Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
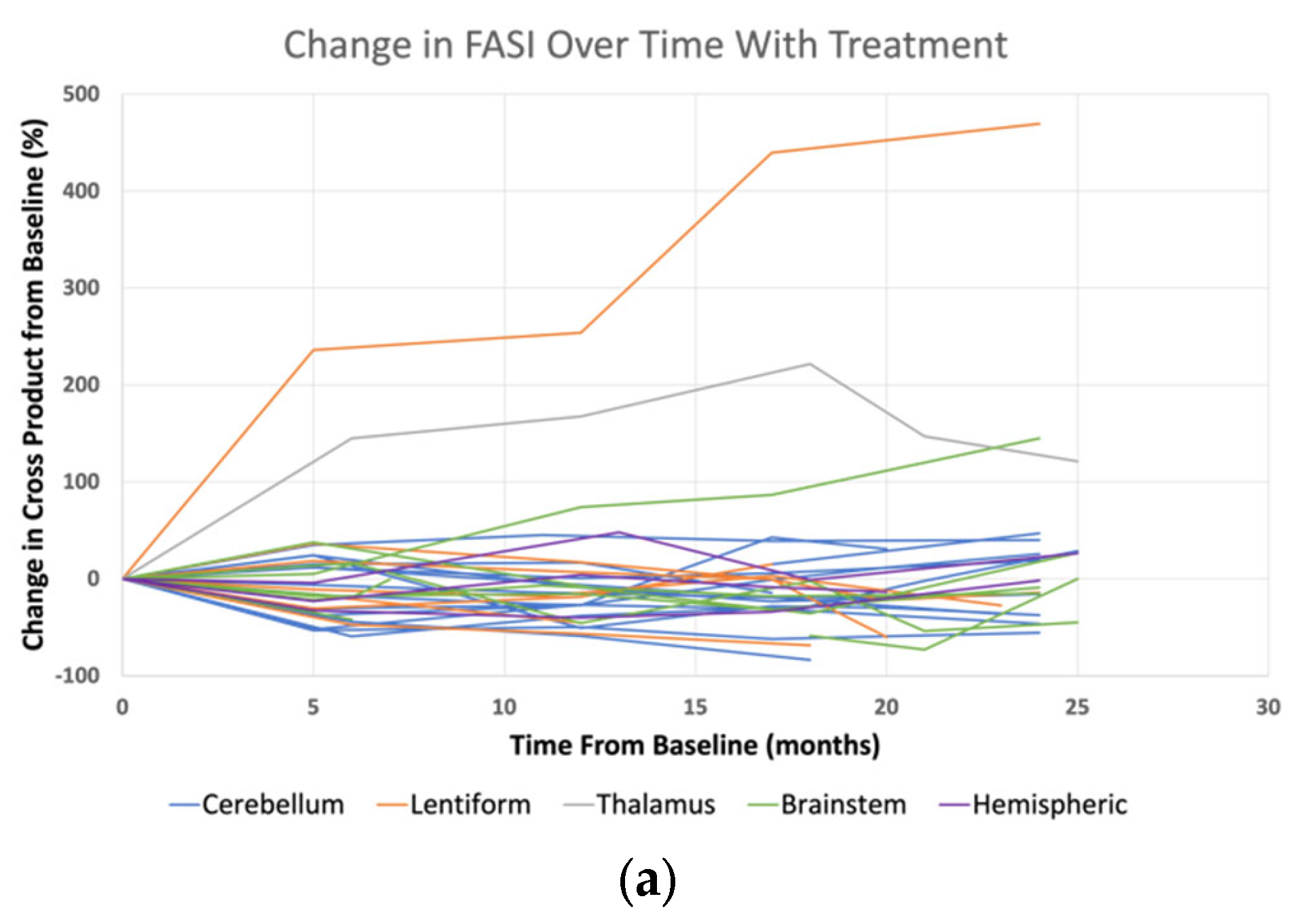
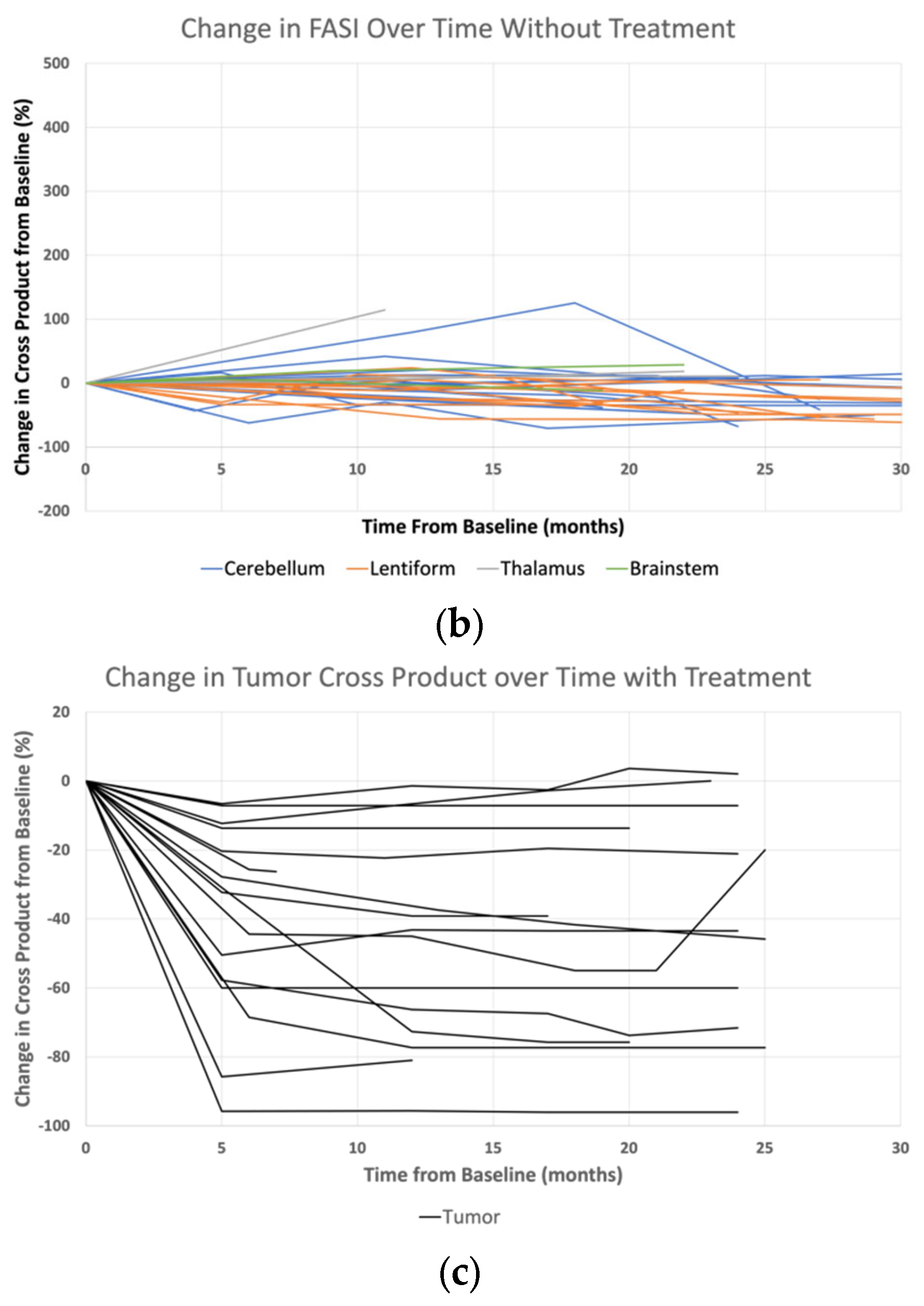
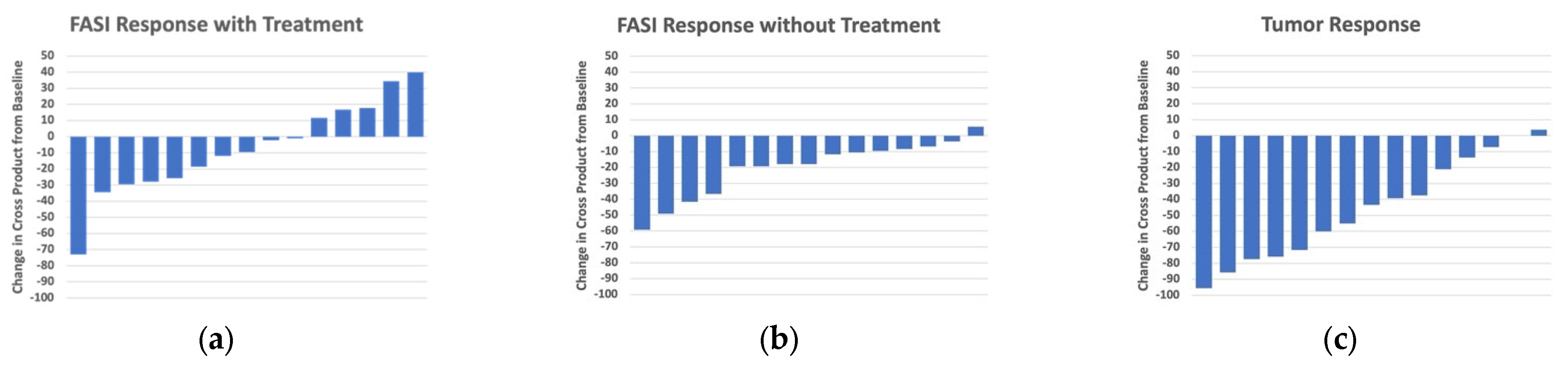
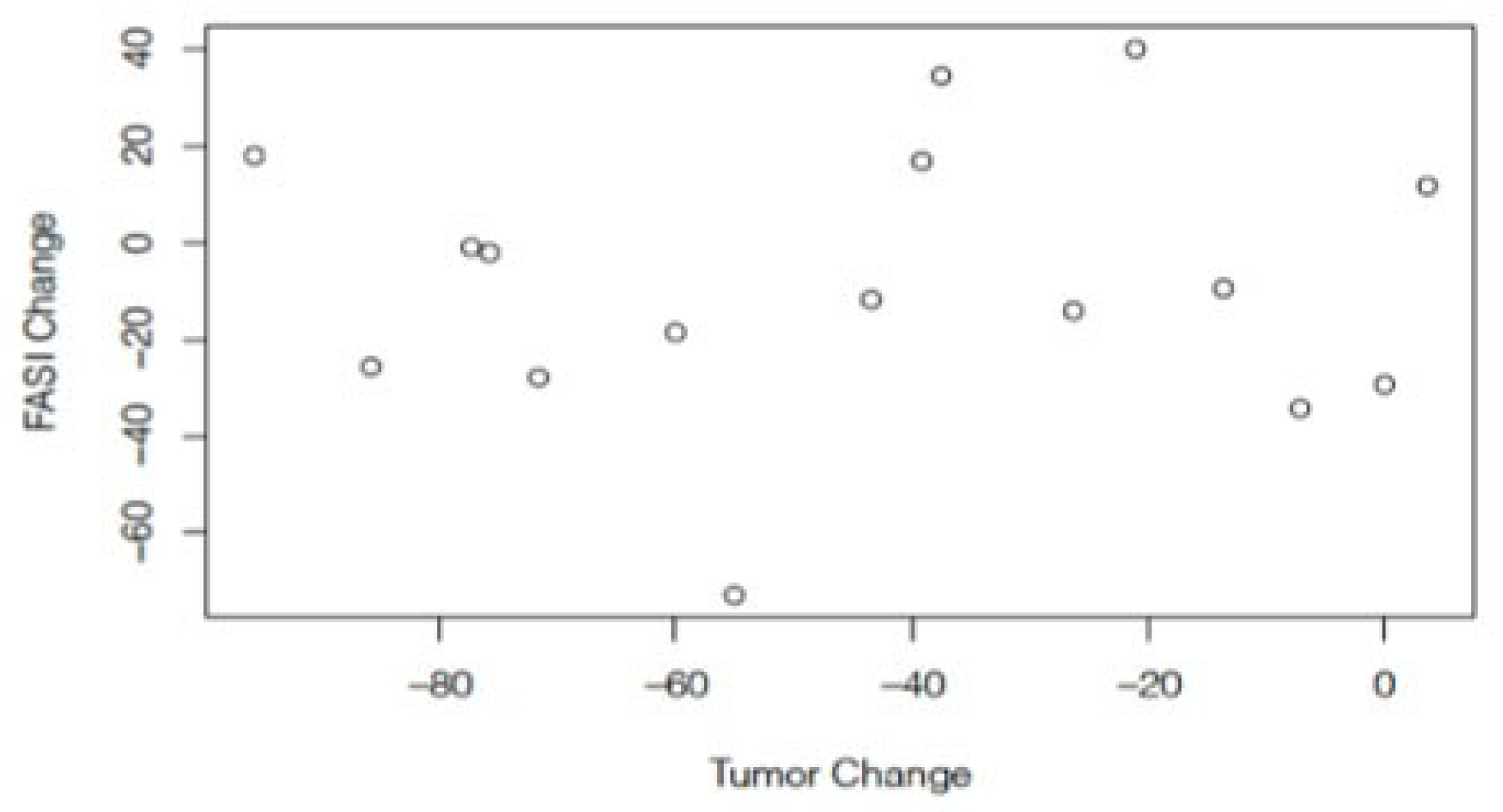
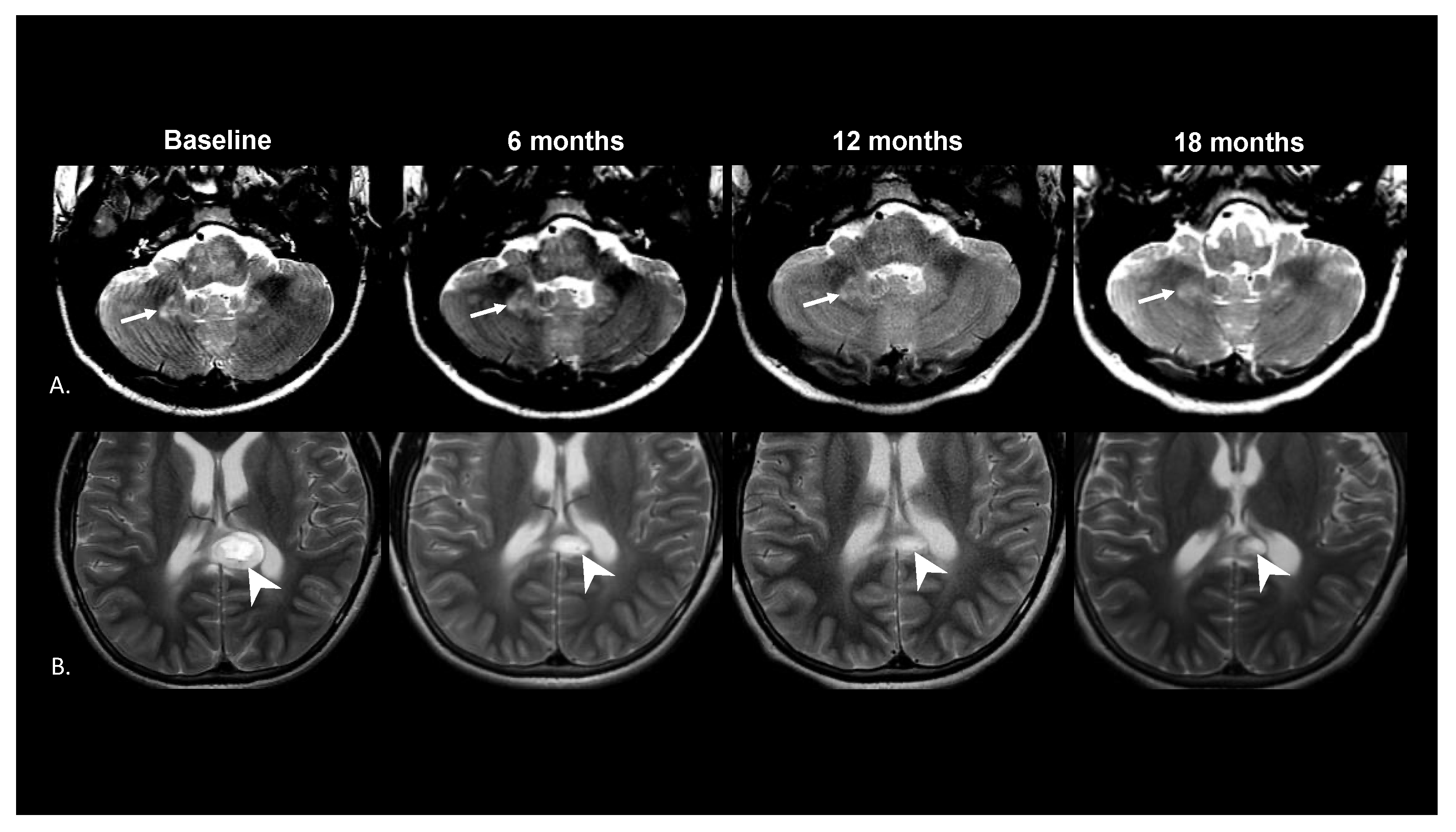
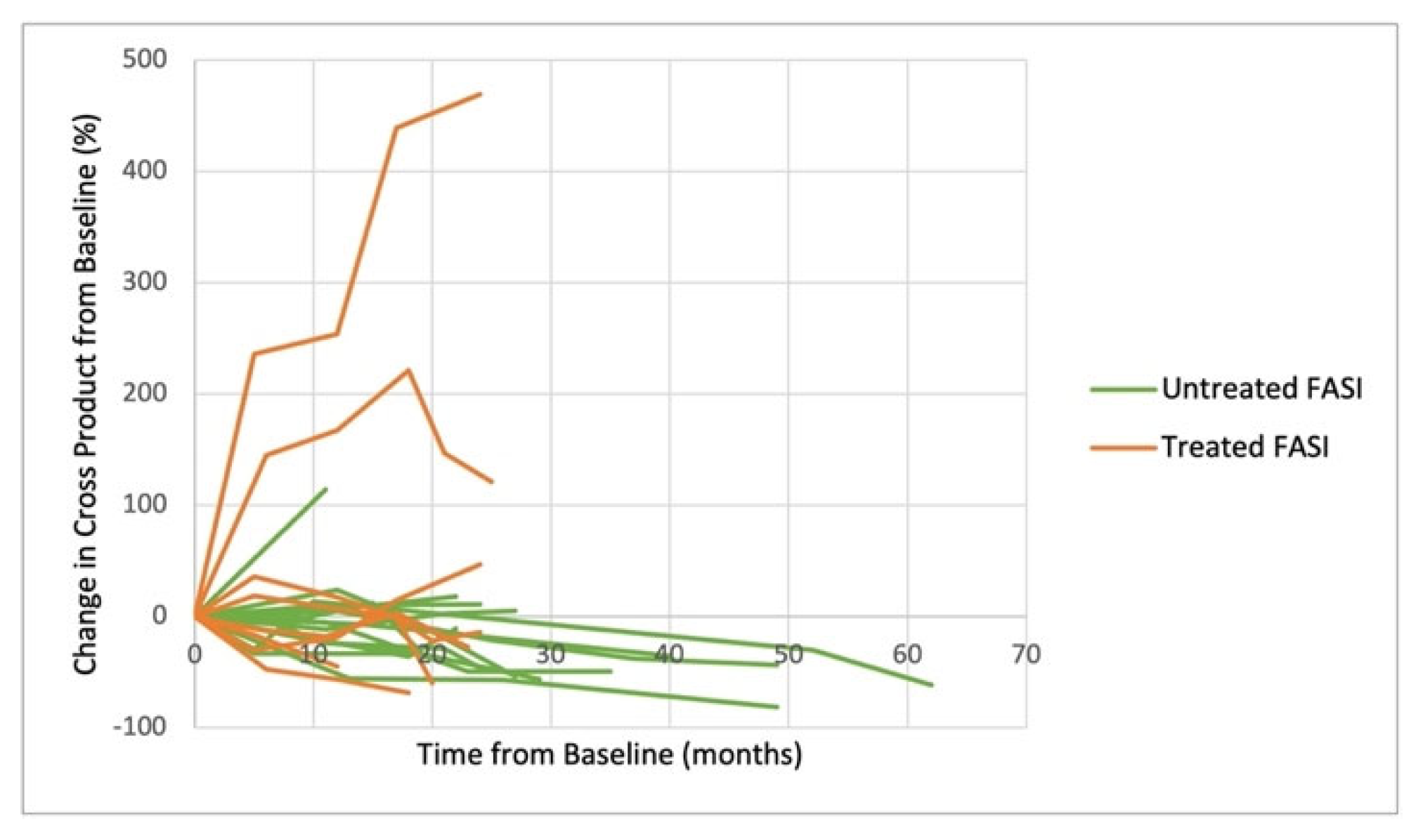
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uusitalo, E.; Leppavirta, J.; Koffert, A.; Suominen, S.; Vahtera, J.; Vahlberg, T.; Poyhonen, M.; Peltonen, J.; Peltonen, S. Incidence and mortality of neurofibromatosis: A total population study in Finland. J. Investig. Dermatol. 2015, 135, 904–906. [Google Scholar] [CrossRef]
- Fangusaro, J.; Onar-Thomas, A.; Young Poussaint, T.; Wu, S.; Ligon, A.H.; Lindeman, N.; Banerjee, A.; Packer, R.J.; Kilburn, L.B.; Goldman, S.; et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol. 2019, 20, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, J.; Shah, A.C.; Sato, A.; Morris, S.M.; McKinstry, R.C.; Listernick, R.; Packer, R.J.; Fisher, M.J.; Gutmann, D.H. A multi-institutional study of brainstem gliomas in children with neurofibromatosis type 1. Neurology 2017, 88, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D.; Blaser, S.; Mukonoweshuro, W.; Armstrong, D.; Milo-Mason, G.; Cheung, S. Neurofibromatosis bright objects in children with neurofibromatosis type 1: A proliferative potential? Pediatrics 1999, 104, e49. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.S.; Hossain, S.; Gorun, S.; Alqublan, L.; Bunge, M.; Rozovsky, K. Cerebellar radiological abnormalities in children with neurofibromatosis type 1: Part 2—A neuroimaging natural history study with clinical correlations. Cerebellum Ataxias 2018, 5, 13. [Google Scholar] [CrossRef]
