The Immune Checkpoint Receptor CD96: A Local and Systemic Immune Modulator in Oral Cancer?
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients Collective
2.2. Processing of Tissue and Blood
2.3. Analysis of CD96 Expression by Quantitative Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-qPCR)
2.4. Detection and Quantitative Immunohistochemical Analysis of CD96 Expression by Immunohistochemistry (IHC)
3. Statistical Analysis
4. Results
4.1. Demographic and Clinico-Histopathological Characteristics of the Study Collective
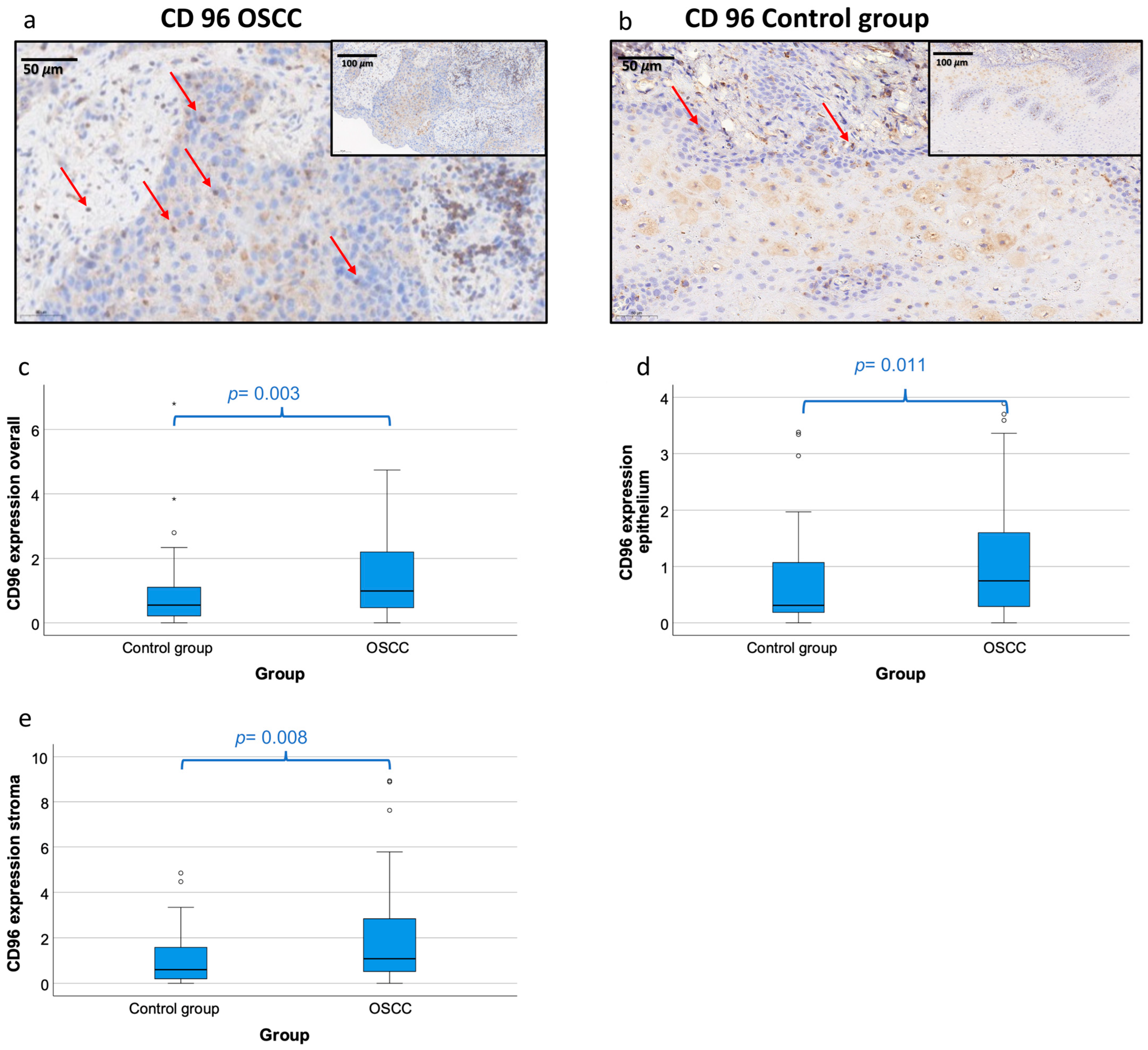
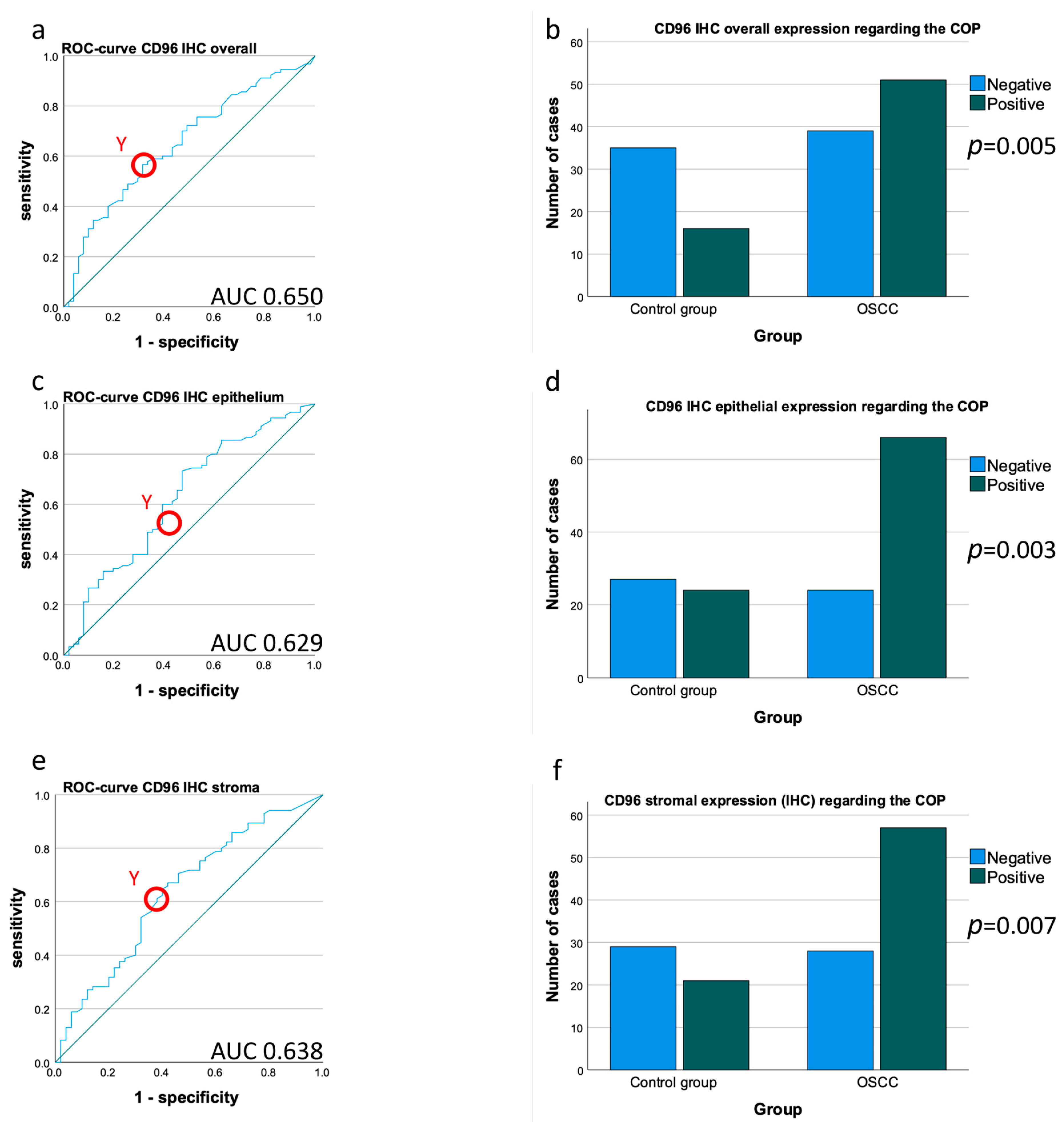
4.2. Immunohistochemical Staining Characteristic of CD96
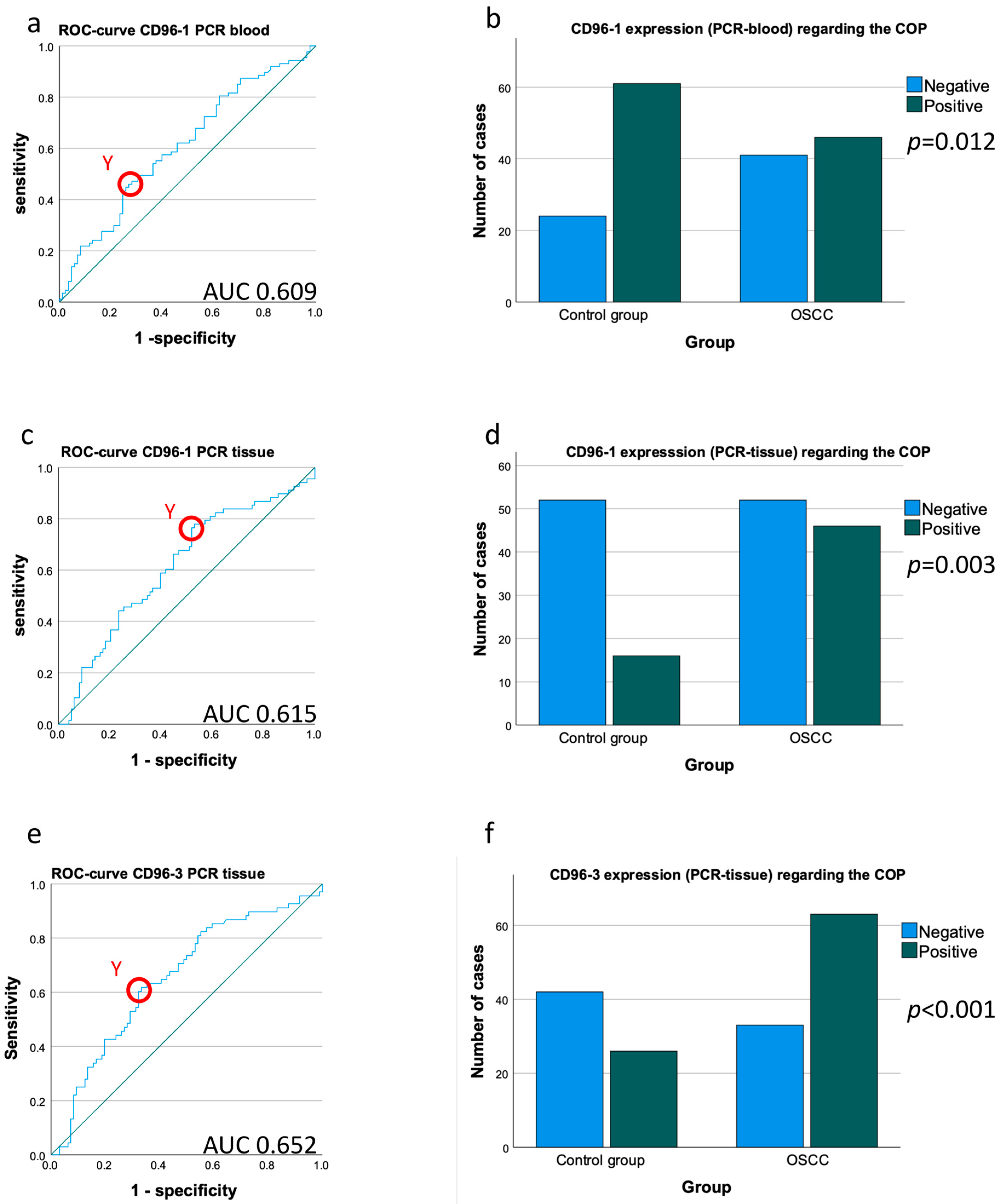
4.3. Comparison of CD96 Expression in Peripheral Blood between OSCC Patients and Healthy Controls
4.4. Comparison of CD96 Expression in Tissue Samples between OSCC Patients and Healthy Controls
4.5. Association CD96 Expression Patterns with Histomorphological Parameters
4.6. Correlations between CD96 mRNA and Protein Expression, Macrophage and Checkpoint Markers
5. Discussion
5.1. CD96 Expression in Tumor Tissue
5.2. Potential Therapeutic Use of CD96 Inhibition
5.3. CD96 Expression in Peripheral Blood
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [PubMed]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Shekarian, T.; Alcazer, V.; Valladeau-Guilemond, J.; Valsesia-Wittmann, S.; Amigorena, S.; Caux, C.; Depil, S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Diao, P.; Jiang, Y.; Li, Y.; Wu, X.; Li, J.; Zhou, C.; Jiang, L.; Zhang, W.; Yan, E.; Zhang, P.; et al. Immune landscape and subtypes in primary resectable oral squamous cell carcinoma: Prognostic significance and predictive of therapeutic response. J. Immunother. Cancer 2021, 9, e002434. [Google Scholar] [CrossRef] [PubMed]
- Uppaluri, R.; Campbell, K.M.; Egloff, A.M.; Zolkind, P.; Skidmore, Z.L.; Nussenbaum, B.; Paniello, R.C.; Rich, J.T.; Jackson, R.; Pipkorn, P.; et al. Neoadjuvant and Adjuvant Pembrolizumab in Resectable Locally Advanced, Human Papillomavirus-Unrelated Head and Neck Cancer: A Multicenter, Phase 2 Trial. Clin. Cancer Res. 2020, 26, 5140–5152. [Google Scholar] [CrossRef]
