Leukocytic Infiltration of Intraductal Carcinoma of the Prostate: An Exploratory Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Ethics
2.2. Selection of the Representative Blocks and Identification of IDC-P
2.3. Immunohistochemistry
2.4. Quantification of Immunohistochemistry Staining
2.5. Clinical-Data Collection and Endpoints
2.6. Statistical Analyses
3. Results
3.1. Clinicopathological Characteristics of Patients
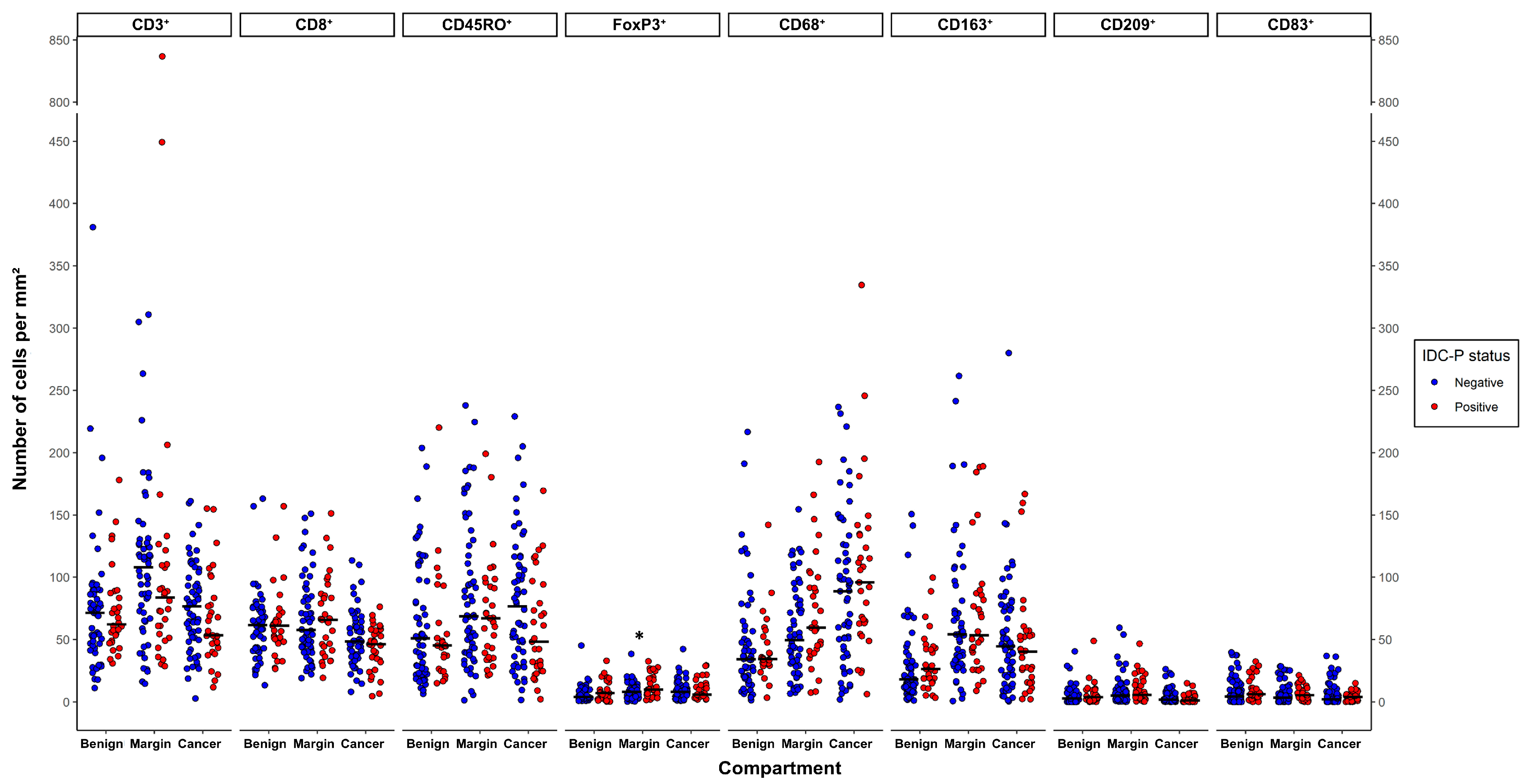
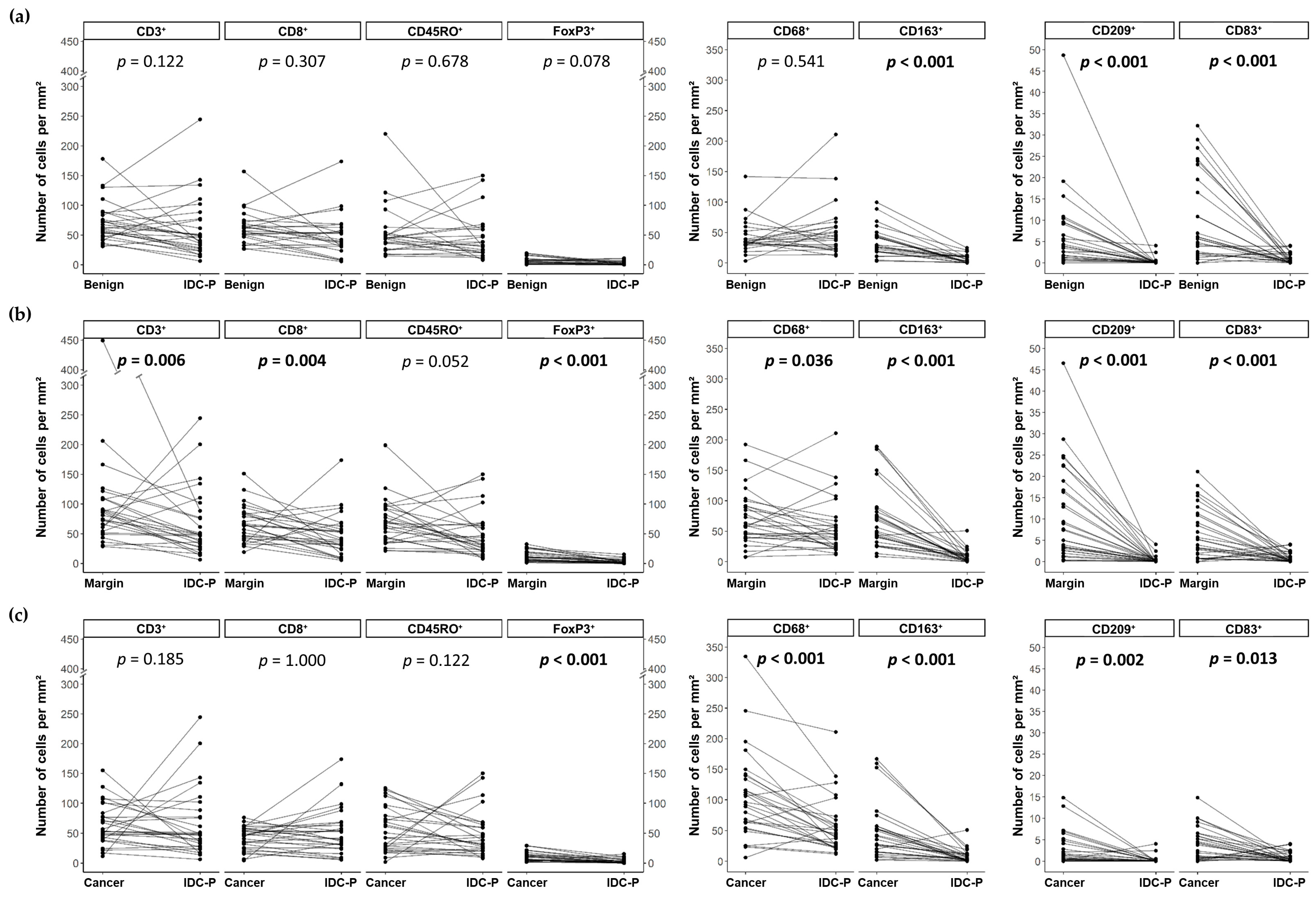
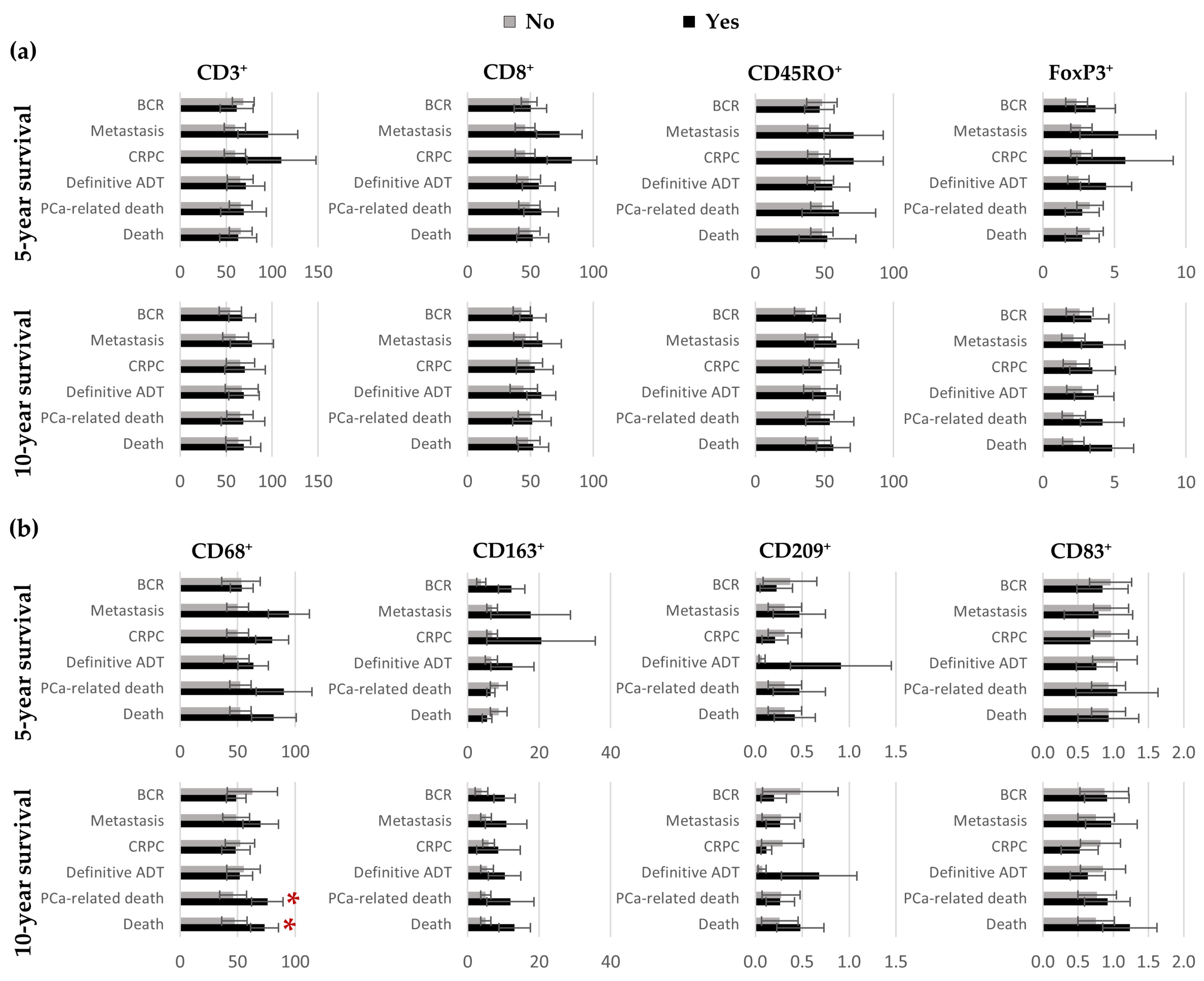
3.2. Quantification of CD3⁺, CD8⁺, CD45RO⁺, FoxP3⁺, CD68⁺, CD163⁺, CD209⁺ and CD83⁺ Cells in Benign Tissues, Margins, Cancer and IDC-P
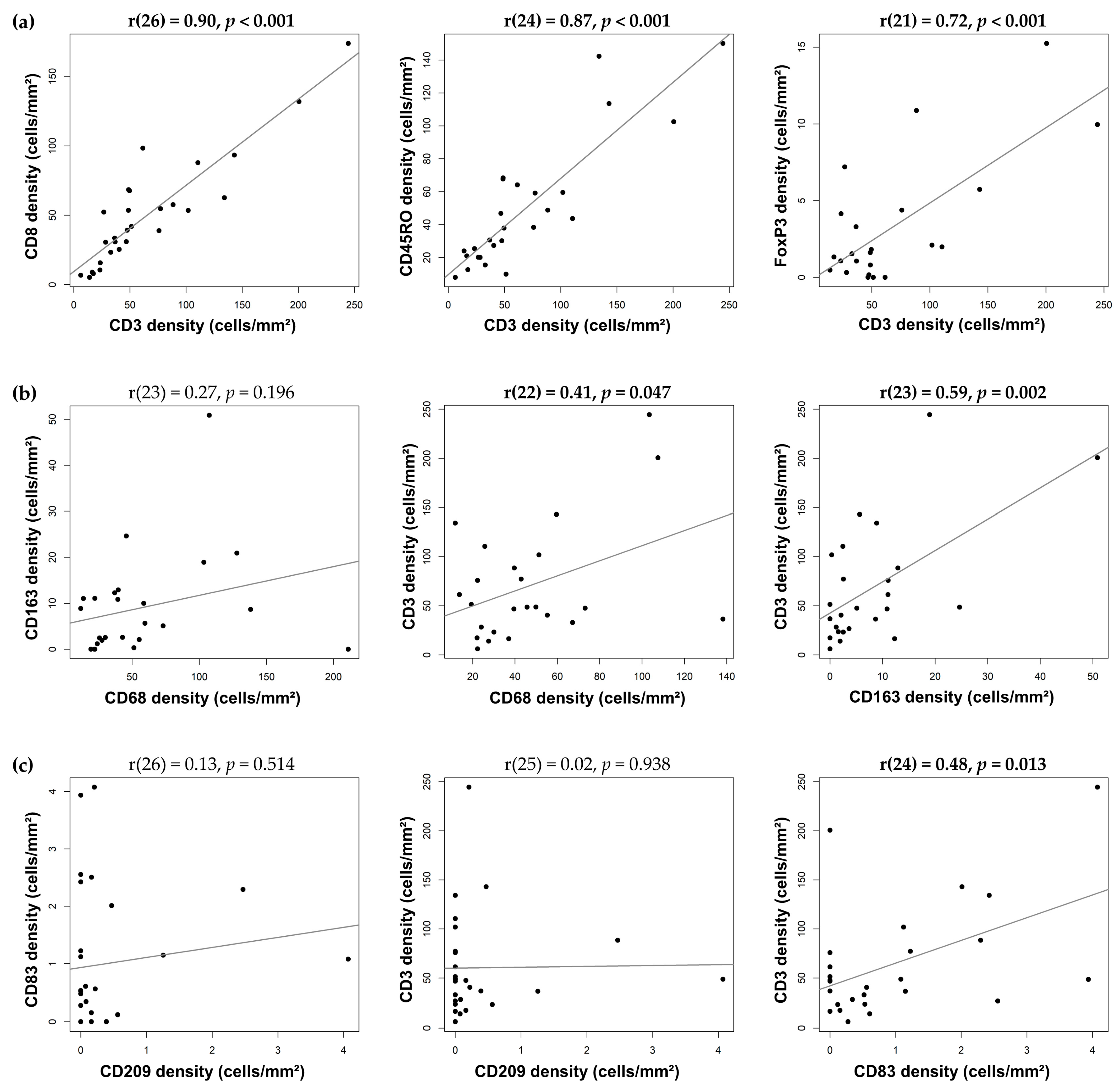
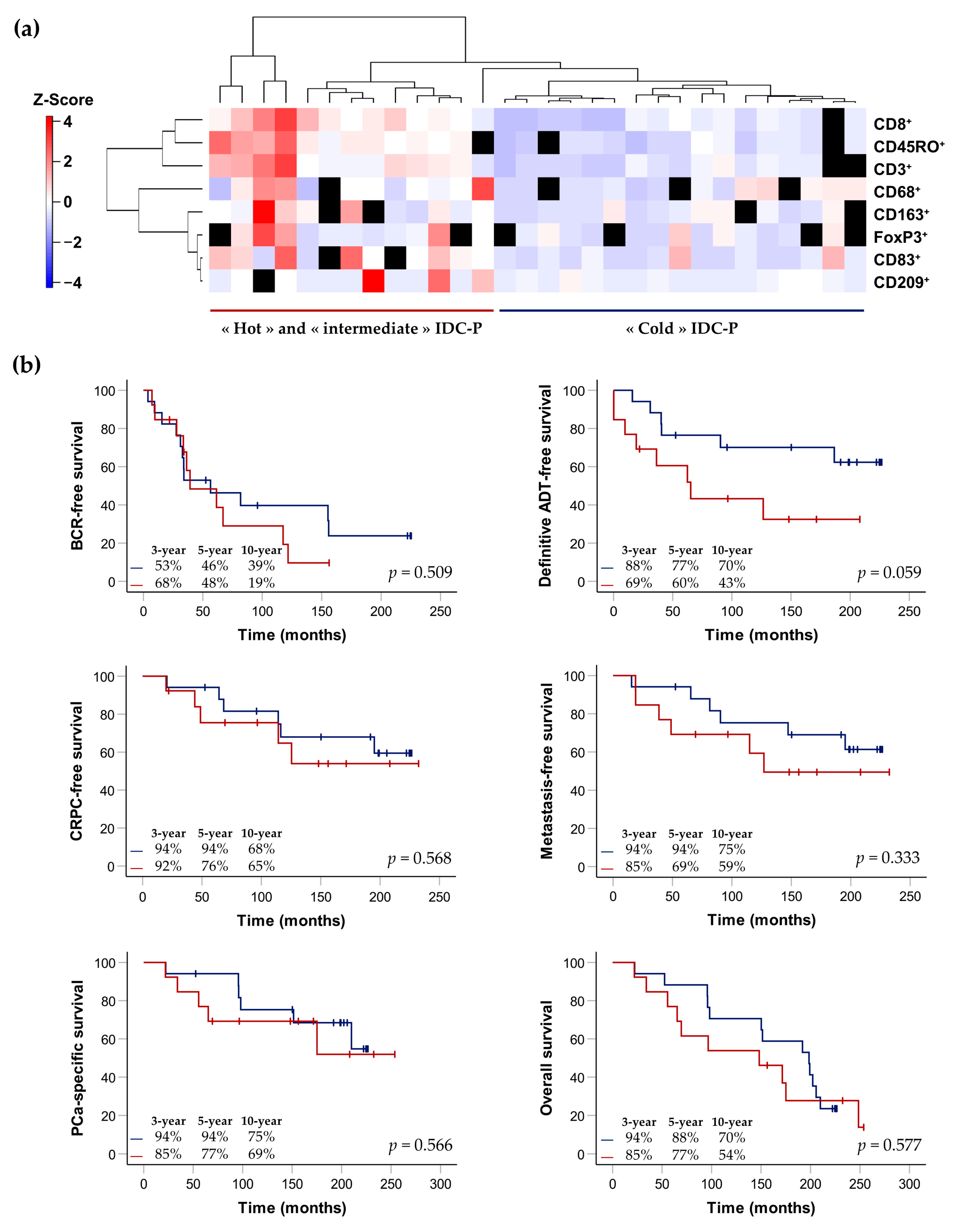
3.3. Correlation between Immune-Cell Densities in IDC-P
3.4. Clusterization of IDC-P-Positive Patients According to Immune-Cell Densities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H.; et al. Advances in cancer immunotherapy 2019—Latest trends. J. Exp. Clin. Cancer Res. 2019, 38, 268. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Borre, M.; Vogelzang, N.J.; Ng, S.; Agarwal, N.; Parker, C.C.; Pook, D.W.; Rathenborg, P.; Flaig, T.W.; Carles, J.; et al. Phase III Trial of PROSTVAC in Asymptomatic or Minimally Symptomatic Metastatic Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2019, 37, 1051–1061. [Google Scholar] [CrossRef]
- Narayan, V.; Barber-Rotenberg, J.S.; Jung, I.Y.; Lacey, S.F.; Rech, A.J.; Davis, M.M.; Hwang, W.T.; Lal, P.; Carpenter, E.L.; Maude, S.L.; et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: A phase 1 trial. Nat. Med. 2022, 28, 724–734. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Ness, N.; Andersen, S.; Valkov, A.; Nordby, Y.; Donnem, T.; Al-Saad, S.; Busund, L.T.; Bremnes, R.M.; Richardsen, E. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014, 74, 1452–1461. [Google Scholar] [CrossRef]
- McArdle, P.A.; Canna, K.; McMillan, D.C.; McNicol, A.M.; Campbell, R.; Underwood, M.A. The relationship between T-lymphocyte subset infiltration and survival in patients with prostate cancer. Br. J. Cancer 2004, 91, 541–543. [Google Scholar] [CrossRef]