- Calvez, S.; Levy, R.; Calvez, R.; Roux, C.J.; Grevent, D.; Purcell, Y.; Beccaria, K.; Blauwblomme, T.; Grill, J.; Dufour, C.; et al. Focal Areas of High Signal Intensity in Children with Neurofibromatosis Type 1: Expected Evolution on MRI. AJNR Am. J. Neuroradiol. 2020, 41, 1733–1739. [Google Scholar] [CrossRef]
- North, K.N.; Riccardi, V.; Samango-Sprouse, C.; Ferner, R.; Moore, B.; Legius, E.; Ratner, N.; Denckla, M.B. Cognitive function and academic performance in neurofibromatosis. 1: Consensus statement from the NF1 Cognitive Disorders Task Force. Neurology 1997, 48, 1121–1127. [Google Scholar] [CrossRef]
- Carvalho, I.; Quintas-Neves, M.; Pinto, J.; Santos, A.F.; Pereira, J. Primary Progressive Multiple Sclerosis in a Portuguese Patient With Neurofibromatosis Type 1. Cureus 2021, 13, e20561. [Google Scholar] [CrossRef]
- Hervey-Jumper, S.L.; Singla, N.; Gebarski, S.S.; Robertson, P.; Maher, C.O. Diffuse pontine lesions in children with neurofibromatosis type 1: Making a case for unidentified bright objects. Pediatr. Neurosurg. 2013, 49, 55–59. [Google Scholar] [CrossRef]
- Santoro, C.; Picariello, S.; Palladino, F.; Spennato, P.; Melis, D.; Roth, J.; Cirillo, M.; Quaglietta, L.; D’Amico, A.; Gaudino, G.; et al. Retrospective Multicentric Study on Non-Optic CNS Tumors in Children and Adolescents with Neurofibromatosis Type 1. Cancers 2020, 12, 1426. [Google Scholar] [CrossRef]