- Hanna, G.J.; A, O.N.; Shin, K.Y.; Wong, K.; Jo, V.Y.; Quinn, C.T.; Cutler, J.M.; Flynn, M.; Lizotte, P.H.; Annino, D.J., Jr.; et al. Neoadjuvant and Adjuvant Nivolumab and Lirilumab in Patients with Recurrent, Resectable Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563–1570. [Google Scholar] [CrossRef]
- Olmos, M.; Lutz, R.; Büntemeyer, T.-O.; Glajzer, J.; Nobis, C.-P.; Ries, J.; Möst, T.; Eckstein, M.; Hecht, M.; Gostian, A.-O.; et al. Case report: Patient specific combination of surgery and immunotherapy in advanced squamous cell carcinoma of the head and neck—a case series and review of literature. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Preidl, R.; Neukam, F.W.; Ries, J. PD-L1 expression in tumor tissue and peripheral blood of patients with oral squamous cell carcinoma. Oncotarget 2017, 8, 112584–112597. [Google Scholar] [CrossRef]
- Wehrhan, F.; Weber, M.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Kesting, M.; Ries, J. PD1 expression and correlation with its ligands in oral cancer specimens and peripheral blood. J. Cranio-Maxillofacial Surg. 2021, 49, 118–125. [Google Scholar] [CrossRef]
- Weber, M.; Wehrhan, F.; Baran, C.; Agaimy, A.; Büttner-Herold, M.; Kesting, M.; Ries, J. Prognostic significance of PD-L2 expression in patients with oral squamous cell carcinoma—A comparison to the PD-L1 expression profile. Cancer Med. 2019, 8, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, C. The Rise of NK Cell Checkpoints as Promising Therapeutic Targets in Cancer Immunotherapy. Front. Immunol. 2019, 10, 2354. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Lutz, R.; Olmos, M.; Glajzer, J.; Baran, C.; Nobis, C.-P.; Möst, T.; Eckstein, M.; Kesting, M.; Ries, J. Beyond PD-L1—Identification of Further Potential Therapeutic Targets in Oral Cancer. Cancers 2022, 14, 1812. [Google Scholar] [CrossRef] [PubMed]