- Richardsen, E.; Uglehus, R.D.; Due, J.; Busch, C.; Busund, L.T. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology 2008, 53, 30–38. [Google Scholar] [CrossRef]
- Kärjä, V.; Aaltomaa, S.; Lipponen, P.; Isotalo, T.; Talja, M.; Mokka, R. Tumour-infiltrating lymphocytes: A prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer. Res. 2005, 25, 4435–4438. [Google Scholar]
- Kaur, H.B.; Guedes, L.B.; Lu, J.; Maldonado, L.; Reitz, L.; Barber, J.R.; De Marzo, A.M.; Tosoian, J.J.; Tomlins, S.A.; Schaeffer, E.M.; et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 2018, 31, 1539–1552. [Google Scholar] [CrossRef]
- Davidsson, S.; Ohlson, A.L.; Andersson, S.O.; Fall, K.; Meisner, A.; Fiorentino, M.; Andrén, O.; Rider, J.R. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod. Pathol. 2013, 26, 448–455. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Diop, M.K.; Grosset, A.A.; Rouleau-Gagné, F.; Al-Saleh, A.; Boblea, T.; Trudel, D. Intraductal Carcinoma of the Prostate as a Cause of Prostate Cancer Metastasis: A Molecular Portrait. Cancers 2022, 14, 820. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Guo, C.C.; Epstein, J.I. Intraductal carcinoma of the prostate on needle biopsy: Histologic features and clinical significance. Mod. Pathol. 2006, 19, 1528–1535. [Google Scholar] [CrossRef]
- Haffner, M.C.; Weier, C.; Xu, M.M.; Vaghasia, A.; Gürel, B.; Gümüşkaya, B.; Esopi, D.M.; Fedor, H.; Tan, H.-L.; Kulac, I.; et al. Molecular evidence that invasive adenocarcinoma can mimic prostatic intraepithelial neoplasia (PIN) and intraductal carcinoma through retrograde glandular colonization. J. Pathol. 2016, 238, 31–41. [Google Scholar] [CrossRef]
- Miyai, K.; Divatia, M.K.; Shen, S.S.; Miles, B.J.; Ayala, A.G.; Ro, J.Y. Heterogeneous clinicopathological features of intraductal carcinoma of the prostate: A comparison between “precursor-like” and “regular type” lesions. Int. J. Clin. Exp. Pathol. 2014, 7, 2518–2526. [Google Scholar]
- Porter, L.H.; Lawrence, M.G.; Ilic, D.; Clouston, D.; Bolton, D.M.; Frydenberg, M.; Murphy, D.G.; Pezaro, C.; Risbridger, G.P.; Taylor, R.A. Systematic Review Links the Prevalence of Intraductal Carcinoma of the Prostate to Prostate Cancer Risk Categories. Eur. Urol. 2017, 72, 492–495. [Google Scholar] [CrossRef] [PubMed]