- Payne, J.M.; Pickering, T.; Porter, M.; Oates, E.C.; Walia, N.; Prelog, K.; North, K.N. Longitudinal assessment of cognition and T2-hyperintensities in NF1: An 18-year study. Am. J. Med. Genet. A 2014, 164A, 661–665. [Google Scholar] [CrossRef]
- Walsh, K.S.; Wolters, P.L.; Widemann, B.C.; Del Castillo, A.; Sady, M.D.; Inker, T.; Roderick, M.C.; Martin, S.; Toledo-Tamula, M.A.; Struemph, K.; et al. Impact of MEK Inhibitor Therapy on Neurocognitive Functioning in NF1. Neurol. Genet. 2021, 7, e616. [Google Scholar] [CrossRef] [PubMed]
- Kraut, M.A.; Gerring, J.P.; Cooper, K.L.; Thompson, R.E.; Denckla, M.B.; Kaufmann, W.E. Longitudinal evolution of unidentified bright objects in children with neurofibromatosis-1. Am. J. Med. Genet. A 2004, 129A, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Pfister, S.; Bouffet, E.; Avery, R.; Bandopadhayay, P.; Bornhorst, M.; Bowers, D.C.; Ellison, D.; Fangusaro, J.; Foreman, N.; et al. Pediatric low-grade gliomas: Implications of the biologic era. Neuro. Oncol. 2017, 19, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.W.; Kieran, M.W.; Bouffet, E.; Alexandrescu, S.; Bandopadhayay, P.; Bornhorst, M.; Ellison, D.; Fangusaro, J.; Fisher, M.J.; Foreman, N.; et al. Pediatric low-grade gliomas: Next biologically driven steps. Neuro. Oncol. 2018, 20, 160–173. [Google Scholar] [CrossRef]
- Prada, C.E.; Hufnagel, R.B.; Hummel, T.R.; Lovell, A.M.; Hopkin, R.J.; Saal, H.M.; Schorry, E.K. The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type 1. J. Pediatr. 2015, 167, 851–856.e1. [Google Scholar] [CrossRef]
