. Apicidin
6. Sirtuin Inhibitors
7. Novel Synthetic HDACIs
8. Statins
9. Pyruvate/Lactate
10. Phytopharmaceuticals
11. Discussion
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society. What Is Cervical Cancer? American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- American Cancer Society. Key Statistics for Cervical Cancer; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- American Cancer Society. Risk Factors for Cervical Cancer; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- American Cancer Society. Signs and Symptoms of Cervical Cancer; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- American Cancer Society. Treatment Options for Cervical Cancer, by Stage; American Cancer Society: Atlanta, GA, USA, 2021. [Google Scholar]
- Davey, C.A.; Sargent, D.F.; Luger, K.; Maeder, A.W.; Richmond, T.J. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J. Mol. Biol. 2002, 319, 1097–1113. [Google Scholar] [CrossRef] [PubMed]
- Luger, K.; Mader, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef]
- Damaskos, C.; Garmpis, N.; Karatzas, T.; Nikolidakis, L.; Kostakis, I.D.; Garmpi, A.; Karamaroudis, S.; Boutsikos, G.; Damaskou, Z.; Kostakis, A.; et al. Histone Deacetylase (HDAC) Inhibitors: Current Evidence for Therapeutic Activities in Pancreatic Cancer. Anticancer Res. 2015, 35, 3129–3135. [Google Scholar] [PubMed]
- Damaskos, C.; Garmpis, N.; Valsami, S.; Kontos, M.; Spartalis, E.; Kalampokas, T.; Kalampokas, E.; Athanasiou, A.; Moris, D.; Daskalopoulou, A.; et al. Histone Deacetylase Inhibitors: An Attractive Therapeutic Strategy Against Breast Cancer. Anticancer Res. 2017, 37, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Wolffe, A.P. Transcription: In tune with the histones. Cell 1994, 77, 13–16. [Google Scholar] [CrossRef]
- Felsenfeld, G. Chromatin as an essential part of the transcriptional mechanism. Nature 1992, 355, 219–224. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Damaskos, C.; Tomos, I.; Garmpis, N.; Karakatsani, A.; Dimitroulis, D.; Garmpi, A.; Spartalis, E.; Kampolis, C.F.; Tsagkari, E.; Loukeri, A.A.; et al. Histone Deacetylase Inhibitors as a Novel Targeted Therapy Against Non-small Cell Lung Cancer: Where Are We Now and What Should We Expect? Anticancer Res. 2018, 38, 37–43. [Google Scholar]
- Garmpi, A.; Damaskos, C.; Garmpis, N.; Kaminiotis, V.V.; Georgakopoulou, V.E.; Spandidos, D.A.; Papalexis, P.; Diamantis, E.; Patsouras, A.; Kyriakos, G.; et al. Role of histone deacetylase inhibitors in diabetic cardiomyopathy in experimental models (Review). Med. Int. (Lond.) 2022, 2, 26. [Google Scholar] [CrossRef]
- Garmpi, A.; Garmpis, N.; Damaskos, C.; Valsami, S.; Spartalis, E.; Lavaris, A.; Patelis, N.; Margonis, G.A.; Apostolou, K.G.; Spartalis, M.; et al. Histone deacetylase inhibitors as a new anticancer option: How far can we go with expectations? delivery systems. J. BUON 2018, 23, 846–861. [Google Scholar]
- Garmpis, N.; Damaskos, C.; Dimitroulis, D.; Kouraklis, G.; Garmpi, A.; Sarantis, P.; Koustas, E.; Patsouras, A.; Psilopatis, I.; Antoniou, E.A.; et al. Clinical Significance of the Histone Deacetylase 2 (HDAC-2) Expression in Human Breast Cancer. J. Pers. Med. 2022, 12, 1672. [Google Scholar] [CrossRef]
- McKenna, N.J.; O’Malley, B.W. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 2002, 108, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Dimitroulis, D.; Spartalis, E.; Margonis, G.A.; Schizas, D.; Deskou, I.; Doula, C.; Magkouti, E.; et al. Targeting Histone Deacetylases in Malignant Melanoma: A Future Therapeutic Agent or Just Great Expectations? Anticancer Res. 2017, 37, 5355–5362. [Google Scholar] [PubMed]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Georgakopoulou, V.E.; Sarantis, P.; Antoniou, E.A.; Karamouzis, M.V.; Nonni, A.; Schizas, D.; Diamantis, E.; et al. Histone Deacetylase Inhibitors in the Treatment of Hepatocellular Carcinoma: Current Evidence and Future Opportunities. J. Pers. Med. 2021, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Kalampokas, E.; Kalampokas, T.; Spartalis, E.; Daskalopoulou, A.; Valsami, S.; Kontos, M.; Nonni, A.; et al. Histone Deacetylases as New Therapeutic Targets in Triple-negative Breast Cancer: Progress and Promises. Cancer Genom. Proteom. 2017, 14, 299–313. [Google Scholar]
- Grunstein, M. Histone acetylation in chromatin structure and transcription. Nature 1997, 389, 349–352. [Google Scholar] [CrossRef]
- Marks, P.A.; Richon, V.M.; Rifkind, R.A. Histone deacetylase inhibitors: Inducers of differentiation or apoptosis of transformed cells. J. Natl. Cancer Inst. 2000, 92, 1210–1216. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Nonni, A.; Georgakopoulou, V.E.; Antoniou, E.; Schizas, D.; Sarantis, P.; Patsouras, A.; Syllaios, A.; et al. Histone Deacetylases and their Inhibitors in Colorectal Cancer Therapy: Current Evidence and Future Considerations. Curr. Med. Chem. 2022, 29, 2979–2994. [Google Scholar] [CrossRef]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Spartalis, E.; Kalampokas, E.; Kalampokas, T.; Margonis, G.A.; Schizas, D.; Andreatos, N.; Angelou, A.; et al. Targeting histone deacetylases in endometrial cancer: A paradigm-shifting therapeutic strategy? Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 950–960. [Google Scholar]
