New Perspectives on Primary Prophylaxis of Invasive Fungal Infection in Children Undergoing Hematopoietic Stem Cell Transplantation: A 10-Year Retrospective Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Transplant Methods
2.3. IFI Definition, Monitoring and Antifungal Treatment
2.4. Clinical Outcomes and Definitions
2.5. Descriptive Statistics and Logistic Regression Analysis
3. Results
3.1. Clinical Characteristics of Patients (Table 1)
| Patient Characterisitcs | Total No. of Patients (%) | Invasive Fungal Infection | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Yes | No | ||||||
| No. of Patients (%) | No. of Patients (%) | ||||||
| 308 | 18 (5.8) | 290 (94.2) | |||||
| Gender | |||||||
| Male | 184 | 8 | 176 | ||||
| Female | 124 | 10 | 114 | ||||
| sd | sd | sd | |||||
| Median patient Age [min-max] | 8.6 (0.1–20) | 5.3 | 11.9 (2–19) | 5.6 | 8.4 (0.1–20) | 5.2 | 0.0089 |
| <10 years old | 188 (61) | 6 (33.3) | 182 (62.4) | 0.014 | |||
| Indication for HCT | |||||||
| Malignant disease | 176 (57) | 13 (72.2) | 163 (56.2) | ||||
| ALL | 86 (27.9) | 5 (27.8) | 81 (27.9) | ||||
| AML | 50 (16.2) | 2 (11.1) | 48 (16.6) | ||||
| CR2 or more | 83 (26.9) | 7 (38.9) | 76 (26.2) | 0.022 | |||
| Non-Malignant disease | 127 (41.2) | 5 (27.8) | 122 (42.1) | ||||
| Primary immunodeficiencies | 43 (14.0) | 1 (5.5) | 42 (14.5) | ||||
| Thalassemia | 18 (5.8) | 0 (0) | 18 (6.2) | ||||
| Sickle cell disease | 16 (5.2) | 0 (0) | 16 (5.5) | ||||
| Metabolic disorders | 5 (1.6) | 0 (0) | 5 (1.7) | ||||
| Type of donor | |||||||
| Matched related donor | 106 (34.4) | 6 (33.3) | 100 (34.5) | ||||
| Unrelated donor | 188 (61.0) | 12 (66.6) | 176 (60.7) | ||||
| Haplo-identical donor | 14 (4.5) | 0 (0) | 14 (4.8) | ||||
| Stem cell source | |||||||
| Bone marrow | 229 (74.3) | 11 (61.1) | 218 (75.2) | ||||
| PBSC | 19 (6.2) | 2 (11.1) | 17 (5.9) | ||||
| Cord blood unit | 56 (18.2) | 5 (27.8) | 51 (17.6) | ||||
| Mixed | 4 (1.3) | 0 (0) | 4 (1.4) | ||||
| Type of conditionning | |||||||