- Billiet, T.; Madler, B.; D’Arco, F.; Peeters, R.; Deprez, S.; Plasschaert, E.; Leemans, A.; Zhang, H.; den Bergh, B.V.; Vandenbulcke, M.; et al. Characterizing the microstructural basis of “unidentified bright objects” in neurofibromatosis type 1: A combined in vivo multicomponent T2 relaxation and multi-shell diffusion MRI analysis. Neuroimage Clin. 2014, 4, 649–658. [Google Scholar] [CrossRef]
- Wilkinson, I.D.; Griffiths, P.D.; Wales, J.K. Proton magnetic resonance spectroscopy of brain lesions in children with neurofibromatosis type 1. Magn. Reson. Imaging 2001, 19, 1081–1089. [Google Scholar] [CrossRef]
- Griffith, J.L.; Morris, S.M.; Mahdi, J.; Goyal, M.S.; Hershey, T.; Gutmann, D.H. Increased prevalence of brain tumors classified as T2 hyperintensities in neurofibromatosis 1. Neurol. Clin. Pract. 2018, 8, 283–291. [Google Scholar] [CrossRef]
- Hyman, S.L.; Shores, A.; North, K.N. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 2005, 65, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.D.; Slopis, J.M.; Schomer, D.; Jackson, E.F.; Levy, B.M. Neuropsychological significance of areas of high signal intensity on brain MRIs of children with neurofibromatosis. Neurology 1996, 46, 1660–1668. [Google Scholar] [CrossRef]
- Goh, W.H.; Khong, P.L.; Leung, C.S.; Wong, V.C. T2-weighted hyperintensities (unidentified bright objects) in children with neurofibromatosis 1: Their impact on cognitive function. J. Child Neurol. 2004, 19, 853–858. [Google Scholar] [CrossRef]
