Intravascular Complications of Central Venous Catheterization by Insertion Site in Acute Leukemia during Remission Induction Chemotherapy Phase: Lower Risk with Peripherally Inserted Catheters in a Single-Center Retrospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Oversight
2.2. Eligibility Criteria and Participants
2.3. Implantation Procedures
2.4. Collected Data
2.5. Outcomes
2.6. Statistical Analyses
3. Results
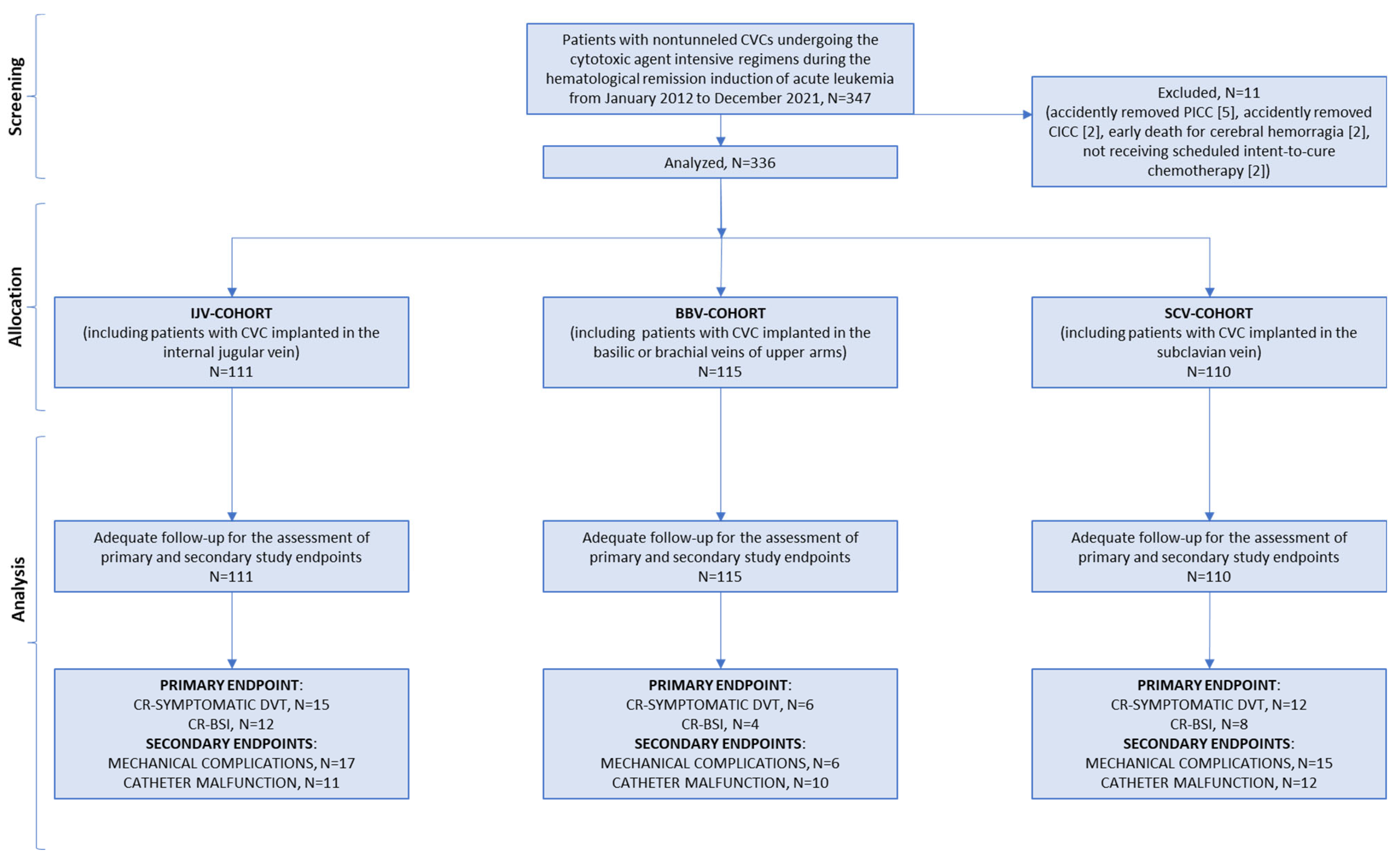
3.1. Participants and Recruitment
3.2. CVC Insertion and Use
3.3. Post-Chemotherapy Aplasia
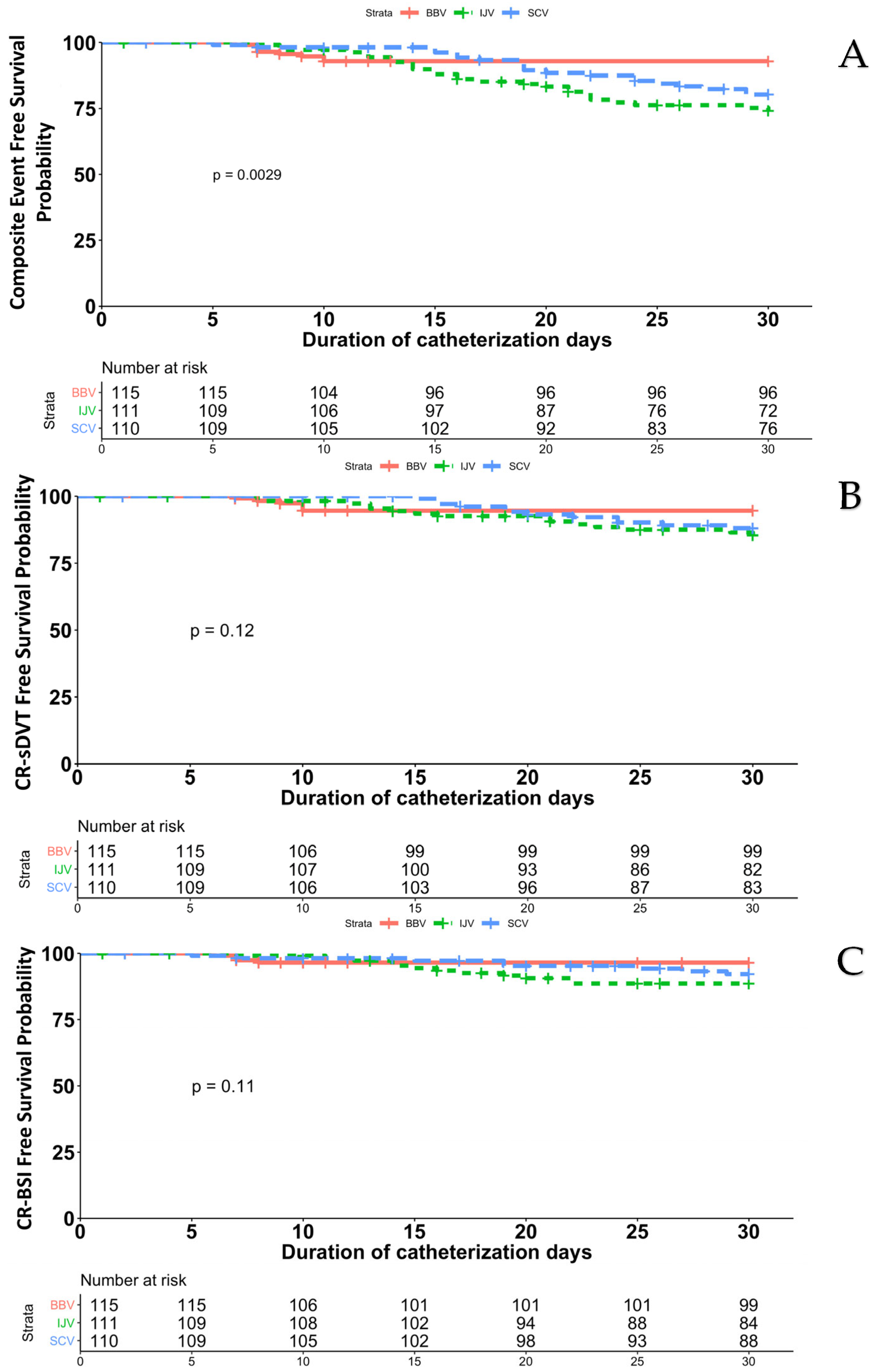
3.4. Catheter-Related Symptomatic Deep-Vein Thrombosis and Blood Stream Infection
3.5. Secondary Endpoints
3.6. Thirty-Day Catheter Removals and Deaths
3.7. Risk Factors for the Occurrence of CVC Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boll, B.; Schalk, E.; Buchheidt, D.; Hasenkamp, J.; Kiehl, M.; Kiderlen, T.R.; Kochanek, M.; Koldehoff, M.; Kostrewa, P.; Claßen, A.Y.; et al. Central venous catheter–related infections in hematology and oncology: 2020 updated guidelines on diagnosis, management, and prevention by the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2021, 100, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, D.; Hansen, E.; Kreil, S.; Nolte, F.; Jawhar, M.; Hecht, A.; Hofmann, W.K.; Klein, S.A. The insertion site is the main risk factor for central venous catheter-related complications in patients with hematologic malignancies. Am. J. Hematol. 2022, 97, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Picardi, M.; Pagliuca, S.; Chiurazzi, F.; Iula, D.; Catania, M.; Rossano, F.; Pane, F. Early ultrasonographic finding of septic thrombophlebitis is the main indicator of central venous catheter removal to reduce infection-related mortality in neutropenic patients with bloodstream infection. Ann. Oncol. 2012, 23, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Rockholt, M.M.; Thorarinsdottir, H.R.; Vladimir, L.; Rundgren, M.; Kander, T. Central venous catheter-related complications in hematologic patients: An observational study. Acta Anaesthesiol. Scand. 2022, 66, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Baier, C.; Linke, L.; Eder, M.; Schwab, F.; Chaberny, I.F.; Vonberg, R.P.; Ebadi, E. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS ONE 2020, 15, e0227772. [Google Scholar] [CrossRef] [PubMed]