- Archer, S.Y.; Hodin, R.A. Histone acetylation and cancer. Curr. Opin. Genet. Dev. 1999, 9, 171–174. [Google Scholar] [CrossRef]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Pergaris, A.; Giaginis, C.; Theocharis, S. Histone Deacetylase Inhibitors: A Promising Therapeutic Alternative for Endometrial Carcinoma. Dis. Markers 2021, 2021, 7850688. [Google Scholar] [CrossRef] [PubMed]
- Giaginis, C.; Damaskos, C.; Koutsounas, I.; Zizi-Serbetzoglou, A.; Tsoukalas, N.; Patsouris, E.; Kouraklis, G.; Theocharis, S. Histone deacetylase (HDAC)-1, -2, -4 and -6 expression in human pancreatic adenocarcinoma: Associations with clinicopathological parameters, tumor proliferative capacity and patients’ survival. BMC Gastroenterol. 2015, 15, 148. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A.; Richon, V.M.; Miller, T.; Kelly, W.K. Histone deacetylase inhibitors. Adv. Cancer Res. 2004, 91, 137–168. [Google Scholar] [PubMed]
- Tasoulas, J.; Giaginis, C.; Patsouris, E.; Manolis, E.; Theocharis, S. Histone deacetylase inhibitors in oral squamous cell carcinoma treatment. Expert Opin. Investig. Drugs 2015, 24, 69–78. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Jenke, R.; Ressing, N.; Hansen, F.K.; Aigner, A.; Buch, T. Anticancer Therapy with HDAC Inhibitors: Mechanism-Based Combination Strategies and Future Perspectives. Cancers 2021, 13, 634. [Google Scholar] [CrossRef]
- Tsuji, N.; Kobayashi, M.; Nagashima, K.; Wakisaka, Y.; Koizumi, K. A new antifungal antibiotic, trichostatin. J. Antibiot. (Tokyo) 1976, 29, 1–6. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, L.J.; Li, X.P.; Wang, J.L.; Chai, G.L.; Wei, L.H. Histone deacetylase inhibitors inducing human cervical cancer cell apoptosis by decreasing DNA-methyltransferase 3B. Chin. Med. J. 2012, 125, 3273–3278. [Google Scholar]
- Liu, J.H.; Cao, Y.M.; Rong, Z.P.; Ding, J.; Pan, X. Trichostatin A Induces Autophagy in Cervical Cancer Cells by Regulating the PRMT5-STC1-TRPV6-JNK Pathway. Pharmacology 2021, 106, 60–69. [Google Scholar] [CrossRef]
- Ma, X.; Ma, Q.; Liu, J.; Tian, Y.; Wang, B.; Taylor, K.M.; Wu, P.; Wang, D.; Xu, G.; Meng, L.; et al. Identification of LIV1, a putative zinc transporter gene responsible for HDACi-induced apoptosis, using a functional gene screen approach. Mol. Cancer Ther. 2009, 8, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Raju, I.; Kaushal, G.P.; Haun, R.S. Epigenetic regulation of KLK7 gene expression in pancreatic and cervical cancer cells. Biol. Chem. 2016, 397, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Meng, L.; Wang, H.; Zhou, J.; Xu, G.; Wang, S.; Xi, L.; Chen, G.; Wang, B.; Zhu, T.; et al. Role of hTERT in apoptosis of cervical cancer induced by histone deacetylase inhibitor. Biochem. Biophys. Res. Commun. 2005, 335, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, X. Histone deacetylase inhibitor, Trichostatin A, activates p21WAF1/CIP1 expression through downregulation of c-myc and release of the repression of c-myc from the promoter in human cervical cancer cells. Biochem. Biophys. Res. Commun. 2004, 324, 860–867. [Google Scholar] [CrossRef]
- Yadav, S.S.; Prasad, S.B.; Das, M.; Kumari, S.; Pandey, L.K.; Singh, S.; Pradhan, S.; Narayan, G. Epigenetic silencing of CXCR4 promotes loss of cell adhesion in cervical cancer. Biomed. Res. Int. 2014, 2014, 581403. [Google Scholar] [CrossRef]
- Lee, J.W.; Yang, D.H.; Park, S.; Han, H.K.; Park, J.W.; Kim, B.Y.; Um, S.H.; Moon, E.Y. Trichostatin A resistance is facilitated by HIF-1alpha acetylation in HeLa human cervical cancer cells under normoxic conditions. Oncotarget 2018, 9, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Lee, J.W.; Lee, J.; Moon, E.Y. Dynamic rearrangement of F-actin is required to maintain the antitumor effect of trichostatin A. PLoS ONE 2014, 9, e97352. [Google Scholar] [CrossRef]
- You, B.R.; Park, W.H. Trichostatin A induces apoptotic cell death of HeLa cells in a Bcl-2 and oxidative stress-dependent manner. Int. J. Oncol. 2013, 42, 359–366. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Kundu, G.C. Transcriptional regulation of human osteopontin promoter by histone deacetylase inhibitor, trichostatin A in cervical cancer cells. Mol. Cancer 2010, 9, 178. [Google Scholar] [CrossRef]
- Danam, R.P.; Howell, S.R.; Brent, T.P.; Harris, L.C. Epigenetic regulation of O6-methylguanine-DNA methyltransferase gene expression by histone acetylation and methyl-CpG binding proteins. Mol. Cancer Ther. 2005, 4, 61–69. [Google Scholar] [CrossRef]