| Myeloablative regimen | 293 (95.1) | 17 (94.4) | 276 (95.2) | ||||
| With Busulfan | 185 (60.1) | 8 (44.4) | 177 (61) | ||||
| With TBI | 79 (25.6) | 9 (50.0) | 70 (24.1) | 0.007 | |||
| RIC | 15 (4.9) | 1 (5.55) | 14 (6.7) | ||||
| Acute GVHD occurrence | 186 (60.4) | 9 (50.0) | 177 (61) | ||||
| severe (grade III–IV) | 48 (15.6) | 4 (22.2) | 44 (15.2) | ||||
| Chronic GVHD occurrence | 69 (22.4) | 4 (22.2) | 65 (22.4) | ||||
| severe (grade III–IV) | 28 (9.1) | 0 (0) | 28 (9.7) | ||||
| High dose steroid administration after HCT * | 170 (55.2) | sd | 11 (61,1) | sd | 159 (54.8) | sd | |
| Mean duration of steroid (days) [min–max] | 90.8 (7–365) | 70 | 46 (30–214) | 70.5 | 91.3 (7–365) | 70.3 | |
| Immunosuppressive treatment used in 2nd line Treatment after high steroid administration | 63 (20.5) | 4 (22.2) | 59 (20.3) | ||||
| Relapse post-aHCT | 36 (11.7) | 2 (11.1) | 34 (11.7) | ||||
| Engraftment failure ** | 16 (5.2) | 2 (11.1) | 14 (4.8) | ||||
| sd | sd | sd | |||||
| Mean neutrophil recovery [min-max] | 22.9 (10–79) | 9.3 | 19.3 (12–31) | 6.2 | 23.2 (10–79) | 9.4 | |
| Antifungal prophylaxis | 50 (16.2) | 2 (11.1) | 48 (16.6) | ||||
| Primary prophylaxis | 23 (7.5) | 1 (5.5) | 22 (7.6) | ||||
| Secondary prophylaxis | 27 (8.8) | 1 (5.5) | 26 (9) | ||||
3.2. IFIs: Incidence and Characteristics of IFI Patients
3.3. Determinants of IFI Identified by Logistic Regression Analysis (Table 4)
| Risk Factors | No. of Patients | Invasive Fungal Infection (IFI) | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | OR | 95% CI | p-Value | OR | 95% CI | p-Value | ||
| Age at SCT | |||||||||
| >10 years old | 120 | 12 | 10 | 0.29 | 0.10–0.81 | 0.014 | 0.29 | 0.09–0.83 | 0.022 |
| Malignant underlying disease | 176 | 13 | 7.4 | 2.02 | 0.70–5.83 | 0.173 | |||
| ≥CR2 or more | 83 | 7 | 8.4 | 2.25 | 0.85–5.91 | 0.107 | |||
| CMV risk reactivation * | 42 | 0 | 0 | NA | NA | NA | |||
| CMV reactivation or disease | 96 | 7 | 7.3 | 1.42 | 0.53–3.79 | 0.486 | |||
| ADV disease | 20 | 2 | 10 | 1.87 | 0.39–8.79 | 0.456 | |||
