Label-Free Quantification Mass Spectrometry Identifies Protein Markers of Chemotherapy Response in High-Grade Serous Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and TISSUE Specimens
2.2. Laser Microdissection and Protein Extraction of the Ovarian Cancer Tissues
2.3. Generation of Carboplatin-Resistant (CBPR) OVCAR-5 Cells
2.4. Protein Digestion
2.5. Identification of Proteins by DDA Nano-LC-ESI-MS/MS
2.6. DDA Nano-LC-ESI-MS/MS Data Analysis
2.7. Quantification of Protein(s) of Interest by DIA Nano-LC-ESI-MS
2.8. Validation by Immunohistochemistry (IHC)
2.9. Online Database Analysis
3. Results
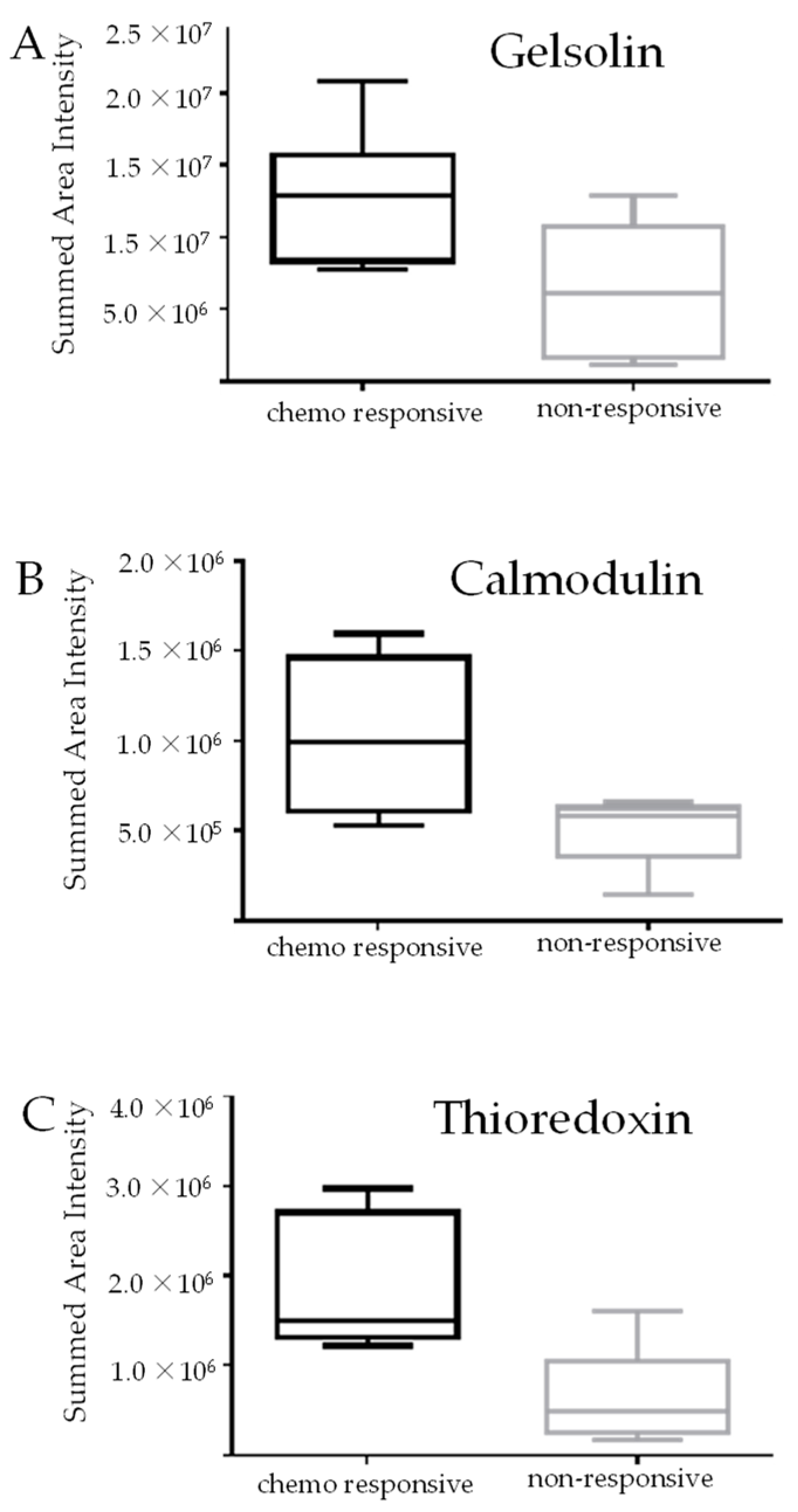
3.1. Identification of Protein Markers of Innate Chemoresistance by DIA LC-MS
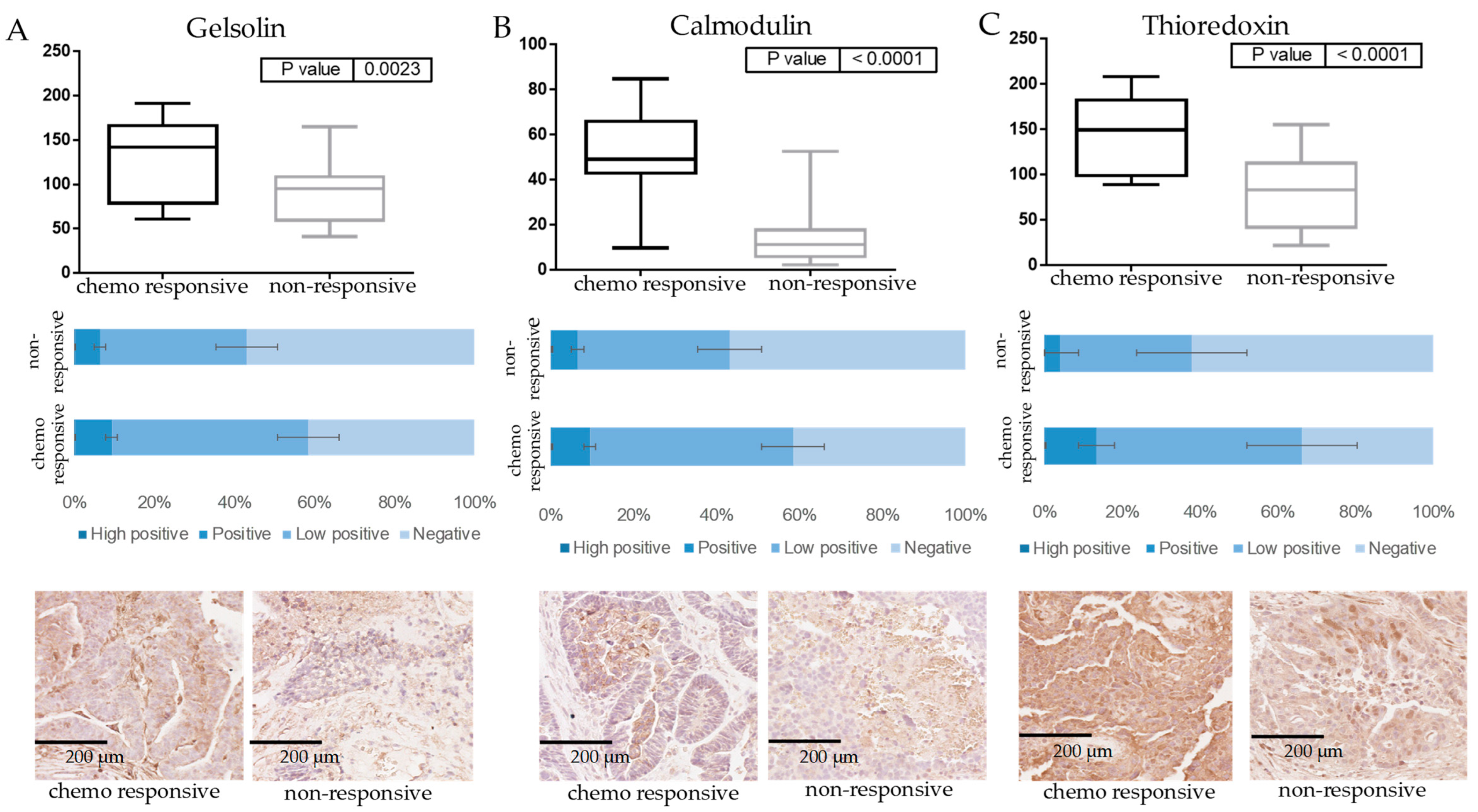
3.2. Verification of Protein Markers of Innate Chemoresistance by IHC
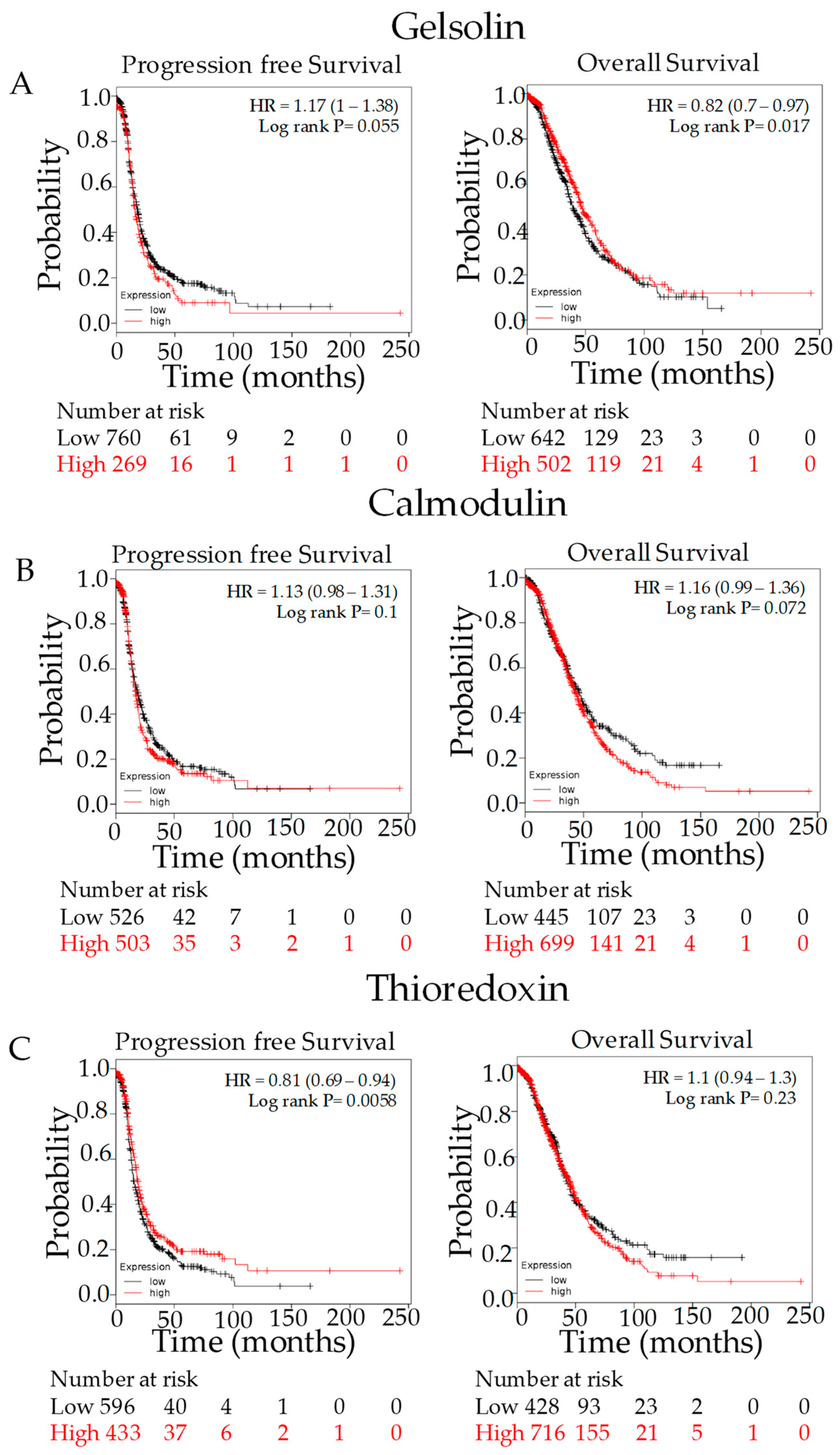
3.3. Marker Expression in Normal Tissues Compared to HGSOC and Survival Analysis
3.4. Kaplan–Meier Outcome Analysis
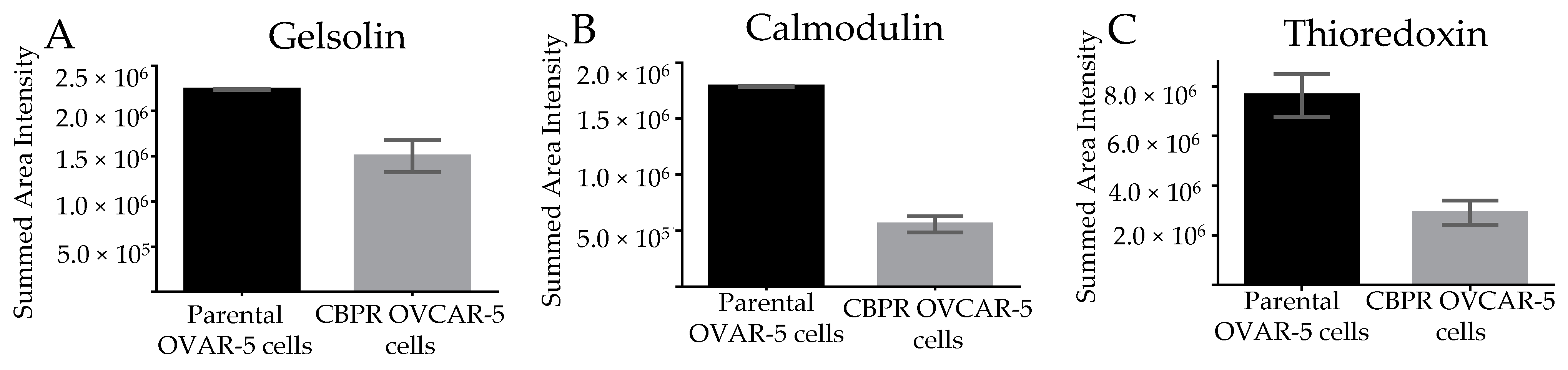
3.5. Marker Expression in Parental OVCAR-5 Cells Compared to Carboplatin-Resistant OVCAR-5 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fund, W.C.R. Ovarian Cancer Statistics. 2022. Available online: https://www.wcrf.org/cancer-trends/ovarian-cancer-statistics/ (accessed on 5 January 2023).
- Australian Institute of Health and Welfare. Ovarian Cancer Statistics in Australia. 2022. Available online: https://www.canceraustralia.gov.au/cancer-types/ovarian-cancer/statistics (accessed on 5 January 2023).
- Ricciardelli, C.; Oehler, M.K. Diverse molecular pathways in ovarian cancer and their clinical significance. Maturitas 2009, 62, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hellström, I.; Raycraft, J.; Hayden-Ledbetter, M.; Ledbetter, J.A.; Schummer, M.; McIntosh, M.; Drescher, C.; Urban, N.; Hellström, K.E. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003, 63, 3695–3700. [Google Scholar] [PubMed]
- Goldie, J.H.; Coldman, A.J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 1979, 63, 1727–1733. [Google Scholar]
- American Cancer Society; The American Cancer Society Medical and Editorial Content Team. Treatment of Invasive Epithelial Ovarian Cancers, by Stage. 2022. Available online: https://www.cancer.org/cancer/ovarian-cancer/treating/by-stage.html#written_by (accessed on 5 January 2023).