- Jung, S.; Yi, L.; Jeong, D.; Kim, J.; An, S.; Oh, T.J.; Kim, C.H.; Kim, C.J.; Yang, Y.; Kim, K.I.; et al. The role of ADCYAP1, adenylate cyclase activating polypeptide 1, as a methylation biomarker for the early detection of cervical cancer. Oncol. Rep. 2011, 25, 245–252. [Google Scholar] [PubMed]
- Narayan, G.; Arias-Pulido, H.; Nandula, S.V.; Basso, K.; Sugirtharaj, D.D.; Vargas, H.; Mansukhani, M.; Villella, J.; Meyer, L.; Schneider, A.; et al. Promoter hypermethylation of FANCF: Disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer Res. 2004, 64, 2994–2997. [Google Scholar] [CrossRef] [PubMed]
- Huisman, C.; van der Wijst, M.G.; Falahi, F.; Overkamp, J.; Karsten, G.; Terpstra, M.M.; Kok, K.; van der Zee, A.G.; Schuuring, E.; Wisman, G.B.; et al. Prolonged re-expression of the hypermethylated gene EPB41L3 using artificial transcription factors and epigenetic drugs. Epigenetics 2015, 10, 384–396. [Google Scholar] [CrossRef]
- Hernandez-Juarez, J.; Vargas-Sierra, O.; Herrera, L.A.; Cantu De Leon, D.; Fernandez-Retana, J.; Perez-Plasencia, C.; Lopez-Camarillo, C.; Gariglio, P.; Diaz-Chavez, J. Sodium-coupled monocarboxylate transporter is a target of epigenetic repression in cervical cancer. Int. J. Oncol. 2019, 54, 1613–1624. [Google Scholar] [PubMed]
- Lin, Z.; Bazzaro, M.; Wang, M.C.; Chan, K.C.; Peng, S.; Roden, R.B. Combination of proteasome and HDAC inhibitors for uterine cervical cancer treatment. Clin. Cancer Res. 2009, 15, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Das, K.; Saha, S.; Mazumdar, M.; Manna, A.; Chakraborty, S.; Mukherjee, S.; Khan, P.; Adhikary, A.; Mohanty, S.; et al. Nuclear matrix protein SMAR1 represses c-Fos-mediated HPV18 E6 transcription through alteration of chromatin histone deacetylation. J. Biol. Chem. 2014, 289, 29074–29085. [Google Scholar] [CrossRef]
- Igaz, N.; Kovacs, D.; Razga, Z.; Konya, Z.; Boros, I.M.; Kiricsi, M. Modulating chromatin structure and DNA accessibility by deacetylase inhibition enhances the anti-cancer activity of silver nanoparticles. Colloids Surf. B Biointerfaces 2016, 146, 670–677. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yan, Q.; Shen, W.; Gurunathan, S. Trichostatin A Enhances the Apoptotic Potential of Palladium Nanoparticles in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1354. [Google Scholar] [CrossRef]
- Yu, J.; Mi, J.; Wang, Y.; Wang, A.; Tian, X. Regulation of radiosensitivity by HDAC inhibitor trichostatin A in the human cervical carcinoma cell line Hela. Eur. J. Gynaecol. Oncol. 2012, 33, 285–290. [Google Scholar]
- Tandon, N.; Ramakrishnan, V.; Kumar, S.K. Clinical use and applications of histone deacetylase inhibitors in multiple myeloma. Clin. Pharmacol. 2016, 8, 35–44. [Google Scholar]
- Marks, P.A.; Breslow, R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat. Biotechnol. 2007, 25, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.K.; Kopp, L.; Bi, K. High-throughput screening compatible cell-based assay for interrogating activated notch signaling. Assay Drug Dev. Technol. 2009, 7, 68–79. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, C.; Tong, A.; Chen, B.; Zeng, Z.; Zhang, P.; Wang, C.; Wei, Y. Proteomic analysis of cervical cancer cells treated with suberonylanilide hydroxamic acid. J. Biosci. 2008, 33, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Yin, S.; Peng, F.; Liu, C.; Liang, H.; Su, J.; Hsiao, W.L.W.; Cai, Y.; Luo, D.; Xia, C. Vorinostat targets UBE2C to reverse epithelial-mesenchymal transition and control cervical cancer growth through the ubiquitination pathway. Eur. J. Pharmacol. 2021, 908, 174399. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Shu, Y.; Ye, G.; Wu, C.; Xu, M.; Gao, R.; Huang, D.; Zhang, J. Histone deacetylase inhibitors inhibit cervical cancer growth through Parkin acetylation-mediated mitophagy. Acta Pharm. Sin. B 2022, 12, 838–852. [Google Scholar] [CrossRef]
- Xia, C.; He, Z.; Cai, Y.; Liang, S. Vorinostat upregulates MICA via the PI3K/Akt pathway to enhance the ability of natural killer cells to kill tumor cells. Eur. J. Pharmacol. 2020, 875, 173057. [Google Scholar] [CrossRef]
- You, B.R.; Park, W.H. Suberoyl bishydroxamic acid-induced apoptosis in HeLa cells via ROS-independent, GSH-dependent manner. Mol. Biol. Rep. 2013, 40, 3807–3816. [Google Scholar] [CrossRef]
- You, B.R.; Park, W.H. Suberoylanilide hydroxamic acid-induced HeLa cell death is closely correlated with oxidative stress and thioredoxin 1 levels. Int. J. Oncol. 2014, 44, 1745–1755. [Google Scholar] [CrossRef]
- Jin, K.L.; Park, J.Y.; Noh, E.J.; Hoe, K.L.; Lee, J.H.; Kim, J.H.; Nam, J.H. The effect of combined treatment with cisplatin and histone deacetylase inhibitors on HeLa cells. J. Gynecol. Oncol. 2010, 21, 262–268. [Google Scholar] [CrossRef]