| No neutrophils engraftment (counted as continous variable) | 8 | 2 | 25 | 0.93 | 0.87–0.98 | 0.011 | 0.93 | 0.87–0.99 | 0.024 |
| No lymphocyte reconstitution (counted as continous variable) | 95 | 11 | 11.6 | 0.99 | 0.98–1 | 0.018 | |||
| Type of donor | |||||||||
| Matched related donor | 106 | 6 | 5.7 | 0.9 | 0.3–2.2 | 0.78 | |||
| Unrelated donor | 188 | 12 | 6.4 | 1.1 | 0.5–2.8 | 0.79 | |||
| Haplo-identical donor | 14 | 0 | 0 | NA | NA | NA | |||
| Stem cell source | |||||||||
| Bone marrow | 229 | 11 | 4.8 | 0.6 | 0.2–1.5 | 0.25 | |||
| PBSC | 19 | 2 | 10.5 | 1.6 | 0.3–7.4 | 0.6 | |||
| Cord blood unit | 56 | 5 | 8.9 | 1.7 | 0.6–4.7 | 0.29 | |||
| Mixed | 4 | 0 | 0 | NA | NA | NA | |||
| Type of conditionning | |||||||||
| Myeloablative regimen | 293 | 17 | 5.8 | 0.4 | 0.1–2.2 | 0.382 | |||
| Acute GVHD occurrence | 186 | 9 | 4.8 | 0.79 | 0.31–1.96 | 0.612 | |||
| severe (grade III–IV) | 48 | 4 | 8.3 | 1.39 | 0.44–4.34 | 0.585 | |||
| Chronic GVHD occurrence | 69 | 4 | 5.8 | 1.4 | 0.5–3.6 | 0.54 | |||
| severe (grade III–IV) | 28 | 0 | 0 | NA | NA | NA | |||
| Total immunosuppressive treatment used other than cyclosporine after HCT ** | 233 | 15 | 6.4 | 1.4 | 0.5–3.6 | 0.54 | |||
| High dose steroid administration after HCT | 170 | 11 | 6.5 | 1.48 | 0.57–3.83 | 0.405 | |||
| Antifungal prophylaxis | 50 | 2 | 4 | 0.79 | 0.25–2.45 | 0.681 | |||
| With prior history of IFI | 27 | 1 | 3.7 | 0.53 | 0.06–4.11 | 0.507 | |||
| Total antiviral drugs | 245 | 18 | 7.3 | 1.6 | 0.6–4.3 | 0.297 | |||
| Rituximab | 101 | 6 | 5.9 | 1.2 | 0.5–2.9 | 0.72 | |||
| Curative Antiviral treatment *** | 144 | 12 | 8.3 | 2.39 | 0.87–6.55 | 0.079 | 2.71 | 0.92–7.99 | 0.069 |
3.4. Secondary Outcomes
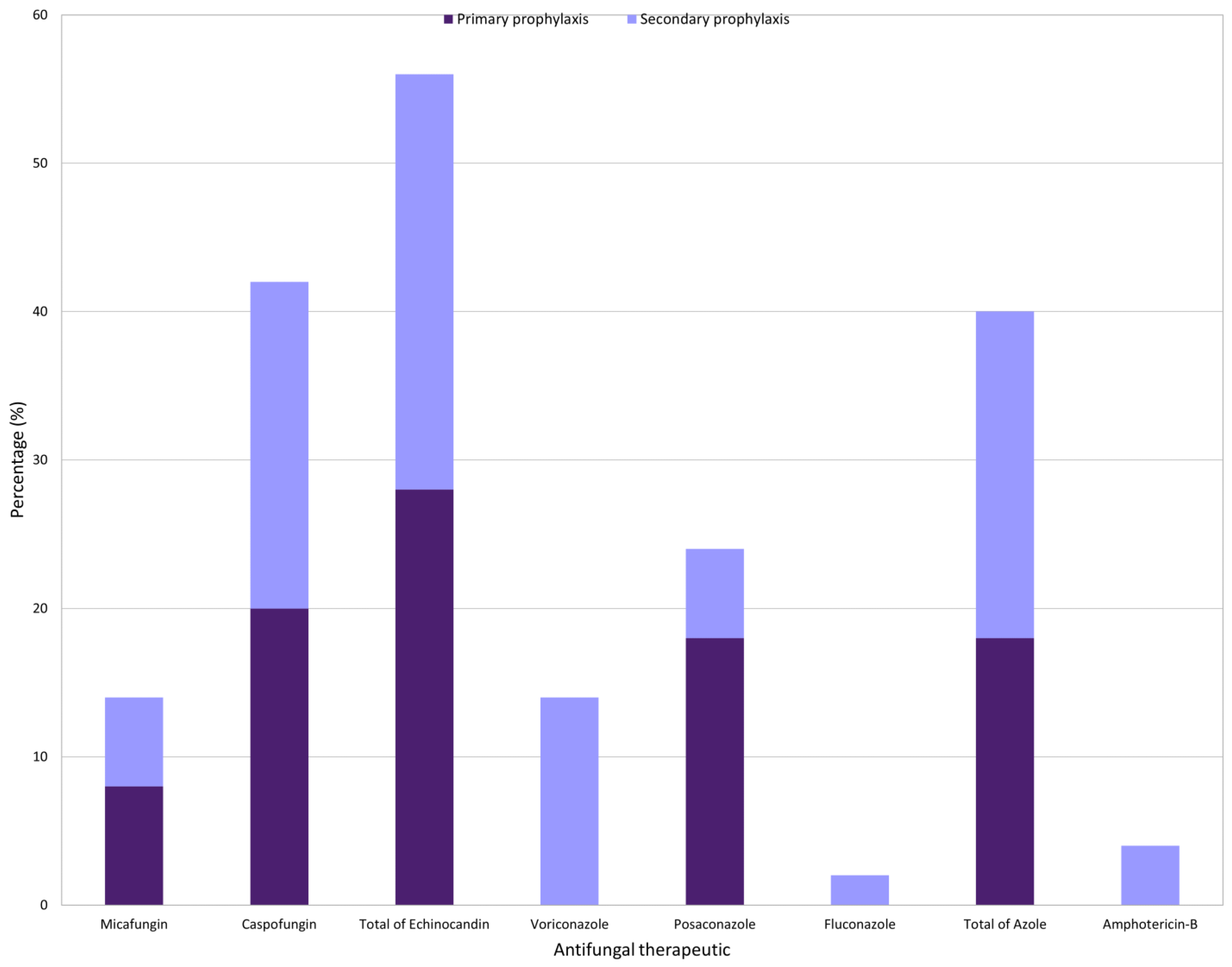
3.5. Antifungal Prophylaxis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biomedicine Agency, Hematopoietic Stem Cells, National HSCT Activity. Available online: https://rams.agence-biomedecine.fr/activite-nationale-de-greffe-de-csh-0 (accessed on 11 December 2022).