- Geerts, W. Central venous catheter–related thrombosis. Hematol. Am. Soc. Hematol. Educ. Prog. 2014, 2014, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.; Furlanetto, J.; Hutka, M.; Gouveia, P.; Wuerstlein, R.; Mariz, J.M.; Pinto, D.; Cardoso, F. Central venous access in oncology: ESMO clinical practice guidelines. Ann. Oncol. 2015, 26 (Suppl. 5), v152–v168. [Google Scholar] [CrossRef]
- Schiffer, C.A.; Mangu, P.B.; Wade, J.C.; Camp-Sorrell, D.; Cope, D.G.; El-Rayes, B.F.; Gorman, M.; Ligibel, J.; Mansfield, P.; Levine, M. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2013, 31, 1357–1370. [Google Scholar] [CrossRef]
- Camera, A.; Villa, M.R.; Pezzullo, L.; Picardi, M.; Rocco, S.; Fontana, R.; Notaro, R.; Rotoli, B. Central venous catheter insertion: A bedside procedure for haematological patients. Eur. J. Haematol. 1996, 56, 93–94. [Google Scholar] [CrossRef]
- Picardi, M.; Della Pepa, R.; Cerchione, C.; Pugliese, N.; Mortaruolo, C.; Trastulli, F.; Giordano, C.; Grimaldi, F.; Zacheo, I.; Raimondo, M.; et al. A Frontline Approach with Peripherally Inserted Versus Centrally Inserted Central Venous Catheters for Remission Induction Chemotherapy Phase of Acute Myeloid Leukemia: A Randomized Comparison. Clin. Lymphoma Myeloma Leuk. 2019, 19, e184–e194. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.M. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Rupp, M.S.; Tung, A.; Connis, R.T. Practice guidelines for central venous access 2020. An update report by the Americal Society of Anesthesiologists task force on central venous access. Anesthesiology 2020, 132, 8–43. [Google Scholar]
- Lamperti, M.; Bodenham, A.R.; Pittiruti, M.; Blaivas, M.; Augoustides, J.G.; Elbarbary, M.; Pirotte, T.; Karakitsos, D.; Ledonne, J.; Doniger, S.; et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 2012, 38, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Scoppettuolo, G.; Pittiruti, M. Ultrasound guided placement of peripherally inserted central venous catheters. In Critical Care Ultrasound; Lumb, P., Karakitsos, D., Eds.; Elsevier-Saunders: Philadelphia, PA, USA, 2014; pp. 89–94. [Google Scholar]