- Rogel, A.; Ibrahim, F.M.; Thirdborough, S.M.; Renart-Depontieu, F.; Birts, C.N.; Buchan, S.L.; Preville, X.; King, E.V.; Al-Shamkhani, A. Fcγ receptor–mediated cross-linking codefines the immunostimulatory activity of anti-human CD96 antibodies. J. Clin. Investig. 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Isayev, O.; Werner, J.; Bazhin, A.V. CD96 as a Potential Immune Regulator in Cancers. Int. J. Mol. Sci. 2023, 24, 1303. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-P.; Xi, C.-H.; Zhu, Y.; Yin, L.-D.; Wei, J.-S.; Zhang, J.-J.; Liu, X.-C.; Guo, S.; Fu, Y.; Miao, Y. Altered expression of CD226 and CD96 on natural killer cells in patients with pancreatic cancer. Oncotarget 2016, 7, 66586–66594. [Google Scholar] [CrossRef] [PubMed]
- Conner, M.; Hance, K.W.; Yadavilli, S.; Smothers, J.; Waight, J.D. Emergence of the CD226 Axis in Cancer Immunotherapy. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Liu, F.; Liu, Z.; Chen, F. CD96 Correlates With Immune Infiltration and Impacts Patient Prognosis: A Pan-Cancer Analysis. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.S.; Park, Y. Hitting the complexity of the TIGIT-CD96-CD112R-CD226 axis for next-generation cancer immunotherapy. BMB Rep. 2021, 54, 2–11. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Q.; Huang, M.; Wen, H.; Lin, R.; Zheng, M.; Qu, K.; Li, K.; Wei, H.; Xiao, W.; et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 2019, 70, 168–183. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Qiu, J.; Qu, X.; Peng, J.; Lu, C.; Zhang, M.; Zhang, M.; Qi, X.; Li, G.; et al. Targeting CD96 overcomes PD-1 blockade resistance by enhancing CD8+ TIL function in cervical cancer. J. Immunother. Cancer 2022, 10, e003667. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Todorovic, J.; Spohn, S.S.K.; Gkika, E.; Becker, C.; Knopf, A.; Zamboglou, C.; Sprave, T.; Werner, M.; Grosu, A.-L.; et al. Prognostic value of tumor-infiltrating immune cells and immune checkpoints in elderly head-and-neck squamous cell carcinoma patients undergoing definitive (chemo)radiotherapy. Radiat. Oncol. 