- Ardura, M.I. Overview of Infections Complicating Pediatric Hematopoietic Cell Transplantation. Infect. Dis. Clin. N. Am. 2018, 32, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Reddy, P. Current and emerging strategies for the prevention of graft-versus-host disease. Nat. Rev. Clin. Oncol. 2014, 11, 536–547. [Google Scholar] [CrossRef]
- Cesaro, S.; Tridello, G. Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur. J. Haematol. 2017, 99, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Kaneda, M. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: A 10-year analysis at a single institution at Japan. J. Pediatr. Hematol. Oncol. 2008, 30, 886–890. [Google Scholar] [CrossRef]
- Mor, M.; Gilad, G. Invasive fungal infections in pediatric oncology. Pediatr. Blood Cancer 2011, 56, 1092–1097. [Google Scholar] [CrossRef]
- Czyżewski, K.; Gałązka, P. Epidemiology and outcome of invasive fungal disease in children after hematopoietic cell transplantation or treated for malignancy: Impact of national programme of antifungal prophylaxis. Mycoses 2019, 62, 990–998. [Google Scholar] [CrossRef]
- Aftandilian, C.; Weinberg, K. Invasive Fungal Disease in Pediatric Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplant. J. Pediatr. Hematol. Oncol. 2016, 38, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.C.; Steinbach, W.J. Risks and outcomes of invasive fungal infections in pediatric patients undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2005, 36, 621–629. [Google Scholar] [CrossRef]
- Gomez, S.; Fynn, A.B. Early bacterial and fungal infection in children receiving allogeneic stem cell transplantation for acute lymphoblastic leukemia in Argentina. Pediatr. Transplant. 2018, 22, e13070. [Google Scholar] [CrossRef]
- Hale, K.A.; Shaw, P.J. Epidemiology of paediatric invasive fungal infections and a case-control study of risk factors in acute leukaemia or post stem cell transplant. Br. J. Haematol. 2010, 149, 263–272. [Google Scholar] [CrossRef]
- Kumar, J.; Singh, A. Prevalence and Predictors of Invasive Fungal Infections in Children with Persistent Febrile Neutropenia Treated for Acute Leukemia—A Prospective Study. Indian J. Pediatr. 2018, 85, 1090–1095. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Fisher, B. Clinical practice guideline for systemic antifungal prophylaxis in pediatric patients with cancer and hematopoietic stem-cell transplantation recipients. J. Clin. Oncol. 2020, 38, 3205–3216. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Schöning, S. Incidence and Outcome of Invasive Fungal Diseases in Children with Hematological Malignancies and/or Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Multicenter Study. Front. Microbiol. 2019, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.; Fisher, B. A Randomized Trial of Caspofungin vs Triazoles Prophylaxis for Invasive Fungal Disease in Pediatric Allogeneic Hematopoietic Cell Transplant. J. Pediatr. Infect. Dis. Soc. 2021, 10, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Garnica, M. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin. Microbiol. Infect. 2013, 19, 745–751. [Google Scholar] [CrossRef]
- Otto, W.R.; Green, A.M. Fungal infections in children with haematologic malignancies and stem cell transplant recipients. Br. J. Haematol. 2020, 189, 607–624. [Google Scholar] [CrossRef]
- Pongas, G.N.; Lewis, R.E. Voriconazole-associated zygomycosis: A significant consequence of evolving antifungal prophylaxis and immunosuppression practices? Clin. Microbiol. Infect. 2009, 15, 93–97. [Google Scholar] [CrossRef]
- Thomas, K.E.; Owens, C.M. The radiological spectrum of invasive aspergillosis in children: A 10-year review. Pediatr. Radiol. 2003, 33, 453–460. [Google Scholar] [CrossRef]