- Kumar, P.; Wasim, L.; Chopra, M.; Chhikara, A. Co-delivery of Vorinostat and Etoposide Via Disulfide Cross-Linked Biodegradable Polymeric Nanogels: Synthesis, Characterization, Biodegradation, and Anticancer Activity. AAPS PharmSciTech 2018, 19, 634–647. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Su, Z.; Yang, L.; Guo, W.; Liu, W.; Zuo, J. Synergistic induction of apoptosis in HeLa cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitor SAHA. Mol. Med. Rep. 2010, 3, 613–619. [Google Scholar]
- Lange, L.; Hemmerich, P.; Spankuch, B. Survival of primary, but not of cancer cells after combined Plk1-HDAC inhibition. Oncotarget 2015, 6, 25801–25814. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, X.; Wang, S.; Zhou, W.; Li, Y.; Lei, W.; Lv, W. Synergistic combination of histone deacetylase inhibitor suberoylanilide hydroxamic acid and oncolytic adenovirus ZD55-TRAIL as a therapy against cervical cancer. Mol. Med. Rep. 2015, 12, 435–441. [Google Scholar] [CrossRef][Green Version]
- Xing, J.; Wang, H.; Xu, S.; Han, P.; Xin, M.; Zhou, J.L. Sensitization of suberoylanilide hydroxamic acid (SAHA) on chemoradiation for human cervical cancer cells and its mechanism. Eur. J. Gynaecol. Oncol. 2015, 36, 117–122. [Google Scholar] [PubMed]
- Prince, H.M.; Bishton, M.J.; Johnstone, R.W. Panobinostat (LBH589): A potent pan-deacetylase inhibitor with promising activity against hematologic and solid tumors. Future Oncol. 2009, 5, 601–612. [Google Scholar] [CrossRef]
- Wasim, L.; Chopra, M. Panobinostat induces apoptosis via production of reactive oxygen species and synergizes with topoisomerase inhibitors in cervical cancer cells. Biomed. Pharmacother. 2016, 84, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Wasim, L.; Chopra, M. Synergistic anticancer effect of panobinostat and topoisomerase inhibitors through ROS generation and intrinsic apoptotic pathway induction in cervical cancer cells. Cell Oncol. (Dordr.) 2018, 41, 201–212. [Google Scholar] [CrossRef]
- Banuelos, C.A.; Banath, J.P.; MacPhail, S.H.; Zhao, J.; Reitsema, T.; Olive, P.L. Radiosensitization by the histone deacetylase inhibitor PCI-24781. Clin. Cancer Res. 2007, 13 Pt 1, 6816–6826. [Google Scholar] [CrossRef]
- Anandan, S.K.; Ward, J.S.; Brokx, R.D.; Denny, T.; Bray, M.R.; Patel, D.V.; Xiao, X.Y. Design and synthesis of thiazole-5-hydroxamic acids as novel histone deacetylase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 5995–5999. [Google Scholar] [CrossRef]
- Korkmaz, I.N.; Ozdemir, H. Synthesis and Anticancer Potential of New Hydroxamic Acid Derivatives as Chemotherapeutic Agents. Appl. Biochem. Biotechnol. 2022, 194, 6349–6366. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chen, Y.F.; Chiu, W.T.; Liu, K.Y.; Liu, Y.L.; Chang, J.Y.; Chang, H.C.; Shen, M.R. Microtubule-associated histone deacetylase 6 supports the calcium store sensor STIM1 in mediating malignant cell behaviors. Cancer Res. 2013, 73, 4500–4509. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.T.; Thomas, S.; Moore, A.; Munster, P.N. Rational therapeutic combinations with histone deacetylase inhibitors for the treatment of cancer. Future Oncol. 2011, 7, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yang, Y.; Lv, J.; Sun, L.; Liu, M. Valproic acid exhibits different cell growth arrest effect in three HPV-positive/negative cervical cancer cells and possibly via inducing Notch1 cleavage and E6 downregulation. Int. J. Oncol. 2016, 49, 422–430. [Google Scholar] [CrossRef]
- Han, B.R.; You, B.R.; Park, W.H. Valproic acid inhibits the growth of HeLa cervical cancer cells via caspase-dependent apoptosis. Oncol. Rep. 2013, 30, 2999–3005. [Google Scholar] [CrossRef]
- Rocha, M.A.; de Campos Vidal, B.; Mello, M.L.S. Sodium Valproate Modulates the Methylation Status of Lysine Residues 4, 9 and 27 in Histone H3 of HeLa Cells. Curr. Mol. Pharmacol. 2022, 16, 197–210. [Google Scholar]
- Zhao, Y.; You, W.; Zheng, J.; Chi, Y.; Tang, W.; Du, R. Valproic acid inhibits the angiogenic potential of cervical cancer cells via HIF-1alpha/VEGF signals. Clin. Transl. Oncol. 2016, 18, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Sami, S.; Hoti, N.; Xu, H.M.; Shen, Z.; Huang, X. Valproic acid inhibits the growth of cervical cancer both in vitro and in vivo. J. Biochem. 2008, 144, 357–362. [Google Scholar] [CrossRef]
- Contis-Montes de Oca, A.; Rodarte-Valle, E.; Rosales-Hernandez, M.C.; Abarca-Rojano, E.; Rojas-Hernandez, S.; Fragoso-Vazquez, M.J.; Mendieta-Wejebe, J.E.; Correa-Basurto, A.M.; Vazquez-Moctezuma, I.; Correa-Basurto, J. N-(2′-Hydroxyphenyl)-2-propylpentanamide (OH-VPA), a histone deacetylase inhibitor, induces the release of nuclear HMGB1 and modifies ROS levels in HeLa cells. Oncotarget 2018, 9, 33368–33381. [Google Scholar] [CrossRef][Green Version]