- Pugliese, N.; Salvatore, P.; Iula, D.V.; Catania, M.R.; Chiurazzi, F.; Della Pepa, R.; Cerchione, C.; Raimondo, M.; Giordano, C.; Simeone, L.; et al. Ultrasonography-driven combination antibiotic therapy with tigecycline significantly increases survival among patients with neutropenic enterocolitis following cytarabine-containing chemotherapy for the remission induction of acute myeloid leukemia. Cancer Med. 2017, 6, 1500–1511. [Google Scholar] [CrossRef]
- Picardi, M.; Della Pepa, R.; Giordano, C.; Pugliese, N.; Mortaruolo, C.; Trastulli, F.; Grimaldi, F.; Zacheo, I.; Raimondo, M.; Sirignano, C.; et al. (1-3)-β-D-Glucan serum increase and small-airway-invasive radiological findings as early signs of pulmonary aspergillosis in high-risk hematologic patients in the posaconazole era: Preliminary observations. Ann. Hematol. 2019, 98, 527–531. [Google Scholar] [CrossRef]
- Della Pepa, R.; Cerchione, C.; Pugliese, N.; Colicchio, R.; Salvatore, P.; Sirignano, C.; Soscia, E.; Pagano, L.; Sanguinetti, M.; Pane, F.; et al. Diagnostic-driven antifungal approach in neutropenic patients at high risk for chronic disseminated candidiasis: Preliminary observations on the role of 1,3-β-D-glucan antigenemia and multiphasic contrast-enhanced computed tomography. Support. Care Cancer 2018, 26, 1691–1694. [Google Scholar] [CrossRef] [PubMed]
- van Rooden, C.J.; Rosendaal, F.R.; Barge, R.M.Y.; van Oostayen, J.A.; van der Meer, F.J.; Meinders, A.E.; Huisman, M.V. Central venous catheter related thrombosis in haematology patients and prediction of risk by screening with Doppler-ultrasound. Br. J. Haematol. 2003, 123, 507–512. [Google Scholar] [CrossRef]
- Cortelezzi, A.; Moia, M.; Falanga, A.; Pogliani, E.M.; Agnelli, G.; Bonizzoni, E.; Gussoni, G.; Barbui, T.; Mannucci, P.M.; CATHEM Study Group. Incidence of thrombotic complications in patients with haematological malignancies with central venous catheters: A prospective multicentre study. Br. J. Haematol. 2005, 129, 811–817. [Google Scholar] [CrossRef]
- Scamuffa, M.C.; Morano, S.G.; Serrao, A.; Bruzzese, A.; Stocchi, F.; Santoro, C.; Vozella, F.; Latagliata, R.; Chistolini, A. PICC-related upper deep venous thrombosis in patients with hematological malignancies. Management of anticoagulant therapy according to the platelet count. J. Thromb. Thrombolysis 2020, 49, 426–430. [Google Scholar] [CrossRef]
- Yates, J.W.; Wallace HJJr Ellison, R.R.; Holland, J.F. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother. Rep. 1973, 57, 485–488. [Google Scholar]