- Burgos, A.; Zaoutis, T.E. Pediatric invasive aspergillosis: A multicenter retrospective analysis of 139 contemporary cases. Pediatrics 2008, 121, e1286–e1294. [Google Scholar] [CrossRef]
- Steinbach, W.J. Pediatric aspergillosis: Disease and treatment differences in children. Pediatr. Infect. Dis. J. 2005, 24, 358–364. [Google Scholar] [CrossRef]
- Groll, H.A.; Pana, D. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet Oncol. 2021, 22, e254–e269. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.D. Galactomannan, β-D-Glucan, and Polymerase Chain Reaction-Based Assays for the Diagnosis of Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2016, 63, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Singhi, S.; Deep, A. Invasive candidiasis in pediatric intensive care units. Indian J. Pediatr. 2009, 76, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Olivier-Gougenheim, L.; Rama, N. Invasive fungal infections in immunocompromised children: Novel insight following a national study. J. Pediatr. 2021, 236, 204–210. [Google Scholar] [CrossRef]
- Hahn, T.; Cummings, K.M. Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect. Control Hosp. Epidemiol. 2002, 23, 525–531. [Google Scholar] [CrossRef]
- Libbrecht, C.; Goutagny, M.P. Impact of a change in protected environment on the occurrence of severe bacterial and fungal infections in children undergoing hematopoietic stem cell transplantation. Eur. J. Haematol. 2016, 97, 70–77. [Google Scholar] [CrossRef]
- Brunet, A.S.; Ploton, C. Low incidence of sepsis due to viridans Streptococci in a ten-year retrospective study of pediatric acute myeloid leukemia. Pediatr. Blood Cancer 2006, 47, 765–772. [Google Scholar] [CrossRef]
- Bleyzac, N.; Cuzzubbo, D. Improved outcome of children transplanted for high-risk leukemia by using a new strategy of cyclosporine-based GVHD prophylaxis. Bone Marrow Transplant. 2016, 51, 698–704. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Cahn, J.Y.; Klein, J.P. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: A joint Société Française de Greffe de Moëlle et Thérapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood 2005, 106, 1495–1500. [Google Scholar] [CrossRef]
- Thomas, E.D.; Storb, R. Bone-marrow transplantation (second of two parts). N. Engl. J. Med. 1975, 292, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Shenep, J.L. Aspergillosis in children with cancer: A 34-year experience. Clin. Infect. Dis. 1999, 29, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Toprak, S.K. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J. Infect. Chemother. 2016, 22, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Hol, J.; Wolfs, T. Predictors of invasive fungal infection in pediatric allogeneic hematopoietic SCT recipients. Bone Marrow Transplant. 2014, 49, 95–101. [Google Scholar] [CrossRef] [PubMed]
| Fungi | Early IFIs | Late IFIs | Total | |||
|---|---|---|---|---|---|---|
| n | % | n | % | N | % | |
| Candidosis | 7 | 2.3 | 0 | 0 | 7 | 2.3 |
| Aspergillosis | 7 | 2.3 | 3 | 1 | 10 | 3.3 |
| Mucormycosis | 2 | 0.6 | 0 | 0 | 2 | 0.6 |
| Trichodermosis | 0 | 0 | 1 | 0.3 | 1 | 0.3 |
| Total | 16 | 5.2 | 4 | 1.3 | 20 * | 6.5 |
| N° | M/F | Age (Years) | Underlying Disease | Status before HSCT | History of IFI Prior HSCT | IFI Classification | Fungus | HSCT—IFI Interval (Days) | Location | Treatment | Antifungal Prophylaxis | Intensive Care | Outcome After One Year of Monitoring |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 13.4 | B-ALL | CR3 | No | Proven | Mucormycosis (rhizomucor) | J20 | Lung | Amphotericin-B, Posaconazole | No | Yes | Deceased |
| 2 | F | 15 | SAA/HPN | _ | No | Proven | Aspergillus fumigatus | J62 | Disseminated | Caspofungin | No | Yes | Deceased |