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Cao, Y.; Jin, T.; Tian, Y.; Dai, C.; Xu, F. The CD112R/CD112 axis: A breakthrough in cancer immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buckle, I.; Guillerey, C. Inhibitory Receptors and Immune Checkpoints Regulating Natural Killer Cell Responses to Cancer. Cancers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Feng, D.; Shi, X.; Xiong, Q.; Zhang, F.; Li, D.; Wei, W.; Yang, L. A Ferroptosis-Related Gene Prognostic Index Associated With Biochemical Recurrence and Radiation Resistance for Patients With Prostate Cancer Undergoing Radical Radiotherapy. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xia, Q.; Di, C.; Li, C.; Si, H.; Zhou, B.; Yu, S.; Li, Y.; Huang, J.; Lu, Y.; et al. Tumor Cell-Intrinsic CD96 Mediates Chemoresistance and Cancer Stemness by Regulating Mitochondrial Fatty Acid beta-Oxidation. Adv. Sci. (Weinh.) 2022, e2202956. [Google Scholar] [CrossRef]
- Dougall, W.C.; Kurtulus, S.; Smyth, M.J.; Anderson, A.C. TIGIT and CD96: New checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef]
- Xu, C.; Fang, H.; Gu, Y.; Yu, K.; Wang, J.; Lin, C.; Zhang, H.; Li, H.; He, H.; Liu, H.; et al. Impact of intratumouralCD96expression on clinical outcome and therapeutic benefit in gastric cancer. Cancer Sci. 2022, 113, 4070–4081. [Google Scholar] [CrossRef]
- Weber, M.; Iliopoulos, C.; Moebius, P.; Büttner-Herold, M.; Amann, K.; Ries, J.; Preidl, R.; Neukam, F.W.; Wehrhan, F. Prognostic significance of macrophage polarization in early stage oral squamous cell carcinomas. Oral Oncol. 2016, 52, 75–84. [Google Scholar] [CrossRef]
- Weber, M.; Büttner-Herold, M.; Hyckel, P.; Moebius, P.; Distel, L.; Ries, J.; Amann, K.; Neukam, F.W.; Wehrhan, F. Small oral squamous cell carcinomas with nodal lymphogenic metastasis show increased infiltration of M2 polarized macrophages—An immunohistochemical analysis. J. Cranio-Maxillofacial Surg. 2014, 42, 1087–1094. [Google Scholar] [CrossRef]
- Kubota, K.; Moriyama, M.; Furukawa, S.; Rafiul, H.; Maruse, Y.; Jinno, T.; Tanaka, A.; Ohta, M.; Ishiguro, N.; Yamauchi, M.; et al. CD163(+)CD204(+) tumor-associated mac-rophages contribute to T cell regulation via interleukin-10 and PD-L1 production in oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1755. [Google Scholar] [CrossRef] [PubMed]