- Pastore, D.; Specchia, G.; Carluccio, P.; Liso, A.; Mestice, A.; Rizzi, R.; Greco, G.; Buquicchio, C.; Liso, V. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: Single-center experience. Ann. Hematol. 2003, 82, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, S.; Messina, M.; Della Starza, I.; Piciocchi, A.; Cafforio, L.; Cavalli, M.; Taherinasab, A.; Ansuinelli, M.; Elia, L.; Albertini Petroni, G.; et al. Philadelphia-like acute lymphoblastic leukemia is associated with minimal residual disease persistence and poor outcome. First report of the minimal residual disease-oriented GIMEMA LAL1913. Haematologica 2021, 106, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Bassan, R.; Pavoni, C.; Intermesoli, T.; Spinelli, O.; Tosi, M.; Audisio, E.; Marmont, F.; Cattaneo, C.; Borlenghi, E.; Cortelazzo, S.; et al. Updated risk-oriented strategy for acute lymphoblastic leukemia in adult patients 18–65 years: NILG ALL 10/07. Blood Cancer J. 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.G.; Wu, O.; Bodenham, A.R.; Agarwal, R.; Menne, T.F.; Jones, B.L.; Heggie, R.; Hill, S.; Dixon-Hughes, J.; CAVA Trial Group; et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): A randomised controlled trial. Lancet 2021, 398, 403–415. [Google Scholar] [CrossRef]
- Dix, C.H.; Yeung, D.T.; Rule, M.L.; Ma, D.D. Essential, but at what risk? A prospective study on central venous access in patients with haematological malignancies. Intern. Med. J. 2012, 42, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Luft, D.; Schmoor, C.; Wilson, C.; Widmer, A.F.; Bertz, H.; Frei, R.; Heim, D.; Dettenkofer, M. Central venous catheter associated bloodstream infection and colonisation of insertion site and catheter tip. What are the rates and risk factors in haematology patients? Ann. Hematol. 2010, 89, 1265–1275. [Google Scholar] [CrossRef]
- Sriskandarajah, P.; Webb, K.; Chisholm, D.; Raobaikady, R.; Davis, K.; Pepper, N.; Ethell, M.E.; Potter, M.N.; Shaw, B.E. Retrospective cohort analysis comparing the incidence of deep vein thromboses between peripherally-inserted and long-term skin tunneled venous catheters in hemato-oncology patients. Thromb. J. 2015, 13, 21. [Google Scholar] [CrossRef]