| 3 | F | 13.4 | Hodgkin | CR2 | No | Proven | Candida dubliniensis Candida albicans | J29 | Disseminated | Amphotericin-B, 5-FU | No | Yes | Deceased |
| 4 | F | 0.8 | SCID | _ | No | Proven | Candida albicans | J166 | Blood | Caspofungin | No | No | Alive |
| 5 | F | 16.2 | Lymphoblastic T Lymphoma | CR2 | No | Proven | Aspergillus flavus | J37 | Lung | Caspofungin | No | Yes | Deceased |
| 6 | F | 7.4 | Fanconi Anemia | _ | No | Proven | Candida parapsilosis | J66 | Blood | Fluconazole | No | Yes | Deceased |
| 7 | F | 15 | AML | CR1 | No | Proven | Candida dubliensis | J5 | Blood | Caspofungin, Fluconazole | No | No | Alive |
| 8 | M | 3 | SAA/MDS | _ | No | Proven | Mucormycosis | J133 | Disseminated | none (palliative cares) | No | Yes | Deceased |
| 9 | M | 18 | Acute CML | CR1 | No | Proven | Trichodermosis | J213 | Lung | Voriconazole | Primary (Posaconazole) | Yes | Alive |
| 10 | M | 14 | B-ALL | CR1 | No | Proven | Candida tropicalis | J48 | Lung | Fluconazole | No | No | Alive |
| 11 | F | 10 | Anaplasic Lymphoma | CR1 | No | Proven | Candida Kefyr | J6 | Blood | Amphotericin-B, Caspofungin | No | No | Alive |
| 12 | M | 16.9 | Hodgkin | CR2 | No | Proven | Candida glabrata Candida albicans | J34 | Lung | Caspofungin | No | No | Alive |
| 13 | F | 8 | MDS | No | Proven | Aspergillus fumigatus | J304 | Intestinal | Amphotericin-B | No | Yes | Deceased | |
| 14 | F | 13.4 | Hodgkin | CR2 | No | Probable | Aspergillosis | J29 | Disseminated | Amphotericin-B, 5-FU | No | Yes | Deceased |
| 15 | F | 19.75 | T-ALL | CR2 | Yes | Probable | Aspergillosis | J90 | Lung | Voriconazole | Secondary (Amphotericin-B) | Yes | Alive |
| 16 | F | 19.75 | T-ALL | CR2 | Yes | Probable | Aspergillosis | J183 | Lung | Amphotericin-B | Secondary (Amphotericin-B) | Yes | Alive |
| 17 | M | 2 | JMML | CR2 | No | Probable | Aspergillosis | J2 | Lung | Amphotericin-B | No | No | Deceased |
| 18 | M | 16.3 | SAA | _ | No | Probable | Aspergillosis | J152 | Lung | Voriconazole | No | Yes | Alive |
| 19 | F | 12 | AML | CR2 | No | Probable | Aspergillosis | J52 | Lung | Voriconazole | No | No | Alive |
| 20 | F | 12.9 | B-ALL | CR1 | No | Probable | Aspergillosis | J244 | Lung | Voriconazole, caspofungin | No | No | Alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricard, N.; Zebali, L.; Renard, C.; Goutagny, M.-P.; Benezech, S.; Bertrand, Y.; Philippe, M.; Domenech, C. New Perspectives on Primary Prophylaxis of Invasive Fungal Infection in Children Undergoing Hematopoietic Stem Cell Transplantation: A 10-Year Retrospective Cohort Study. Cancers 2023, 15, 2107. https://doi.org/10.3390/cancers15072107
Ricard N, Zebali L, Renard C, Goutagny M-P, Benezech S, Bertrand Y, Philippe M, Domenech C. New Perspectives on Primary Prophylaxis of Invasive Fungal Infection in Children Undergoing Hematopoietic Stem Cell Transplantation: A 10-Year Retrospective Cohort Study. Cancers. 2023; 15(7):2107. https://doi.org/10.3390/cancers15072107
Chicago/Turabian StyleRicard, Noémi, Lelia Zebali, Cécile Renard, Marie-Pierre Goutagny, Sarah Benezech, Yves Bertrand, Michael Philippe, and Carine Domenech. 2023. "New Perspectives on Primary Prophylaxis of Invasive Fungal Infection in Children Undergoing Hematopoietic Stem Cell Transplantation: A 10-Year Retrospective Cohort Study" Cancers 15, no. 7: 2107. https://doi.org/10.3390/cancers15072107
APA StyleRicard, N., Zebali, L., Renard, C., Goutagny, M.-P., Benezech, S., Bertrand, Y., Philippe, M., & Domenech, C. (2023). New Perspectives on Primary Prophylaxis of Invasive Fungal Infection in Children Undergoing Hematopoietic Stem Cell Transplantation: A 10-Year Retrospective Cohort Study. Cancers, 15(7), 2107. https://doi.org/10.3390/cancers15072107