- Blake, S.J.; Stannard, K.; Liu, J.; Allen, S.; Yong, M.C.R.; Mittal, D.; Aguilera, A.R.; Miles, J.J.; Lutzky, V.P.; De Andrade, L.F.; et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016, 6, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Choy, F.C.; Sanny, A.; Murakami, T.; Tan, A.H.-M.; Lam, K.-P. An Inhibitory Role for Human CD96 Endodomain in T Cell Anti-Tumor Responses. Cells 2023, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Lepletier, A.; Madore, J.; Aguilera, A.R.; Stannard, K.; Blake, S.J.; Whitehall, V.L.; Liu, C.; Bettington, M.L.; Takeda, K.; et al. CD96 Is an Immune Checkpoint That Regulates CD8+ T-cell Antitumor Function. Cancer Immunol. Res. 2019, 7, 559–571. [Google Scholar] [CrossRef]
- Ito, M.; Yajima, S.; Suzuki, T.; Oshima, Y.; Nanami, T.; Sumazaki, M.; Shiratori, F.; Funahashi, K.; Tochigi, N.; Shimada, H. High serum PD-L1 level is a poor prognostic biomarker in surgically treated esophageal cancer. Cancer Med. 2020, 9, 1321–1327. [Google Scholar] [CrossRef]


| (a) | Patients (OSCC) | Healthy Controls | |||
|---|---|---|---|---|---|
| n | % of cases | n | % of cases | ||
| Number of cases PCR | 183 | 98 | 54 | 85 | 46 |
| Gender | Male | 65 | 66.3 | 51 | 60 |
| Female | 33 | 33.7 | 34 | 40 | |
| Mean age ± SD | 62.66 ± 11.7 years | 49.7 ± 19.7 years | |||
| Range of age | 31–93 years | 18–88 years | |||
| % of cases | |||||
| T-Status | T1 | 28 | 28.6 | ||
| T2 | 33 | 33.7 | |||
| T3 | 14 | 14.3 | |||
| T4 | 18 | 18.4 | |||
| Unknown | 5 | 5 | |||
| N-Status | N0 | 53 | 54.1 | ||
| N1 | 13 | 13.3 | |||
| N2a | 13 | 13.3 | |||
| N2b | 15 | 15.3 | |||
| N2c | 3 | 3 | |||
| Unknown | 1 | 1 | |||
| L-Status | L0 | 70 | 71.4 | ||
| L1 | 18 | 18.4 | |||
| Unknown | 10 | 10.2 | |||
| Pn-Status | Pn0 | 59 | 60.2 | ||
| Pn1 | 28 | 28.6 | |||
| Unknown | 11 | 11.2 | |||
| Grading | G1 | 9 | 9.2 | ||
| G2 | 46 | 47 | |||
| G3 | 37 | 37.7 | |||
| Unknown | 6 | 6.1 | |||
| (b) | Patients (OSCC) | Healthy Controls | |||
| n | % of cases | n | % of cases | ||
| Number of cases IHC | 141 | 90 | 64 | 51 | 36 |
| Gender | Male | 62 | 69 | 38 | 74.5 |
| Female | 28 | 31 | 13 | 25.5 | |