- Chopra, V.; O’Horo, J.C.; Rogers, M.A.M.; Maki, D.G.; Safdar, N. The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2013, 34, 908–918. [Google Scholar] [CrossRef]
- Refaei, M.; Fernandes, B.; Brandwein, J.; Goodyear, M.D.; Pokhrel, A.; Wu, C. Incidence of catheter-related thrombosis in acute leukemia patients: A comparative, retrospective study of the safety of peripherally inserted vs. centrally inserted central venous catheters. Ann. Hematol. 2016, 95, 2057–2064. [Google Scholar] [CrossRef]
- Chopra, V.; Anand, S.; Hickner, A.; Buist, M.; Rogers, M.A.; Saint, S.; Flanders, S.A. Risk of venous thromboembolism associated with peripherally inserted central catheters: A systematic review and meta-analysis. Lancet 2013, 382, 311–325. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (%) | BBV-Cohort (%) | IJV-Cohort (%) | SCV-Cohort (%) | p Value |
|---|---|---|---|---|---|
| No. of patients | 336 | 115 | 111 | 110 | |
| Median age, years Range | 53 18–72 | 53 18–72 | 54 18–72 | 54.5 22–70 | 0.71 |
| Male | 170 (51) | 58 (50) | 56 (51) | 56 (51) | 0.67 |
| Prothrombotic risk factors | |||||
| BMI > 25 | 36 (11) | 13 (11) | 12 (11) | 11 (10) | 0.96 |
| Smoke | 60 (18) | 22 (19) | 20 (18) | 18 (16) | 0.85 |
| Hypertension | 69 (19) | 23 (20) | 24 (21) | 22 (20) | 0.97 |
| Diabetes | 26 (7) | 9 (8) | 10 (9) | 7 (6) | 0.71 |
| Hematological disease | |||||
| AML | 208 (62) | 71 (62) | 69 (62) | 68 (62) | |
| ALL | 128 (38) | 44 (38) | 42 (38) | 42 (38) | 0.98 |
| ECOG status | |||||
| 0–1 | 286 (85) | 97 (84) | 95 (85) | 94 (85) | |
| 2–3 | 50 (15) | 18 (16) | 16 (15) | 16 (15) | 0.97 |
| Blood cell count | |||||
| WBC, ×103/mm3 | |||||
| Median, range | 3.4, 0.2–147 | 3.2, 0.97–96 | 3.9, 1.0–147 | 3.5, 0.2–100 | 0.75 |
| Neutrophils, ×103/mm3 | |||||
| Median, range | 0.5, 0.4–12.34 | 0.45, 0.4–7.5 | 0.56, 0.8–12.34 | 0.48, 0.75–5.5 | 0.69 |
| Hemoglobin, g/dL | |||||
| Median, range | 9.5, 5.9–12.6 | 9.7, 5.9–12.1 | 9.5, 6.6–12.6 | 9.3, 6–12 | 0.81 |
| Platelets, ×103/mm3 | |||||
| Median, range | 41, 3.0–275 | 36, 3.0–275 | 42.5, 9.0–232 | 40, 5.0–200 | 0.92 |
| Variable | Total (%) n = 336 | BBV-Cohort (%) n = 115 | IJV-Cohort (%) n = 111 | SCV-Cohort (%) n = 110 |
|---|---|---|---|---|
| Device insertion place | ||||
| Hematology ward bedside | 120 (35) | 92 (80) | 17 (15) | 11 (10) |
| ICU | 216 (65) | 23 (20) | 94 (95) | 99 (90) |
| Device type | ||||