- Sixto-Lopez, Y.; Rosales-Hernandez, M.C.; Contis-Montes de Oca, A.; Fragoso-Morales, L.G.; Mendieta-Wejebe, J.E.; Correa-Basurto, A.M.; Abarca-Rojano, E.; Correa-Basurto, J. N-(2′-Hydroxyphenyl)-2-Propylpentanamide (HO-AAVPA) Inhibits HDAC1 and Increases the Translocation of HMGB1 Levels in Human Cervical Cancer Cells. Int. J. Mol. Sci. 2020, 21, 5873. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Ivan, C.; Huang, J.; Shen, D.Y.; Kavanagh, J.J.; Bast, R.C., Jr.; Fu, S.; Hu, W.; Sood, A.K. Enhanced Cytotoxic Effects of Combined Valproic Acid and the Aurora Kinase Inhibitor VE465 on Gynecologic Cancer Cells. Front. Oncol. 2013, 3, 58. [Google Scholar] [CrossRef]
- Mora-Garcia Mde, L.; Duenas-Gonzalez, A.; Hernandez-Montes, J.; De la Cruz-Hernandez, E.; Perez-Cardenas, E.; Weiss-Steider, B.; Santiago-Osorio, E.; Ortiz-Navarrete, V.F.; Rosales, V.H.; Cantu, D.; et al. Up-regulation of HLA class-I antigen expression and antigen-specific CTL response in cervical cancer cells by the demethylating agent hydralazine and the histone deacetylase inhibitor valproic acid. J. Transl. Med. 2006, 4, 55. [Google Scholar] [CrossRef]
- Franko-Tobin, L.G.; Mackey, L.V.; Huang, W.; Song, X.; Jin, B.; Luo, J.; Morris, L.M.; Liu, M.; Fuselier, J.A.; Coy, D.H.; et al. Notch1-mediated tumor suppression in cervical cancer with the involvement of SST signaling and its application in enhanced SSTR-targeted therapeutics. Oncologist 2012, 17, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Segura-Pacheco, B.; Avalos, B.; Rangel, E.; Velazquez, D.; Cabrera, G. HDAC inhibitor valproic acid upregulates CAR in vitro and in vivo. Genet. Vaccines Ther. 2007, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Bonifati, S.; Hristov, G.; Marttila, T.; Valmary-Degano, S.; Stanzel, S.; Schnolzer, M.; Mougin, C.; Aprahamian, M.; Grekova, S.P.; et al. Synergistic combination of valproic acid and oncolytic parvovirus H-1PV as a potential therapy against cervical and pancreatic carcinomas. EMBO Mol. Med. 2013, 5, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Mani, E.; Medina, L.A.; Isaac-Olive, K.; Duenas-Gonzalez, A. Radiosensitization of cervical cancer cells with epigenetic drugs hydralazine and valproate. Eur. J. Gynaecol. Oncol. 2014, 35, 140–142. [Google Scholar]
- Zhou, A.M.; Wang, M.M.; Su, Y.; Yu, Z.H.; Liu, H.K.; Su, Z. Switching the Mode of Cell Death between Apoptosis and Autophagy by Histone Deacetylase 6 Inhibition Levels. ChemMedChem 2023, 18, e202200614. [Google Scholar] [CrossRef]
- Feng, D.; Cao, Z.; Li, C.; Zhang, L.; Zhou, Y.; Ma, J.; Liu, R.; Zhou, H.; Zhao, W.; Wei, H.; et al. Combination of valproic acid and ATRA restores RARbeta2 expression and induces differentiation in cervical cancer through the PI3K/Akt pathway. Curr. Mol. Med. 2012, 12, 342–354. [Google Scholar] [CrossRef]
- Feng, D.; Wu, J.; Tian, Y.; Zhou, H.; Zhou, Y.; Hu, W.; Zhao, W.; Wei, H.; Ling, B.; Ma, C. Targeting of histone deacetylases to reactivate tumour suppressor genes and its therapeutic potential in a human cervical cancer xenograft model. PLoS ONE 2013, 8, e80657. [Google Scholar] [CrossRef]
- de la Cruz-Hernandez, E.; Perez-Cardenas, E.; Contreras-Paredes, A.; Cantu, D.; Mohar, A.; Lizano, M.; Duenas-Gonzalez, A. The effects of DNA methylation and histone deacetylase inhibitors on human papillomavirus early gene expression in cervical cancer, an in vitro and clinical study. Virol. J. 2007, 4, 18. [Google Scholar] [CrossRef]
- De la Cruz-Hernandez, E.; Perez-Plasencia, C.; Perez-Cardenas, E.; Gonzalez-Fierro, A.; Trejo-Becerril, C.; Chavez-Blanco, A.; Taja-Chayeb, L.; Vidal, S.; Gutierrez, O.; Dominguez, G.I.; et al. Transcriptional changes induced by epigenetic therapy with hydralazine and magnesium valproate in cervical carcinoma. Oncol. Rep. 2011, 25, 399–407. [Google Scholar]
- Chavez-Blanco, A.; Segura-Pacheco, B.; Perez-Cardenas, E.; Taja-Chayeb, L.; Cetina, L.; Candelaria, M.; Cantu, D.; Gonzalez-Fierro, A.; Garcia-Lopez, P.; Zambrano, P.; et al. Histone acetylation and histone deacetylase activity of magnesium valproate in tumor and peripheral blood of patients with cervical cancer. A phase I study. Mol. Cancer. 2005, 4, 22. [Google Scholar] [CrossRef]
- Chavez-Blanco, A.; Perez-Plasencia, C.; Perez-Cardenas, E.; Carrasco-Legleu, C.; Rangel-Lopez, E.; Segura-Pacheco, B.; Taja-Chayeb, L.; Trejo-Becerril, C.; Gonzalez-Fierro, A.; Candelaria, M.; et al. Antineoplastic effects of the DNA methylation inhibitor hydralazine and the histone deacetylase inhibitor valproic acid in cancer cell lines. Cancer Cell Int. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Coronel, J.; Cetina, L.; Pacheco, I.; Trejo-Becerril, C.; Gonzalez-Fierro, A.; de la Cruz-Hernandez, E.; Perez-Cardenas, E.; Taja-Chayeb, L.; Arias-Bofill, D.; Candelaria, M.; et al. A double-blind, placebo-controlled, randomized phase III trial of chemotherapy plus epigenetic therapy with hydralazine valproate for advanced cervical cancer. Preliminary results. Med. Oncol. 2011, 28 (Suppl. S1), S540–S546. [Google Scholar] [CrossRef] [PubMed]