- DiPaolo, D.P.; Zimmerman, R.A.; Rorke, L.B.; Zackai, E.H.; Bilaniuk, L.T.; Yachnis, A.T. Neurofibromatosis type 1: Pathologic substrate of high-signal-intensity foci in the brain. Radiology 1995, 195, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Ferraz-Filho, J.R.; Jose da Rocha, A.; Muniz, M.P.; Souza, A.S.; Goloni-Bertollo, E.M.; Pavarino-Bertelli, E.C. Unidentified bright objects in neurofibromatosis type 1: Conventional MRI in the follow-up and correlation of microstructural lesions on diffusion tensor images. Eur. J. Paediatr. Neurol. 2012, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Russo, C.; Cascone, D.; Mazio, F.; Santoro, C.; Covelli, E.M.; Cinalli, G. Non-Oncological Neuroradiological Manifestations in NF1 and Their Clinical Implications. Cancers 2021, 13, 1831. [Google Scholar] [CrossRef]
- Maaly, M.; Baghdady, A.; El-wakeel, A.; Mousa, W. The role of diffusion-weighted MRI in the evaluation and differentiation of space-occupying brain lesions. Menoufia Med. J. 2016, 29, 303. [Google Scholar] [CrossRef]
- Szudek, J.; Friedman, J.M. Unidentified bright objects associated with features of neurofibromatosis 1. Pediatr. Neurol. 2002, 27, 123–127. [Google Scholar] [CrossRef]
- Gill, D.S.; Hyman, S.L.; Steinberg, A.; North, K.N. Age-related findings on MRI in neurofibromatosis type 1. Pediatr. Radiol. 2006, 36, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Ostendorf, A.P.; McKinstry, R.C.; Shimony, J.S.; Gutmann, D.H. Teaching NeuroImages: T2 hyperintensities in neurofibromatosis type 1. Neurology 2013, 80, e215–e216. [Google Scholar] [CrossRef]