- Iyer, L.; Ratain, M.J. Pharmacogenetics and cancer chemotherapy. Eur. J. Cancer 1998, 34, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Tate Thigpen, J. Contemporary phase III clinical trial endpoints in advanced ovarian cancer: Assessing the pros and cons of objective response rate, progression-free survival, and overall survival. Gynecol. Oncol. 2015, 136, 121–129. [Google Scholar] [CrossRef]
- Markman, M.; Bookman, M.A. Second-line treatment of ovarian cancer. Oncologist 2000, 5, 26–35. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Kristeleit, R.S. Optimal treatment for relapsing ovarian cancer. Ann. Oncol. 2010, 21 (Suppl. 7), vii218–vii222. [Google Scholar] [CrossRef]
- Foley, O.W.; Rauh-Hain, J.A.; del Carmen, M.G. Recurrent epithelial ovarian cancer: An update on treatment. Oncology 2013, 27, 288–294, 298. [Google Scholar]
- Sharma, S.; Michael, H.N.; Federica, D.N.; Louise, A.K.; Pauline, A.W.; Stuart, J.M.; Bernard, R.H.; Alan, L.; Richard, O.; Andrew, C.H.; et al. Outcome of ATP-based tumor chemosensitivity assay directed chemotherapy in heavily pre-treated recurrent ovarian carcinoma. BMC Cancer 2003, 3, 19. [Google Scholar] [CrossRef]
- Litan, A.; Langhans, S.A. Cancer as a channelopathy: Ion channels and pumps in tumor development and progression. Front. Cell Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef]
- Roy, L.; Cowden Dahl, K.D. Can Stemness and Chemoresistance Be Therapeutically Targeted via Signaling Pathways in Ovarian Cancer? Cancers 2018, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Song, C.; Zhang, J.; Gao, Y.; Qi, Y.; Zhao, Z.; Yuan, C. Revisiting chemoresistance in ovarian cancer: Mechanism, biomarkers, and precision medicine. Genes Dis. 2022, 9, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Vikramdeo, K.S.; Sudan, S.K.; Singh, S.; Wilhite, A.; Dasgupta, S.; Rocconi, R.P.; Singh, A.P. Platinum-resistant ovarian cancer: From drug resistance mechanisms to liquid biopsy-based biomarkers for disease management. Semin. Cancer Biol. 2021, 77, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Acland, M.; Lokman, N.A.; Young, C.; Anderson, D.; Condina, M.; Desire, C.; Noye, T.M.; Wang, W.; Ricciardelli, C.; Creek, D.J.; et al. Chemoresistant Cancer Cell Lines Are Characterized by Migratory, Amino Acid Metabolism, Protein Catabolism and IFN1 Signalling Perturbations. Cancers 2022, 14, 2763. [Google Scholar] [CrossRef]
- Park, S.-J.; Yoon, B.-H.; Kim, S.-Y. GENT2: An updated gene expression database for normal and tumor tissues. BMC Med. Genom. 2019, 12, 101. [Google Scholar] [CrossRef]
- Meding, S.; Martin, K.; Gustafsson, O.J.R.; Eddes, J.S.; Hack, S.; Oehler, M.K.; Hoffmann, P. Tryptic Peptide Reference Data Sets for MALDI Imaging Mass Spectrometry on Formalin-fixed Ovarian Cancer Tissues. J. Proteome Res. 2012, 12, 308–315. [Google Scholar] [CrossRef]
- Mittal, P.; Klingler-Hoffmann, M.; Arentz, G.; Winderbaum, L.; Lokman, N.; Zhang, C.; Anderson, L.; Scurry, J.; Leung, Y.; Stewart, C.J.; et al. Lymph node metastasis of primary endometrial cancers: Associated proteins revealed by MALDI imaging. Proteomics 2016, 16, 1793–1801. [Google Scholar] [CrossRef]