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate histone deacetylase inhibitors. Biores. Open Access 2012, 1, 192–198. [Google Scholar] [CrossRef]
- Darvas, K.; Rosenberger, S.; Brenner, D.; Fritsch, C.; Gmelin, N.; Krammer, P.H.; Rosl, F. Histone deacetylase inhibitor-induced sensitization to TNFalpha/TRAIL-mediated apoptosis in cervical carcinoma cells is dependent on HPV oncogene expression. Int. J. Cancer 2010, 127, 1384–1392. [Google Scholar] [CrossRef]
- Finzer, P.; Kuntzen, C.; Soto, U.; zur Hausen, H.; Rosl, F. Inhibitors of histone deacetylase arrest cell cycle and induce apoptosis in cervical carcinoma cells circumventing human papillomavirus oncogene expression. Oncogene 2001, 20, 4768–4776. [Google Scholar] [CrossRef]
- Finzer, P.; Stohr, M.; Seibert, N.; Rosl, F. Phenylbutyrate inhibits growth of cervical carcinoma cells independent of HPV type and copy number. J. Cancer Res. Clin. Oncol. 2003, 129, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.; Zawatzky, R.; Rosl, F. Genetic redundancy in human cervical carcinoma cells: Identification of cells with “normal” properties. Int. J. Cancer 2007, 120, 2119–2126. [Google Scholar] [CrossRef]
- Decrion-Barthod, A.Z.; Bosset, M.; Plissonnier, M.L.; Marchini, A.; Nicolier, M.; Launay, S.; Pretet, J.L.; Rommelaere, J.; Mougin, C. Sodium butyrate with UCN-01 has marked antitumour activity against cervical cancer cells. Anticancer Res. 2010, 30, 4049–4061. [Google Scholar] [PubMed]
- Park, J.K.; Cho, C.H.; Ramachandran, S.; Shin, S.J.; Kwon, S.H.; Kwon, S.Y.; Cha, S.D. Augmentation of sodium butyrate-induced apoptosis by phosphatidylinositol 3-kinase inhibition in the human cervical cancer cell-line. Cancer Res. Treat. 2006, 38, 112–117. [Google Scholar] [CrossRef]
- Almotairy, A.R.Z.; Gandin, V.; Morrison, L.; Marzano, C.; Montagner, D.; Erxleben, A. Antitumor platinum(IV) derivatives of carboplatin and the histone deacetylase inhibitor 4-phenylbutyric acid. J. Inorg. Biochem. 2017, 177, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, M.; Sun, H.; Yang, W.; Ye, M.; Li, H.; Meng, Y. 4SC-202 exerts an anti-tumor effect in cervical cancer by targeting PRLR signaling pathway. J. Mol. Histol. 2022, 53, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Choi, C.H.; Lee, H.J.; Oh, S.J.; Woo, S.R.; Hong, S.O.; Noh, K.H.; Cho, H.; Chung, E.J.; Kim, J.H.; et al. HDAC1 Upregulation by NANOG Promotes Multidrug Resistance and a Stem-like Phenotype in Immune Edited Tumor Cells. Cancer Res. 2017, 77, 5039–5053. [Google Scholar] [CrossRef]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bednarek, M.A.; et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef] [PubMed]
- Luczak, M.W.; Jagodzinski, P.P. Apicidin down-regulates human papillomavirus type 16 E6 and E7 transcripts and proteins in SiHa cervical cancer cells. Cancer Lett. 2008, 272, 53–60. [Google Scholar] [CrossRef]
- You, J.S.; Kang, J.K.; Lee, E.K.; Lee, J.C.; Lee, S.H.; Jeon, Y.J.; Koh, D.H.; Ahn, S.H.; Seo, D.W.; Lee, H.Y.; et al. Histone deacetylase inhibitor apicidin downregulates DNA methyltransferase 1 expression and induces repressive histone modifications via recruitment of corepressor complex to promoter region in human cervix cancer cells. Oncogene 2008, 27, 1376–1386. [Google Scholar] [CrossRef]
- Eun, D.W.; Ahn, S.H.; You, J.S.; Park, J.W.; Lee, E.K.; Lee, H.N.; Kang, G.M.; Lee, J.C.; Choi, W.S.; Seo, D.W.; et al. PKCepsilon is essential for gelsolin expression by histone deacetylase inhibitor apicidin in human cervix cancer cells. Biochem. Biophys. Res. Commun. 2007, 354, 769–775. [Google Scholar] [CrossRef]
- Dai, Y.; Faller, D.V. Transcription Regulation by Class III Histone Deacetylases (HDACs)-Sirtuins. Transl. Oncogenomics 2008, 3, 53–65. [Google Scholar]
- Kuhlmann, N.; Chollet, C.; Baldus, L.; Neundorf, I.; Lammers, M. Development of Substrate-Derived Sirtuin Inhibitors with Potential Anticancer Activity. ChemMedChem 2017, 12, 1703–1714. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, P.U.; Thakur, S.; Kiran, S.; Sen, B.; Sharma, S.; Rao, V.V.; Poongothai, A.R.; Ramakrishna, G. Expression/localization patterns of sirtuins (SIRT1, SIRT2, and SIRT7) during progression of cervical cancer and effects of sirtuin inhibitors on growth of cervical cancer cells. Tumour Biol. 2015, 36, 6159–6171. [Google Scholar] [CrossRef]