- Feldmann, R.; Schuierer, G.; Wessel, A.; Neveling, N.; Weglage, J. Development of MRI T2 hyperintensities and cognitive functioning in patients with neurofibromatosis type 1. Acta Paediatr. 2010, 99, 1657–1660. [Google Scholar] [CrossRef]
- Fisher, M.J.; Jones, D.T.W.; Li, Y.; Guo, X.; Sonawane, P.S.; Waanders, A.J.; Phillips, J.J.; Weiss, W.A.; Resnick, A.C.; Gosline, S.; et al. Integrated molecular and clinical analysis of low-grade gliomas in children with neurofibromatosis type 1 (NF1). Acta Neuropathol. 2021, 141, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Schwetye, K.E.; Gutmann, D.H. Cognitive and behavioral problems in children with neurofibromatosis type 1: Challenges and future directions. Expert Rev. Neurother. 2014, 14, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Baudou, E.; Nemmi, F.; Biotteau, M.; Maziero, S.; Assaiante, C.; Cignetti, F.; Vaugoyeau, M.; Audic, F.; Peran, P.; Chaix, Y. Are morphological and structural MRI characteristics related to specific cognitive impairments in neurofibromatosis type 1 (NF1) children? Eur. J. Paediatr. Neurol. 2020, 28, 89–100. [Google Scholar] [CrossRef]
- Chabernaud, C.; Sirinelli, D.; Barbier, C.; Cottier, J.P.; Sembely, C.; Giraudeau, B.; Deseille-Turlotte, G.; Lorette, G.; Barthez, M.A.; Castelnau, P. Thalamo-striatal T2-weighted hyperintensities (unidentified bright objects) correlate with cognitive impairments in neurofibromatosis type 1 during childhood. Dev. Neuropsychol. 2009, 34, 736–748. [Google Scholar] [CrossRef]
- Piscitelli, O.; Digilio, M.C.; Capolino, R.; Longo, D.; Di Ciommo, V. Neurofibromatosis type 1 and cerebellar T2-hyperintensities: The relationship to cognitive functioning. Dev. Med. Child Neurol. 2012, 54, 49–51. [Google Scholar] [CrossRef]