| Mean age ± SD | 63.4 ± 12.4 years | 48.7 ± 19.1 years | |||
| Range of age | 33–89 years | 18–87 years | |||
| % of cases | |||||
| T-Status | T1 | 17 | 18.9 | ||
| T2 | 34 | 37.8 | |||
| T3 | 14 | 15.5 | |||
| T4 | 23 | 25.6 | |||
| Unknown | 2 | 2.2 | |||
| N-Status | N0 | 50 | 55.6 | ||
| N1 | 13 | 14.5 | |||
| N2a | 10 | 11.1 | |||
| N2b | 12 | 13.3 | |||
| N2c | 2 | 2.2 | |||
| Unknown | 3 | 3.3 | |||
| L-Status | L0 | 60 | 66.7 | ||
| L1 | 16 | 17.8 | |||
| Unknown | 14 | 15.5 | |||
| Pn-Status | Pn0 | 52 | 57.8 | ||
| Pn1 | 26 | 28.9 | |||
| Unknown | 12 | 13.3 | |||
| Grading | G1 | 4 | 4.5 | ||
| G2 | 47 | 52.2 | |||
| G3 | 30 | 33.3 | |||
| Unknown | 9 | 10 | |||
| Primer | Sequence (5′ to 3′) | Primer (bp) | Amplicon (bp) | Annealing Temp. (°C) | Accession |
|---|---|---|---|---|---|
| CD96_1 s | ACCTCCAGTGGGACAGATACC | 21 | 91 | 60 | NM_198196.3 * |
| CD96_1 as | GAAGTGTTGAGCCTGCACCT | 20 | – | – | |
| CD96_3 s | GCATGGTCGGTGGAGGATAA | 20 | 130 | 60 | NM_001318889 ** |
| CD96_3 as | GGACTGGAGAGAGGTGGAGT | 20 | – | – | |
| PD1 s | AAACCCTGGTGGTTGGTGTC | 20 | 105 | – | NM_005018.2 |
| PD1 as | CTCCTATTGTCCCTCGTGCG | 20 | – | – | |
| PD-L1 s | AGCTATGGTGGTGCCGACTA | 20 | 152 | 60 | NM_014143.3 § |
| PD-L1 s | CAGATGACTTCGGCCTTGGG | 20 | – | – | NM_001314029.1 |
| CD68 s | TGGGTGGGATCATCTCCAGT | 20 | 100 | 60 | NM_001040059.1 $ NM_001251.2 $ |
| CD68 as | TAGGCTGTCTGCACCAGTTG | 20 | – | 60 | |
| CD163 s | CTTGGGGTTGTTCTGTTGGC | 20 | 92 | 60 | NM_004244 ++ |
| CD163 as | CCTCTTGAGGAAACTGCAAGC | 21 | – | 60 | |
| GAPDH s | GACCCCTTCATTGACCTCAACTA | 23 | 102 | 60 | NM_002046.5 |
| GAPDH as | GAATTTGCCATGGGTGGAAT | 20 | – | – |
| n | Mean | SD | p-Value | AUC | FC | COP | No. of Cases | + | − | % Pos. Cases | p-Value χ2 Test | Sensitivity | Specificity | Pos. Predictive Value | Neg. Predictive Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT-qPCR CD96_1 blood | ∆CT | 0.014 | 0.609 | 0.84 | 4.29 | 172 | 0.012 | 0.529 | 0.282 | 0.43 | 0.369 | |||||
| controls | 85 | 3.99 | 0.70 | 85 | 61 | 24 | 71.8 | |||||||||
| patients | 87 | 4.24 | 0.73 | 87 | 46 | 41 | 52.9 | |||||||||