- Wossner, N.; Alhalabi, Z.; Gonzalez, J.; Swyter, S.; Gan, J.; Schmidtkunz, K.; Zhang, L.; Vaquero, A.; Ovaa, H.; Einsle, O.; et al. Sirtuin 1 Inhibiting Thiocyanates (S1th)-A New Class of Isotype Selective Inhibitors of NAD(+) Dependent Lysine Deacetylases. Front. Oncol. 2020, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Borutinskaite, V.V.; Navakauskiene, R.; Magnusson, K.E. Retinoic acid and histone deacetylase inhibitor BML-210 inhibit proliferation of human cervical cancer HeLa cells. Ann. NY Acad. Sci. 2006, 1091, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Borutinskaite, V.V.; Magnusson, K.E.; Navakauskiene, R. Histone deacetylase inhibitor BML-210 induces growth inhibition and apoptosis and regulates HDAC and DAPC complex expression levels in cervical cancer cells. Mol. Biol. Rep. 2012, 39, 10179–10186. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Jegan, A.; Premnath, D.; Periasamy, V.S.; Muthusubramanian, S.; Vasanthkumar, S. Synthesis, molecular docking and cytotoxicity evaluation of novel 2-(4-amino-benzosulfonyl)-5H-benzo[b]carbazole-6,11-dione derivatives as histone deacetylase (HDAC8) inhibitors. Bioorg. Chem. 2014, 53, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.K.; Liu, S.T.; Chang, C.C.; Huang, S.M. Regulatory mechanisms of fluvastatin and lovastatin for the p21 induction in human cervical cancer HeLa cells. PLoS ONE 2019, 14, e0214408. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wu, Y.; Zhai, Y.; Hu, B.; Ma, W.; Yang, W.; Yu, Q.; Chen, Z.; Workman, J.L.; Yu, X.; et al. Exogenous pyruvate represses histone gene expression and inhibits cancer cell proliferation via the NAMPT-NAD+-SIRT1 pathway. Nucleic Acids Res. 2019, 47, 11132–11150. [Google Scholar] [CrossRef]
- Wagner, W.; Ciszewski, W.M.; Kania, K.D. L- and D-lactate enhance DNA repair and modulate the resistance of cervical carcinoma cells to anticancer drugs via histone deacetylase inhibition and hydroxycarboxylic acid receptor 1 activation. Cell Commun. Signal. 2015, 13, 36. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Reddy, D.B.; Padukudru, M.A.; Chitturi, C.M.K.; Vimalambike, M.G.; Madhunapantula, S.V. Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of Histone Deacetylases (HDAC). PLoS ONE 2017, 12, e0186208. [Google Scholar] [CrossRef]
- Bishayee, K.; Sikdar, S.; Khuda-Bukhsh, A.R. Evidence of an Epigenetic Modification in Cell-cycle Arrest Caused by the Use of Ultra-highly-diluted Gonolobus Condurango Extract. J. Pharmacopunct. 2013, 16, 7–13. [Google Scholar] [CrossRef]
- Saenglee, S.; Jogloy, S.; Patanothai, A.; Leid, M.; Senawong, T. Cytotoxic effects of peanut phenolics possessing histone deacetylase inhibitory activity in breast and cervical cancer cell lines. Pharmacol. Rep. 2016, 68, 1102–1110. [Google Scholar] [CrossRef]
- Senawong, T.; Misuna, S.; Khaopha, S.; Nuchadomrong, S.; Sawatsitang, P.; Phaosiri, C.; Surapaitoon, A.; Sripa, B. Histone deacetylase (HDAC) inhibitory and antiproliferative activities of phenolic-rich extracts derived from the rhizome of Hydnophytum formicarum Jack.: Sinapinic acid acts as HDAC inhibitor. BMC Complement. Altern. Med. 2013, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Mukherjee, S. Reversal of resistance towards cisplatin by curcumin in cervical cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, M.G.; Thirugnanasampandan, R.; NagaSundaram, N.; Bhuvaneswari, G. Molecular Docking and Dynamic Simulation Studies of Terpenoids of I. wightii (Bentham) H. Hara against Acetylcholinesterase and Histone Deacetylase3 Receptors. Curr. Comput. Aided Drug Des. 2018, 14, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Pawar, J.S.; Ghosh, I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021, 181, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Phaosiri, C.; Yenjai, C.; Senawong, T.; Senawong, G.; Saenglee, S.; Somsakeesit, L.O.; Kumboonma, P. Histone Deacetylase Inhibitory Activity and Antiproliferative Potential of New [6]-Shogaol Derivatives. Molecules 2022, 27, 3332. [Google Scholar] [CrossRef]
- Raina, R.; Almutary, A.G.; Bagabir, S.A.; Afroze, N.; Fagoonee, S.; Haque, S.; Hussain, A. Chrysin Modulates Aberrant Epigenetic Variations and Hampers Migratory Behavior of Human Cervical (HeLa) Cells. Front. Genet. 2021, 12, 768130. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F. HTP Nutraceutical Screening for Histone Deacetylase Inhibitors and Effects of HDACis on Tumor-suppressing miRNAs by Trichostatin A and Grapeseed (Vitis vinifera) in HeLa cells. Cancer Genom. Proteom. 2017, 14, 17–33. [Google Scholar] [CrossRef][Green Version]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Ansari, M.Z.; Al Mutery, A.; Ashraf, M.; Nasab, R.; Rai, S.; Rais, N.; Hussain, A. Genistein Induces Alterations of Epigenetic Modulatory Signatures in Human Cervical Cancer Cells. Anticancer Agents Med. Chem. 2018, 18, 412–421. [Google Scholar] [CrossRef]
- Kedhari Sundaram, M.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell Biochem. 2019, 120, 18357–18369. [Google Scholar] [CrossRef]