| Single lumen | 20 (6) | 20 (17) | NA | NA |
| Double lumen | 111 (33) | 90 (78) | 10 (9) | 11 (10) |
| Triple lumen | 205 (61) | 5 (5) | 101 (91) | 99 (90) |
| 4 French | 40 (10) | 40 (35) | NA | NA |
| 5 French | 70 (21) | 70 (61) | NA | NA |
| 6 French | 5 (1) | 5 (4) | NA | NA |
| 7 French | 177 (53) | NA | 96 (86) | 94 (85) |
| 8 French | 32 (9) | NA | 15 (14) | 16 (15) |
| Venous access | ||||
| Basilic | 73 (22) | 73 (63) | NA | NA |
| Brachial | 42 (12) | 42 (37) | NA | NA |
| Internal jugular | 111 (33) | NA | 111 (100) | NA |
| Subclavian | 110 (33) | NA | NA | 110 (100) |
| Right side | 227 (67) | 88 (76) | 74 (67) | 65 (59) |
| Left side | 109 (33) | 27 (24) | 37 (33) | 45 (41) |
| Attempts at venipuncture, n | ||||
| Median, range | 1, 1–4 | 1, 1–4 | 1, 1–3 | 1, 1–3 |
| Tip location | ||||
| Lower third of superior vena cava | 84 (25) | 22 (19) | 31 (28) | 31 (28) |
| Cavoatrial junction | 252 (75) | 93 (81) | 80 (72) | 79 (72) |
| Interval from CVC implantation to chemotherapy start, day | ||||
| Median | 1 | 1 | 1 | 1 |
| Range | 0.5–1.5 | 0.5–1 | 0.5–1.5 | 0.5–1 |
| Chemotherapy regimen | ||||
| Cytarabine-based | 188 (56) | 63 (55) | 62 (56) | 63 (57) |
| Fludarabine-based | 20 (6) | 7 (6) | 7 (6) | 6 (5.5) |
| MTX-asparaginase-based | 128 (38) | 45 (39) | 42 (38) | 41 (37) |
| Chemotherapy-induced hematological toxicity | ||||
| Severe neutropenia | 336 (100) | 115 (100) | 111 (100) | 110 (100) |
| Severe thrombocytopenia | 336 (100) | 115 (100) | 111 (100) | 110 (100) |
| Duration of catheterization—days | ||||
| Median | 30 | 30 | 30 | 30 |
| Range | 1–30 | 6–30 | 1–30 | 2–30 |
| Outcome | Internal Jugular versus Basilic/Brachial (BB) | Subclavian versus Basilic/Brachial (BB) | Subclavian versus Internal Jugular | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jugular | BB | Hazard Ratio (95% CI) * | p Value | Subclavian | BB | Hazard Ratio (95% CI) * | p Value | Jugular | Subclavian | Hazard Ratio (95% CI) * | p Value | |
| Number | Number | Number | ||||||||||
| Catheters | 111 | 115 | 110 | 115 | 110 | 111 | ||||||
| Catheter-days | 2812 | 3050 | 2902 | 3050 | 2812 | 2902 | ||||||
| Primary composite outcome ** | 27 | 8 | 3.6 (1.6–7.9) | 0.0016 | 20 | 8 | 2.6 (1.2–5.9) | 0.02 | 27 | 20 | 0.7 (0.4–1.3) | 0.23 |