- Dombi, E.; Baldwin, A.; Marcus, L.J.; Fisher, M.J.; Weiss, B.; Kim, A.; Whitcomb, P.; Martin, S.; Aschbacher-Smith, L.E.; Rizvi, T.A.; et al. Activity of Selumetinib in Neurofibromatosis Type 1-Related Plexiform Neurofibromas. N. Engl. J. Med. 2016, 375, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Perreault, S.; Larouche, V.; Tabori, U.; Hawkin, C.; Lippe, S.; Ellezam, B.; Decarie, J.C.; Theoret, Y.; Metras, M.E.; Sultan, S.; et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer 2019, 19, 1250. [Google Scholar] [CrossRef]
| Selumetinib Treated Subjects | Untreated Controls | ||||||
|---|---|---|---|---|---|---|---|
| Pair # | Age at Initial MRI (Years) | Gender | Sum of Cross Products of all FASI (mm2) | Pair # | Age at Initial MRI (Years) | Gender | Sum of Cross Products of All FASI (mm2) |
| Located in Cerebellum Unless Otherwise Specified | Located in Cerebellum Unless Otherwise Specified | ||||||
| 1 | 3.5 | Male | 72.33 | 1 | 2.9 | Male | 81.13 |
| 2 | 5.7 | Male | 346.54 | 2 | 4.8 | Male | 67.6 |
| 3 | 6.7 | Female | 77.2 | 3 | 5.7 | Male | 74.1 |
| 4 | 6.8 | Male | 134.73 | 4 | 6.3 | Female | 127.56 |
| 5 | 7.3 | Male | 152.44 | 5 | 6.3 | Female | 411.09 |
| 6 | 7.5 | Female | 14.4 | 6 | 7.3 | Female | 239.17 |
| 7 | 9.8 | Male | 92.07 | 7 | 9 | Male | 92.78 |
| 8 | 10.2 | Female | 52.52 | 8 | 10 | Male | 134.89 |
| 9 | 11.7 | Male | 119.1 | 9 | 11.1 | Male | 216.83 |
| 10 | 11.8 | Male | 159.88 | 10 | 11.4 | Female | 159.3 |