- Kedhari Sundaram, M.; Haque, S.; Somvanshi, P.; Bhardwaj, T.; Hussain, A. Epigallocatechin gallate inhibits HeLa cells by modulation of epigenetics and signaling pathways. 3 Biotech 2020, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, Q.; Cai, W.; Ruan, W. Trends of cervical cancer at global, regional, and national level: Data from the Global Burden of Disease study 2019. BMC Public Health 2021, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Survival Rates for Cervical Cancer; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- Psilopatis, I.; Kokkali, S.; Palamaris, K.; Digklia, A.; Vrettou, K.; Theocharis, S. Organoids: A New Chapter in Sarcoma Diagnosis and Treatment. Int. J. Mol. Sci. 2022, 23, 11271. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Sykaras, A.G.; Mandrakis, G.; Vrettou, K.; Theocharis, S. Patient-Derived Organoids: The Beginning of a New Era in Ovarian Cancer Disease Modeling and Drug Sensitivity Testing. Biomedicines 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Psilopatis, I.; Mantzari, A.; Vrettou, K.; Theocharis, S. The Role of Patient-Derived Organoids in Triple-Negative Breast Cancer Drug Screening. Biomedicines 2023, 11, 773. [Google Scholar] [CrossRef]


| HDACI | Class | Target HDAC Class |
|---|---|---|
| Hydroxamic acids | Trichostatin A | Pan |
| Suberoylanilide hydroxamic acid | Pan | |
| Panobinostat | Pan | |
| Abexinostat | Pan | |
| Short-chain fatty acids | Valproic Acid | I, IIa |
| Sodium butyrate | I, II | |
| Phenylbutyrate | I, II | |
| Benzamides | Domatinostat | I |
| Cyclic peptides | Romidepsin | I |
| Apicidin | I |
| HDACI | Major Effects on Cervical Cancer | References |
|---|---|---|
| Trichostatin A | Apoptosis induction Decreased methyltransferase levels Cell viability impairment p21 activation Improved cell adhesion In vivo tumor growth inhibition | [34,35,36,37,38,39,40,41,42,43,44] |
| Vorinostat | Reversed epithelial-mesenchymal transition Apoptosis and mitophagy induction Sensitization to natural killer cell-mediated cytolytic reactions | [57,58,59,60,61,62,63] |
| Valproic acid | Cell proliferation suppression Apoptosis induction Cell cycle arrest Epithelial-mesenchymal transition induction Angiogenesis suppression In vivo tumor growth inhibition | [78,79,80,81,82] |
| Sodium butyrate | Apoptosis induction Cell cycle arrest | [100,101,102] |
| Phytopharmaceutical | Major Effects on Cervical Cancer | References |
|---|---|---|
| Caffeic acid | Apoptosis induction Cancer cell death by ROS generation Cell cycle arrest | [123] |
| Condurango 30C | Cell cycle arrest Cytotoxicity induction DNA synthesis reduction | [124] |
| Peanut phenolic | Apoptosis induction Cell cycle arrest | [125] |
| Hydnophytum formicarium Jack. rhizome extract | Apoptosis induction | [126] |
| Curcumin | Cell cycle arrest | [127] |
| Terpenoid abietic acid | Apoptosis induction | [128] |
| [6]-shogaol (4) | Antiproliferative activity | [130] |
| EGCG | Interaction with promoter hypermethylation and transcriptional expression of tumor suppressor genes | [136] |
| HDACI | Synergistic Agent | References |
|---|---|---|
| Trichostatin A | 5-Aza-dC Alkylating agents Demethylation inducers Pyruvate Vorinostat Bortezomib Curcumin Silver nanoparticles Palladium nanoparticles Radiation | [45,46,47,48,49,50,51,52,53,54] |
| Vorinostat | Cisplatin Etoposide Bortezomib SBE13 ZD55-TRAIL Radiation | [64,65,66,67,68,69] |
| Panobinostat | Topoisomerase inhibitors Topotecan Etoposide | [71,72] |
| Abexinostat | Radiation | [73] |
| Valproic acid | VE465 Hydralazine CPT-SST Adenovirus H-1PV Cisplatin Adriamycin Gemcitabine Topotecan Retinoic acid Radiation | [85,86,87,88,89,90,91,92,93,94,95,97,98] |
| Sodium butyrate | UCN-01 Wortmannin LY294002 | [103,104,105] |
| BML-210 | Retinoic acid | [117,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psilopatis, I.; Garmpis, N.; Garmpi, A.; Vrettou, K.; Sarantis, P.; Koustas, E.; Antoniou, E.A.; Dimitroulis, D.; Kouraklis, G.; Karamouzis, M.V.; et al. The Emerging Role of Histone Deacetylase Inhibitors in Cervical Cancer Therapy. Cancers 2023, 15, 2222. https://doi.org/10.3390/cancers15082222
Psilopatis I, Garmpis N, Garmpi A, Vrettou K, Sarantis P, Koustas E, Antoniou EA, Dimitroulis D, Kouraklis G, Karamouzis MV, et al. The Emerging Role of Histone Deacetylase Inhibitors in Cervical Cancer Therapy. Cancers. 2023; 15(8):2222. https://doi.org/10.3390/cancers15082222
Chicago/Turabian StylePsilopatis, Iason, Nikolaos Garmpis, Anna Garmpi, Kleio Vrettou, Panagiotis Sarantis, Evangelos Koustas, Efstathios A. Antoniou, Dimitrios Dimitroulis, Gregory Kouraklis, Michail V. Karamouzis, and et al. 2023. "The Emerging Role of Histone Deacetylase Inhibitors in Cervical Cancer Therapy" Cancers 15, no. 8: 2222. https://doi.org/10.3390/cancers15082222
APA StylePsilopatis, I., Garmpis, N., Garmpi, A., Vrettou, K., Sarantis, P., Koustas, E., Antoniou, E. A., Dimitroulis, D., Kouraklis, G., Karamouzis, M. V., Marinos, G., Kontzoglou, K., Nonni, A., Nikolettos, K., Fleckenstein, F. N., Zoumpouli, C., & Damaskos, C. (2023). The Emerging Role of Histone Deacetylase Inhibitors in Cervical Cancer Therapy. Cancers, 15(8), 2222. https://doi.org/10.3390/cancers15082222