| Symptomatic deep-vein thrombosis | 15 | 6 | 2.6 (1.1–6.7) | 0.032 | 12 | 6 | 2.1 (0.8–5.5) | 0.15 | 15 | 12 | 0.8 (0.4–1.7) | 0.53 |
| Bloodstream infection | 12 | 4 | 3.1 (1.0–9.6) | 0.047 | 8 | 4 | 2.1 (0.6–7.0) | 0.22 | 12 | 8 | 0.6 (0.3–1.6) | 0.35 |
| Secondary outcome | ||||||||||||
| Major mechanical complications | 17 | 6 | 3.7 (1.5–9.5) | 0.006 | 15 | 6 | 3.1 (1.2–8.1) | 0.017 | 17 | 15 | 0.8 (0.4–1.7) | 0.59 |
| Arterial injury | 3 | - | - | 0.99 | 3 | - | - | 0.99 | 3 | 3 | 0.9 (0.2–4.7) | 0.95 |
| Haematoma | 9 | 3 | 3.9 (1.1–14.7) | 0.04 | 7 | 3 | 2.9 (0.7–11.2) | 0.12 | 9 | 7 | 0.7 (0.3–1.9) | 0.52 |
| Pneumothorax | 2 | - | - | 0.99 | 2 | - | - | 0.99 | 2 | 2 | 0.9 (0.1–6.7) | 0.95 |
| Neurologic damage | 3 | 3 | 1.1 (0.2–5.6) | 0.88 | 3 | 3 | 1.2 (0.2–5.8) | 0.85 | 3 | 3 | 0.9 (0.2–4.8) | 0.96 |
| Catheter malfunction | 11 | 10 | 1.2 (0.53–2.9) | 0.62 | 12 | 10 | 1.4 (0.6–3.2) | 0.46 | 11 | 12 | 1.0 (0.5–2.4) | 0.95 |
| Total (n = 336 Patients) | BBV-Cohort (n = 115 Patients) | IJV-Cohort (n = 111) | SCV-Cohort (n = 110) | |
|---|---|---|---|---|
| Catheter-related deep vein thromboses | ||||
| Number of events | 33 | 6 | 15 | 12 |
| Thrombosis symptoms/clinical signs | ||||
| Yes | 33 | 6 | 15 | 12 |
| Ultrasonography diagnoses | ||||
| Yes | 33 | 6 | 15 | 12 |
| French thrombosed catheters | ||||
| 5 Fr | 6 | 6 | - | - |
| 7 Fr | 12 | - | 7 | 5 |
| 8 Fr | 15 | - | 8 | 7 |
| Thrombus site | ||||
| Basilic vein | 3 | 3 | - | - |
| Brachial vein | 3 | 3 | - | - |
| Axillary vein | 25 | 2 | 13 | 10 |
| Subclavian vein | 20 | 2 | 10 | 8 |
| Internal jugular vein | 7 | - | 5 | 2 |
| Brachiocephalic vein | 6 | - | 4 | 2 |
| Thrombosis in multiple sites | 25 | 4 | 11 | 10 |
| Thrombus size (mm) | ||||
| Median (range) | 20 (5–80) | 20 (5–50) | 25 (5–80) | 20 (5–70) |
| Anti-thrombotic specific therapy * | ||||
| Yes | 19 | 3 | 10 | 6 |
| No | 14 | 3 | 5 | 6 |
| Catheter-related blood stream infections | ||||
| Number of events | 24 | 4 | 8 | 12 |
| Causative pathogens of blood stream infection | ||||
| Gram-positive | 14 | 2 | 6 | 6 |
| Staphylococcus haemolyticus | 7 | 2 | 3 | 2 |
| Staphylococcus epidermidis | 4 | - | 2 | 2 |
| Staphylococcus aureus | 2 | - | 1 | 1 |
| Enterococcus spp. | 1 | - | - | 1 |
| MDR gram-positive bacteria ** | 5 | - | 3 | 2 |
| Gram-negative | 6 | 2 | 1 | 3 |
| Escherichia coli | 3 | 2 | 1 | - |