| 11 * | 12.9 | Male | 606.48 | 11* | 12.9 | Male | 105.18 |
| 12 | 13.3 | Female | 142.65 | 12 | 13.8 | Male | 310.55 |
| 13 | 14.3 | Male | 240.8 | 13 | 14.6 | Female | 115.6 |
| 14 | 14.3 | Female | 267.56 | 14 | 14.2 | Female | 21.07 |
| 15 | 15.8 | Female | 121.06 | 15 | 15.7 | Female | 75 |
| 16 | 16.6 | Female | 162.33 | 16 | 16.5 | Male | 170.3 |
| Complete Response | Partial Response | Stable Disease | Progressive Disease | |
|---|---|---|---|---|
| FASI (treated) | 0 | 1 | 12 | 2 |
| FASI (untreated) | 0 | 1 | 14 | 0 |
| LGG (treated) | 0 | 7 | 8 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillay-Smiley, N.; Leach, J.; Lane, A.; Hummel, T.; Fangusaro, J.; de Blank, P. Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B. Cancers 2023, 15, 2109. https://doi.org/10.3390/cancers15072109
Pillay-Smiley N, Leach J, Lane A, Hummel T, Fangusaro J, de Blank P. Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B. Cancers. 2023; 15(7):2109. https://doi.org/10.3390/cancers15072109
Chicago/Turabian StylePillay-Smiley, Natasha, James Leach, Adam Lane, Trent Hummel, Jason Fangusaro, and Peter de Blank. 2023. "Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B" Cancers 15, no. 7: 2109. https://doi.org/10.3390/cancers15072109
APA StylePillay-Smiley, N., Leach, J., Lane, A., Hummel, T., Fangusaro, J., & de Blank, P. (2023). Evaluating Focal Areas of Signal Intensity (FASI) in Children with Neurofibromatosis Type-1 (NF1) Treated with Selumetinib on Pediatric Brain Tumor Consortium (PBTC)-029B. Cancers, 15(7), 2109. https://doi.org/10.3390/cancers15072109