- McNeal, J.E.; Yemoto, C.E.M. Spread of Adenocarcinoma Within Prostatic Ducts and Acini: Morphologic and Clinical Correlations. Am. J. Surg. Pathol. 1996, 20, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G.; Soh, S.; Chakraborty, S.; Scardino, P.T.; Wheeler, T.M. Patterns of high-grade prostatic intraepithelial neoplasia associated with clinically aggressive prostate cancer. Hum. Pathol. 1998, 29, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.J.; Chan, W.C.; Edgar, S.G.; Robinson, E.; Dodd, N.; Hoscek, S.; Mundy, I.P. Prediction of pathological stage and clinical outcome in prostate cancer: An improved pre-operative model incorporating biopsy-determined intraductal carcinoma. Br. J. Urol. 1998, 81, 413–418. [Google Scholar] [CrossRef]
- Zhao, T.; Liao, B.; Yao, J.; Liu, J.; Huang, R.; Shen, P.; Peng, Z.; Gui, H.; Chen, X.; Zhang, P.; et al. Is there any prognostic impact of intraductal carcinoma of prostate in initial diagnosed aggressively metastatic prostate cancer? Prostate 2015, 75, 225–232. [Google Scholar] [CrossRef]
- Kato, M.; Kimura, K.; Hirakawa, A.; Kobayashi, Y.; Ishida, R.; Kamihira, O.; Majima, T.; Funahashi, Y.; Sassa, N.; Matsukawa, Y.; et al. Prognostic parameter for high risk prostate cancer patients at initial presentation. Prostate 2018, 78, 11–16. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Sun, G.; Zhang, M.; Chen, J.; Shen, P.; Liu, Z.; Liao, B.; Zhang, X.; Gong, J.; et al. The Prognostic Value of the Proportion and Architectural Patterns of Intraductal Carcinoma of the Prostate in Patients with De Novo Metastatic Prostate Cancer. J. Urol. 2019, 201, 759–768. [Google Scholar] [CrossRef]
- Kato, M.; Tsuzuki, T.; Kimura, K.; Hirakawa, A.; Kinoshita, F.; Sassa, N.; Ishida, R.; Fukatsu, A.; Kimura, T.; Funahashi, Y.; et al. The presence of intraductal carcinoma of the prostate in needle biopsy is a significant prognostic factor for prostate cancer patients with distant metastasis at initial presentation. Mod. Pathol. 2016, 29, 166–173. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, G.; Liao, B.; Zhang, X.; Armstrong, C.M.; Yin, X.; Liu, J.; Chen, J.; Yang, Y.; Zhao, P.; et al. Novel nomograms for castration-resistant prostate cancer and survival outcome in patients with de novo bone metastatic prostate cancer. BJU Int. 2018, 122, 994–1002. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, P.; Sun, G.; Chen, N.; Liu, J.; Tang, X.; Huang, R.; Cai, D.; Gong, J.; Zhang, X.; et al. The prognostic implication of intraductal carcinoma of the prostate in metastatic castration-resistant prostate cancer and its potential predictive value in those treated with docetaxel or abiraterone as first-line therapy. Oncotarget 2017, 8, 55374–55383. [Google Scholar] [CrossRef]
- Dinerman, B.F.; Khani, F.; Golan, R.; Bernstein, A.N.; Cosiano, M.F.; Margolis, D.J.; Hu, J.C. Population-based study of the incidence and survival for intraductal carcinoma of the prostate. Urol. Oncol. 2017, 35, 673.e9–673.e14. [Google Scholar] [CrossRef]
- Saeter, T.; Vlatkovic, L.; Waaler, G.; Servoll, E.; Nesland, J.M.; Axcrona, K.; Axcrona, U. Intraductal Carcinoma of the Prostate on Diagnostic Needle Biopsy Predicts Prostate Cancer Mortality: A Population-Based Study. Prostate 2017, 77, 859–865. [Google Scholar] [CrossRef]
- Tom, M.C.; Nguyen, J.K.; Lucianò, R.; Mian, O.Y.; Stephans, K.L.; Ciezki, J.P.; Smile, T.D.; Wei, W.; McKenney, J.K.; Magi-Galluzzi, C.; et al. Impact of Cribriform Pattern and Intraductal Carcinoma on Gleason 7 Prostate Cancer Treated with External Beam Radiotherapy. J. Urol. 2019, 202, 710–716. [Google Scholar] [CrossRef]
- Kimura, K.; Tsuzuki, T.; Kato, M.; Saito, A.M.; Sassa, N.; Ishida, R.; Hirabayashi, H.; Yoshino, Y.; Hattori, R.; Gotoh, M. Prognostic value of intraductal carcinoma of the prostate in radical prostatectomy specimens. Prostate 2014, 74, 680–687. [Google Scholar] [CrossRef]
- van Leenders, G.; van der Kwast, T.H.; Iczkowski, K.A. The 2019 International Society of Urological Pathology Consensus Conference on Prostate Cancer Grading. Eur. Urol. 2021, 79, 707–709. [Google Scholar] [CrossRef]
- Epstein, J.I.; Amin, M.B.; Fine, S.W.; Algaba, F.; Aron, M.; Baydar, D.E.; Beltran, A.L.; Brimo, F.; Cheville, J.C.; Colecchia, M.; et al. The 2019 Genitourinary Pathology Society (GUPS) White Paper on Contemporary Grading of Prostate Cancer. Arch. Pathol. Lab. Med. 2021, 145, 461–493. [Google Scholar] [CrossRef]