- Acland, M.; Mittal, P.; Arentz, G.; Whitehead, F.; Hoffmann, P.; Klingler-Hoffmann, M.; Oehler, M.K. A Protocol for the Acquisition of Comprehensive Proteomics Data from Single Cases Using Formalin-Fixed Paraffin Embedded Sections. Methods Protoc. 2022, 5, 57. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Ween, M.P.; Lokman, N.A.; Tan, I.A.; Pyragius, C.E.; Oehler, M.K. Chemotherapy-induced hyaluronan production: A novel chemoresistance mechanism in ovarian cancer. BMC Cancer 2013, 13, 476. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, X.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the MaxQuant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Klingler-Hoffmann, M.; Arentz, G.; Winderbaum, L.; Kaur, G.; Anderson, L.; Scurry, C., Jr.; Leung, Y.; Stewart, C.J.; Carter, J.; et al. Annexin A2 and alpha actinin 4 expression correlates with metastatic potential of primary endometrial cancer. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 846–857. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An Open Source Plugin for the Quantitative Evaluation and Automated Scoring of Immunohistochemistry Images of Human Tissue Samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Gyorffy, B.; Lánczky, A.; Szallasi, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef]
- Lee, Y.; Miron, A.; Drapkin, R.; Nucci, M.R.; Medeiros, F.; Saleemuddin, A.; Garber, J.; Birch, C.; Mou, H.; Gordon, R.W.; et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 2007, 211, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, T.M.; Burdette, J.E.; Dean, M. Fallopian tube initiation of high grade serous ovarian cancer and ovarian metastasis: Mechanisms and therapeutic implications. Cancer Lett. 2020, 476, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Pierredon, S.; Ribaux, P.; Tille, J.-C.; Petignat, P.; Cohen, M. Comparative secretome of ovarian serous carcinoma: Gelsolin in the spotlight. Oncol. Lett. 2017, 13, 4965–4973. [Google Scholar] [CrossRef]
- Abedini, M.R.; Wang, P.W.; Huang, Y.; Tsang, B.K. Cell fate regulation by gelsolin in human gynecologic cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 14442–14447. [Google Scholar] [CrossRef]
- Kim, S.I.; Hwangbo, S.; Dan, K.; Kim, H.S.; Chung, H.H.; Kim, J.-W.; Park, N.H.; Song, Y.-S.; Han, D.; Lee, M. Proteomic Discovery of Plasma Protein Biomarkers and Development of Models Predicting Prognosis of High-Grade Serous Ovarian Carcinoma. Mol. Cell. Proteom. 2023, 22, 100502. [Google Scholar] [CrossRef]
- Asare-Werehene, M.; Nakka, K.; Reunov, A.; Chiu, C.-T.; Lee, W.-T.; Abedini, M.R.; Wang, P.-W.; Shieh, D.-B.; Dilworth, F.J.; Carmona, E.; et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene 2019, 39, 1600–1616. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Yong, S.; Xu, F.; Zhou, T.; McDonald, J.M.; Chen, Y. Calmodulin antagonists promote TRA-8 therapy of resistant pancreatic cancer. Oncotarget 2015, 6, 25308–25319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H.; Li, W.; Xu, H.; Yang, X.; Gan, L. Thioredoxin 1 upregulates FOXO1 transcriptional activity in drug resistance in ovarian cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-J.; Cha, M.-K.; Kim, I.-H. Thioredoxin 1 as a serum marker for ovarian cancer and its use in combination with CA125 for improving the sensitivity of ovarian cancer diagnoses. Biomarkers 2014, 19, 604–610. [Google Scholar] [CrossRef]