| RT-qPCR CD96_3 blood | ∆CT | 0.095 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | ||
| controls | 85 | 3.40 | 0.74 | |||||||||||||
| patients | 87 | 3.59 | 0.74 | |||||||||||||
| RT-qPCR CD96_1 tissue | ∆CT | 0.012 | 0.615 | 1.28 | 6.52 | 166 | 0.003 | 0.469 | 0.765 | 0.742 | 0.5 | |||||
| controls | 68 | 7.20 | 1.48 | 68 | 16 | 52 | 23.5 | |||||||||
| patients | 98 | 6.84 | 1.48 | 98 | 46 | 52 | 46.9 | |||||||||
| RT-qPCR CD96_3 tissue | ∆CT | <0.001 | 0.652 | 1.52 | 9.27 | 164 | 0.656 | 0.618 | 0.708 | 0.56 | ||||||
| controls | 68 | 9.38 | 1.46 | 68 | 26 | 42 | 38.2 | <0.001 | ||||||||
| patients | 96 | 8.78 | 1.55 | 96 | 63 | 33 | 65.6 | |||||||||
| IHC CD96 tissue overall | LI | 0.003 | 0.650 | 1.53 | 0.905 | 141 | 0.005 | 0.567 | 0.686 | 0.761 | 0.473 | |||||
| controls | 51 | 0.91 | 1.15 | 51 | 16 | 35 | 31.4 | |||||||||
| patients | 90 | 1.39 | 1.15 | 90 | 51 | 39 | 56.7 | |||||||||
| IHC CD96 tissue epithelium | LI | 0.011 | 0.629 | 1.28 | 0.325 | 141 | 0.003 | 0.733 | 0.529 | 0.733 | 0.529 | |||||
| controls | 51 | 0.85 | 1.41 | 51 | 24 | 27 | 47.1 | |||||||||
| patients | 90 | 1.09 | 1.00 | 90 | 66 | 24 | 73.3 | |||||||||
| IHC CD96 tissue stroma | LI | 0.008 | 0.638 | 1.39 | 0.715 | 135 | 0.007 | 0.671 | 0.58 | 0.731 | 0.509 | |||||
| controls | 50 | 1.52 | 3.72 | 50 | 21 | 29 | 42.0 | |||||||||
| patients | 85 | 2.11 | 2.94 | 85 | 57 | 28 | 67.1 |
| N | Mean | SD | p-Value | ||
|---|---|---|---|---|---|
| PCR CD96_1 tissue | 93 | 0.258 | |||
| T-status | T1-T2 | 61 | 6.9 | 1.41 | |
| T3-T4 | 32 | 6.60 | 1.38 | ||
| IHC CD96 | 88 | 0.264 | |||
| T1-T2 | 51 | 1.52 | 1.23 | ||
| T3-T4 | 37 | 1.22 | 1.03 | ||
| PCR CD96_1 tissue | 94 | 0.417 | |||
| N-status | N0 | 53 | 6.91 | 1.46 | |
| N+ | 41 | 6.64 | 1.31 | ||
| IHC CD96 | 87 | 0.315 | |||
| N0 | 50 | 1.52 | 1.23 | ||
| N+ | 37 | 1.26 | 1.06 | ||
| PCR CD96_1 tissue | 88 | 0.975 | |||
| L-status | L0 | 70 | 6.77 | 1.44 | |
| L1 | 18 | 6.80 | 1.47 | ||
| IHC CD96 | 80 | 0.336 | |||
| L0 | 64 | 1.50 | 1.19 | ||
| L1 | 16 | 1.25 | 1.11 | ||
| PCR CD96_1 tissue | 87 | 0.322 | |||
| Pn-status | Pn0 | 59 | 6.68 | 1.28 | |