- Varma, M. Intraductal Carcinoma of the Prostate: A Guide for the Practicing Pathologist. Adv. Anat. Pathol. 2021, 28, 276–287. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017; p. 1032. [Google Scholar]
- Scher, H.I.; Halabi, S.; Tannock, I.; Morris, M.; Sternberg, C.N.; Carducci, M.A.; Eisenberger, M.A.; Higano, C.; Bubley, G.J.; Dreicer, R.; et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008, 26, 1148–1159. [Google Scholar] [CrossRef]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and elaboration. PLoS Med. 2012, 9, e1001216. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; p. 276. [Google Scholar]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef] [PubMed]
- Warnes, G.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; et al. gplots: Various R Programming Tools for Plotting Data; R Package Version 3.1.3; CRAN: Wien, Austria, 2022. [Google Scholar]
- Chua, M.L.K.; Lo, W.; Pintilie, M.; Murgic, J.; Lalonde, E.; Bhandari, V.; Mahamud, O.; Gopalan, A.; Kweldam, C.F.; van Leenders, G.; et al. A Prostate Cancer “Nimbosus”: Genomic Instability and SChLAP1 Dysregulation Underpin Aggression of Intraductal and Cribriform Subpathologies. Eur. Urol. 2017, 72, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Vesalainen, S.; Lipponen, P.; Talja, M.; Syrjänen, K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur. J. Cancer 1994, 30, 1797–1803. [Google Scholar] [CrossRef] [PubMed]
- Irani, J.; Goujon, J.M.; Ragni, E.; Peyrat, L.; Hubert, J.; Saint, F.; Mottet, N. High-grade inflammation in prostate cancer as a prognostic factor for biochemical recurrence after radical prostatectomy. Pathologist Multi Center Study Group. Urology 1999, 54, 467–472. [Google Scholar] [CrossRef]
- Flammiger, A.; Bayer, F.; Cirugeda-Kühnert, A.; Huland, H.; Tennstedt, P.; Simon, R.; Minner, S.; Bokemeyer, C.; Sauter, G.; Schlomm, T.; et al. Intratumoral T but not B lymphocytes are related to clinical outcome in prostate cancer. Apmis 2012, 120, 901–908. [Google Scholar] [CrossRef]
- Flammiger, A.; Weisbach, L.; Huland, H.; Tennstedt, P.; Simon, R.; Minner, S.; Bokemeyer, C.; Sauter, G.; Schlomm, T.; Trepel, M. High tissue density of FOXP3+ T cells is associated with clinical outcome in prostate cancer. Eur. J. Cancer 2013, 49, 1273–1279. [Google Scholar] [CrossRef]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-de-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef]