| Klebsiella pneumonia | 3 | - | - | 3 |
| MDR gram-negative bacteria *** | 3 | - | 2 | 1 |
| Candida parapsilosis **** | 4 | - | 1 | 3 |
| Antimicrobial prophylaxis | ||||
| Levofloxacin (500 mg daily orally) | 336 | 115 | 111 | 110 |
| Posaconazole (200 mg three times daily orally) | 208 | 71 | 69 | 68 |
| Fluconazole (200 mg daily) | 128 | 44 | 42 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picardi, M.; Giordano, C.; Della Pepa, R.; Pugliese, N.; Esposito, M.; Abagnale, D.P.; Giannattasio, M.L.; Lisi, D.; Lamagna, M.; Grimaldi, F.; et al. Intravascular Complications of Central Venous Catheterization by Insertion Site in Acute Leukemia during Remission Induction Chemotherapy Phase: Lower Risk with Peripherally Inserted Catheters in a Single-Center Retrospective Study. Cancers 2023, 15, 2147. https://doi.org/10.3390/cancers15072147
Picardi M, Giordano C, Della Pepa R, Pugliese N, Esposito M, Abagnale DP, Giannattasio ML, Lisi D, Lamagna M, Grimaldi F, et al. Intravascular Complications of Central Venous Catheterization by Insertion Site in Acute Leukemia during Remission Induction Chemotherapy Phase: Lower Risk with Peripherally Inserted Catheters in a Single-Center Retrospective Study. Cancers. 2023; 15(7):2147. https://doi.org/10.3390/cancers15072147
Chicago/Turabian StylePicardi, Marco, Claudia Giordano, Roberta Della Pepa, Novella Pugliese, Maria Esposito, Davide Pio Abagnale, Maria Luisa Giannattasio, Dario Lisi, Martina Lamagna, Francesco Grimaldi, and et al. 2023. "Intravascular Complications of Central Venous Catheterization by Insertion Site in Acute Leukemia during Remission Induction Chemotherapy Phase: Lower Risk with Peripherally Inserted Catheters in a Single-Center Retrospective Study" Cancers 15, no. 7: 2147. https://doi.org/10.3390/cancers15072147
APA StylePicardi, M., Giordano, C., Della Pepa, R., Pugliese, N., Esposito, M., Abagnale, D. P., Giannattasio, M. L., Lisi, D., Lamagna, M., Grimaldi, F., Muccioli Casadei, G., Ciriello, M., Persico, M., Gargiulo, G., & Pane, F. (2023). Intravascular Complications of Central Venous Catheterization by Insertion Site in Acute Leukemia during Remission Induction Chemotherapy Phase: Lower Risk with Peripherally Inserted Catheters in a Single-Center Retrospective Study. Cancers, 15(7), 2147. https://doi.org/10.3390/cancers15072147