| Pn1 | 28 | 7.18 | 1.60 | ||
| IHC CD96 | 78 | 0.090 | |||
| Pn0 | 52 | 1.61 | 1.24 | ||
| Pn1 | 26 | 1.12 | 1.02 | ||
| PCR CD96_1 tissue | 92 | 0.606 | |||
| grading | G1 | 9 | 6.56 | 1.17 | |
| G2 | 46 | 6.65 | 1.38 | ||
| G3 | 37 | 7.01 | 1.51 | ||
| IHC CD96 | 81 | 0.445 | |||
| G1 | 4 | 2.27 | 1.76 | ||
| G2 | 47 | 1.42 | 1.16 | ||
| G3 | 30 | 1.35 | 1.13 |
| CD96_1 PCR Tissue | ||
|---|---|---|
| CD96 IHC tissue | Spearman correlation | −0.631 |
| p-value | 0.005 * | |
| n | 18 | |
| CD68 PCR tissue | Spearman correlation | 0.174 |
| p-value | 0.269 | |
| n | 27 | |
| CD163 PCR tissue | Spearman correlation | 0.483 |
| p-value | 0.006 * | |
| n | 31 | |
| PD-1 PCR tissue | Spearman correlation | 0.636 |
| p-value | <0.001 * | |
| n | 129 | |
| PDL1_4 PCR tissue | Spearman correlation | 0.523 |
| p-value | <0.001 * | |
| n | 155 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trumet, L.; Weber, M.; Hahn, A.; Kunater, L.; Geppert, C.; Glajzer, J.; Struckmeier, A.-K.; Möst, T.; Lutz, R.; Kesting, M.; et al. The Immune Checkpoint Receptor CD96: A Local and Systemic Immune Modulator in Oral Cancer? Cancers 2023, 15, 2126. https://doi.org/10.3390/cancers15072126
Trumet L, Weber M, Hahn A, Kunater L, Geppert C, Glajzer J, Struckmeier A-K, Möst T, Lutz R, Kesting M, et al. The Immune Checkpoint Receptor CD96: A Local and Systemic Immune Modulator in Oral Cancer? Cancers. 2023; 15(7):2126. https://doi.org/10.3390/cancers15072126
Chicago/Turabian StyleTrumet, Leah, Manuel Weber, Alina Hahn, Lina Kunater, Carol Geppert, Jacek Glajzer, Ann-Kristin Struckmeier, Tobias Möst, Rainer Lutz, Marco Kesting, and et al. 2023. "The Immune Checkpoint Receptor CD96: A Local and Systemic Immune Modulator in Oral Cancer?" Cancers 15, no. 7: 2126. https://doi.org/10.3390/cancers15072126
APA StyleTrumet, L., Weber, M., Hahn, A., Kunater, L., Geppert, C., Glajzer, J., Struckmeier, A.-K., Möst, T., Lutz, R., Kesting, M., & Ries, J. (2023). The Immune Checkpoint Receptor CD96: A Local and Systemic Immune Modulator in Oral Cancer? Cancers, 15(7), 2126. https://doi.org/10.3390/cancers15072126