- Calagua, C.; Ficial, M.; Jansen, C.S.; Hirz, T.; Del Balzo, L.; Wilkinson, S.; Lake, R.; Ku, A.T.; Voznesensky, O.; Sykes, D.B.; et al. A Subset of Localized Prostate Cancer Displays an Immunogenic Phenotype Associated with Losses of Key Tumor Suppressor Genes. Clin. Cancer Res. 2021, 27, 4836–4847. [Google Scholar] [CrossRef]
- Nawaz, S.; Heindl, A.; Koelble, K.; Yuan, Y. Beyond immune density: Critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod. Pathol. 2015, 28, 766–777. [Google Scholar] [CrossRef]
- Heindl, A.; Sestak, I.; Naidoo, K.; Cuzick, J.; Dowsett, M.; Yuan, Y. Relevance of Spatial Heterogeneity of Immune Infiltration for Predicting Risk of Recurrence After Endocrine Therapy of ER+ Breast Cancer. J. Natl. Cancer Inst. 2018, 110, 166–175. [Google Scholar] [CrossRef]
- Leinonen, K.A.; Saramäki, O.R.; Furusato, B.; Kimura, T.; Takahashi, H.; Egawa, S.; Suzuki, H.; Keiger, K.; Ho Hahm, S.; Isaacs, W.B.; et al. Loss of PTEN is associated with aggressive behavior in ERG-positive prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 2333–2344. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef]
- Chakraborty, G.; Armenia, J.; Mazzu, Y.Z.; Nandakumar, S.; Stopsack, K.H.; Atiq, M.O.; Komura, K.; Jehane, L.; Hirani, R.; Chadalavada, K.; et al. Significance of BRCA2 and RB1 Co-loss in Aggressive Prostate Cancer Progression. Clin. Cancer Res. 2020, 26, 2047–2064. [Google Scholar] [CrossRef]
- Trudel, D.; Downes, M.R.; Sykes, J.; Kron, K.J.; Trachtenberg, J.; van der Kwast, T.H. Prognostic impact of intraductal carcinoma and large cribriform carcinoma architecture after prostatectomy in a contemporary cohort. Eur. J. Cancer 2014, 50, 1610–1616. [Google Scholar] [CrossRef]
- Kwon, J.T.W.; Bryant, R.J.; Parkes, E.E. The tumor microenvironment and immune responses in prostate cancer patients. Endocr. Relat. Cancer 2021, 28, T95–T107. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
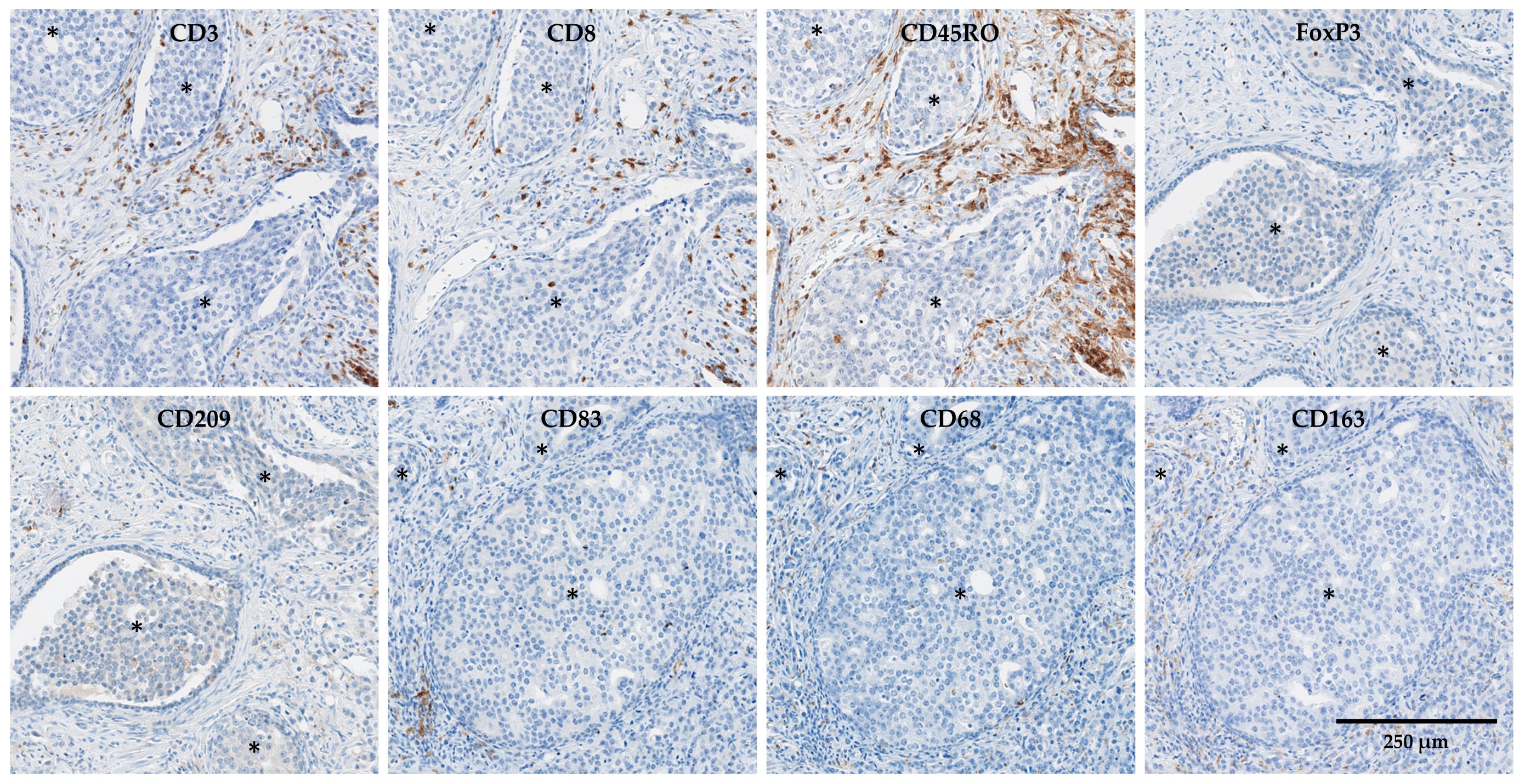


| Antibody | Clone | Company | Dilution | Incubation Time |
|---|---|---|---|---|
| Anti-CD3 | SP7 | Abcam, Toronto, ON, Canada | 1/500 | Overnight |