- Criscuolo, D.; Avolio, R.; Parri, M.; Romano, S.; Chiarugi, P.; Matassa, D.S.; Esposito, F. Decreased Levels of GSH Are Associated with Platinum Resistance in High-Grade Serous Ovarian Cancer. Antioxidants 2022, 11, 1544. [Google Scholar] [CrossRef]
- Wangpaichitr, M.; Sullivan, E.J.; Theodoropoulos, G.; Wu, C.; You, M.; Feun, L.G.; Lampidis, T.J.; Kuo, M.T.; Savaraj, N. The Relationship of Thioredoxin-1 and Cisplatin Resistance: Its Impact on ROS and Oxidative Metabolism in Lung Cancer Cells. Mol. Cancer Ther. 2012, 11, 604–615. [Google Scholar] [CrossRef]
- Huang, D.; Savage, S.R.; Calinawan, A.P.; Lin, C.; Zhang, B.; Wang, P.; Starr, T.K.; Birrer, M.J.; Paulovich, A.G. A highly annotated database of genes associated with platinum resistance in cancer. Oncogene 2021, 40, 6395–6405. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]

| Patient | First Treatment | Grade | Category | Age at Diagnosis (Years) |
|---|---|---|---|---|
| 1 | Carboplatin/paclitaxel | 3 | Complete response | 63 |
| 2 | Carboplatin | 3 | Complete response | 69 |
| 3 | Carboplatin/paclitaxel | 3 | Complete response | 61 |
| 4 | Carboplatin/paclitaxel | 3 | Complete response | 64 |
| 5 | Carboplatin | 3 | Complete response | 60 |
| 6 | Carboplatin/paclitaxel | 3 | Complete response | 59 |
| 7 | Carboplatin/paclitaxel | 3 | Non-response/incomplete response | 78 |
| 8 | Carboplatin/paclitaxel | 3 | Non-response/incomplete response | 66 |
| 9 | Carboplatin/paclitaxel | 3 | Non-response/incomplete response | 61 |
| 10 | Carboplatin/paclitaxel | 3 | Non-response/incomplete response | 44 |
| 11 | Carboplatin/paclitaxel | 3 | Non-response/incomplete response | 75 |
| 12 | Carboplatin | 3 | Non-response/incomplete response | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arentz, G.; Mittal, P.; Klingler-Hoffmann, M.; Condina, M.R.; Ricciardelli, C.; Lokman, N.A.; Kaur, G.; Oehler, M.K.; Hoffmann, P. Label-Free Quantification Mass Spectrometry Identifies Protein Markers of Chemotherapy Response in High-Grade Serous Ovarian Cancer. Cancers 2023, 15, 2172. https://doi.org/10.3390/cancers15072172
Arentz G, Mittal P, Klingler-Hoffmann M, Condina MR, Ricciardelli C, Lokman NA, Kaur G, Oehler MK, Hoffmann P. Label-Free Quantification Mass Spectrometry Identifies Protein Markers of Chemotherapy Response in High-Grade Serous Ovarian Cancer. Cancers. 2023; 15(7):2172. https://doi.org/10.3390/cancers15072172
Chicago/Turabian StyleArentz, Georgia, Parul Mittal, Manuela Klingler-Hoffmann, Mark R. Condina, Carmela Ricciardelli, Noor A. Lokman, Gurjeet Kaur, Martin K. Oehler, and Peter Hoffmann. 2023. "Label-Free Quantification Mass Spectrometry Identifies Protein Markers of Chemotherapy Response in High-Grade Serous Ovarian Cancer" Cancers 15, no. 7: 2172. https://doi.org/10.3390/cancers15072172
APA StyleArentz, G., Mittal, P., Klingler-Hoffmann, M., Condina, M. R., Ricciardelli, C., Lokman, N. A., Kaur, G., Oehler, M. K., & Hoffmann, P. (2023). Label-Free Quantification Mass Spectrometry Identifies Protein Markers of Chemotherapy Response in High-Grade Serous Ovarian Cancer. Cancers, 15(7), 2172. https://doi.org/10.3390/cancers15072172