| Anti-CD8 | 4B11 | Novocastra, Newcastle upon Tyne, England | 1/600 | Overnight |
| Anti-CD45RO | UCHL-1 | Abcam, Toronto, ON, Canada | 1/6000 | Overnight |
| Anti-FoxP3 | 236A/E7 | Abcam, Toronto, ON, Canada | 1/600 | Overnight |
| Anti-CD68 | KP1 | Abcam, Toronto, ON, Canada | 1/800 | 2 h |
| Anti-CD163 | 2G12 | Abcam, Toronto, ON, Canada | 1/2000 | 1 h |
| Anti-CD209 | 120612 | R&D Systems, Minneapolis, MN, USA | 1/80 | Overnight |
| Anti-CD83 | 1H4B | Novocastra, Newcastle-upon-Tyne, UK | 1/200 | Overnight |
| Characteristics | Total Cohort | IDC-P Status | ||
|---|---|---|---|---|
| Negative | Positive | p-Value | ||
| n = 96 (%) | n = 63 (%) | n = 33 (%) | ||
| Mean age at diagnosis (SD) | 63.5 (5) | 62.8 (5) | 64.9 (5) | 0.064 a |
| Mean pre-operative PSA (SD) | 13.4 (14) | 13.6 (16) | 12.9 (10) | 0.889 b |
| Stage pT, n (%) | <0.001 b | |||
| pT2 | 32 (33) | 26 (41) | 6 (18) | |
| pT3a | 32 (33) | 24 (38) | 8 (24) | |
| pT3b | 30 (31) | 13 (21) | 17 (52) | |
| pT4 | 2 (2) | 0 | 2 (6) | |
| Grade group, n (%) | 0.001 c | |||
| 1 | 45 (47) | 37 (59) | 8 (24) | |
| 2 | 24 (25) | 14 (22) | 10 (30) | |
| 3 | 9 (9) | 6 (10) | 3 (9) | |
| 4 | 10 (10) | 3 (5) | 7 (21) | |
| 5 | 8 (8) | 3 (5) | 5 (15) | |
| Lymph-node involvement, n (%) | 27 (28) | 13 (21) | 14 (42) | 0.032 d |
| Lymphovascular invasion, n (%) | 25 (26) | 14 (22) | 11 (33) | 0.332 d |
| Positive margins, n (%) | 75 (78) | 49 (78) | 26 (79) | 1.000 d |
| Biochemical recurrence, n (%) | 46 (48) | 22 (35) | 24 (73) | <0.001 d |
| Castration-resistant PCa, n (%) | 17 (18) | 5 (8) | 12 (36) | <0.001 d |
| Metastasis, n (%) | 17 (18) | 4 (6) | 13 (39) | <0.001 d |
| PCa-related death, n (%) | 14 (15) | 3 (5) | 11 (33) | <0.001 e |
| Overall death, n (%) | 50 (52) | 26 (41) | 24 (73) | 0.005 d |
| Median follow-up in years (IQR) | 15.5 (10–19) | 15.8 (10–19) | 14.3 (8–18) | 0.105 b |
| Characteristics | Total IDC-P | CD68/CD163/CD209 Hotspots | ||||
|---|---|---|---|---|---|---|
| Cold n = 17 | Hot and Intermediate n = 13 | p-Value | Cold and Intermediate n = 22 | Hot n = 7 | p-Value | |
| Mean age at diagnosis (SD) | 64.6 (5) | 65.5 (6) | 0.650 a | 65.9 (5) | 61.7 (7) | 0.149 a |
| Mean pre-operative PSA (SD) | 11.4 (9) | 13.5 (10) | 0.476 a | 13.4 (10) | 8.7 (4) | 0.524 a |
| Stage pT *, n (%) | 1.000 a | 0.162 a | ||||
| pT2 | 2 (12) | 3 (23) | 4 (8) | 1 (14) | ||
| pT3a | 6 (35) | 2 (15) | 8 (36) | 0 (0) | ||
| pT3b-pT4 | 9 (53) | 8 (62) | 10 (46) | 6 (86) | ||
| Grade group *, n (%) | 0.575 a | 0.182 a | ||||
| 1–2 | 10 (59) | 6 (46) | 14 (64) | 2 (29) | ||
| 3 | 1 (6) | 1 (8) | 1 (5) | 1 (14) | ||
| 4–5 | 6 (35) | 6 (46) | 7 (32) | 4 (57) | ||
| Lymph-node involvement, n (%) | 6 (35) | 7 (54) | 0.460 b | 8 (36) | 4 (57) | 0.403 c |
| Lymphovascular invasion, n (%) | 5 (29) | 5 (39) | 0.694 c | 6 (27) | 3 (50) | 0.352 c |
| Positive margins, n (%) | 13 (77) | 12 (92) | 0.355 c | 17 (77) | 7 (100) | 0.296 c |
| Extraprostatic extension, n (%) | 15 (88) | 7 (54) | 0.049 c | 17 (77) | 4 (57) | 0.357 c |
| Seminal vesicle invasion, n (%) | 10 (59) | 8 (62) | 1.000 b | 11 (50) | 6 (86) | 0.187 c |
| Median follow-up in years (IQR) | 16.5 (8–18) | 12.4 (5–16) | 0.263 a | 15.3 (8–19) | 8.2 (2–17) | 0.149 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diop, M.-K.; Molina, O.E.; Birlea, M.; LaRue, H.; Hovington, H.; Têtu, B.; Lacombe, L.; Bergeron, A.; Fradet, Y.; Trudel, D. Leukocytic Infiltration of Intraductal Carcinoma of the Prostate: An Exploratory Study. Cancers 2023, 15, 2217. https://doi.org/10.3390/cancers15082217
Diop M-K, Molina OE, Birlea M, LaRue H, Hovington H, Têtu B, Lacombe L, Bergeron A, Fradet Y, Trudel D. Leukocytic Infiltration of Intraductal Carcinoma of the Prostate: An Exploratory Study. Cancers. 2023; 15(8):2217. https://doi.org/10.3390/cancers15082217
Chicago/Turabian StyleDiop, Mame-Kany, Oscar Eduardo Molina, Mirela Birlea, Hélène LaRue, Hélène Hovington, Bernard Têtu, Louis Lacombe, Alain Bergeron, Yves Fradet, and Dominique Trudel. 2023. "Leukocytic Infiltration of Intraductal Carcinoma of the Prostate: An Exploratory Study" Cancers 15, no. 8: 2217. https://doi.org/10.3390/cancers15082217
APA StyleDiop, M.-K., Molina, O. E., Birlea, M., LaRue, H., Hovington, H., Têtu, B., Lacombe, L., Bergeron, A., Fradet, Y., & Trudel, D. (2023). Leukocytic Infiltration of Intraductal Carcinoma of the Prostate: An Exploratory Study. Cancers, 15(8), 2217. https://doi.org/10.3390/cancers15082217






