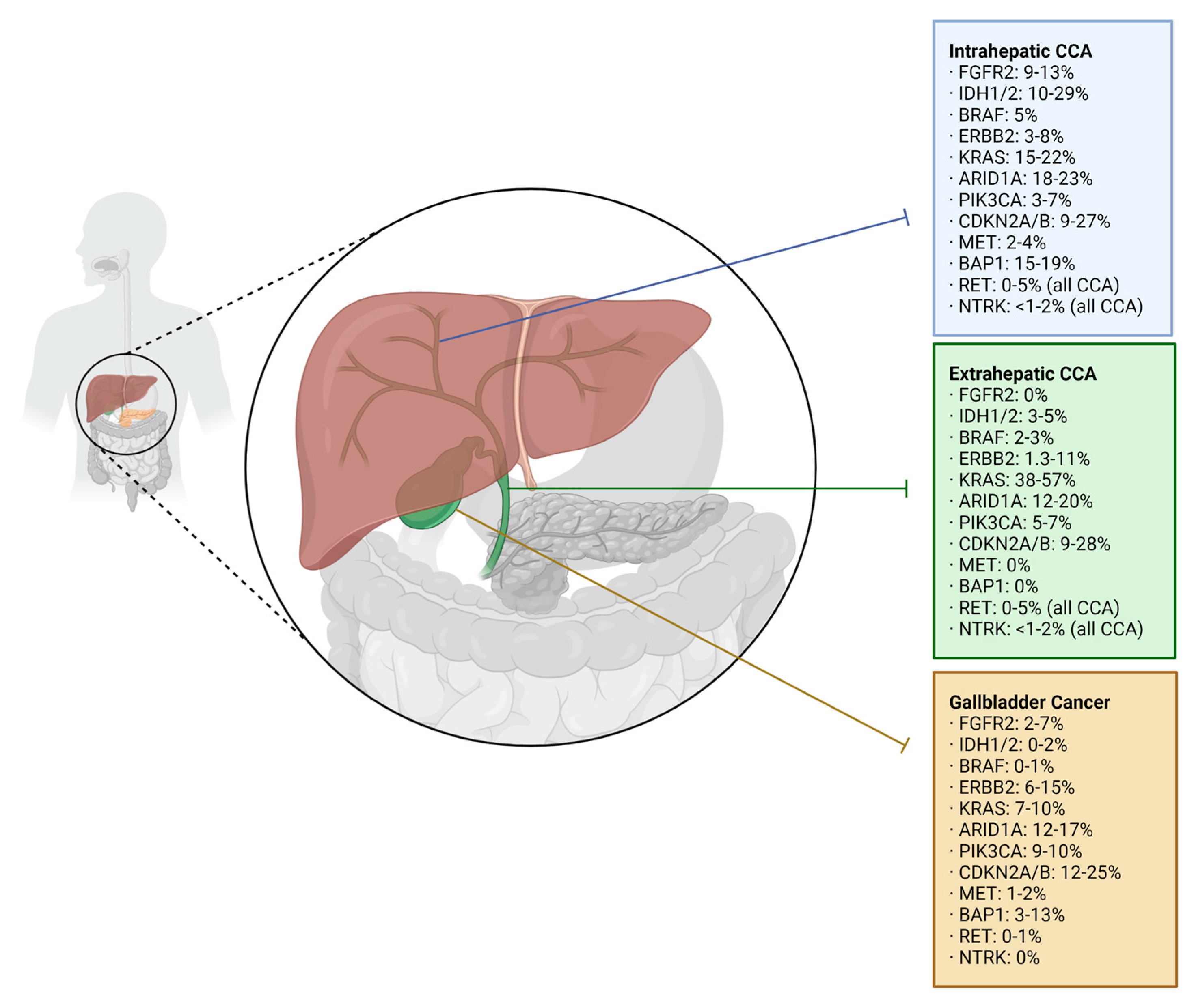
Precision Oncology Targets in Biliary Tract Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor-Specific Treatments
2.1. Fibroblast Growth Factor Receptor (FGFR) Inhibitors
2.2. Isocitrate Dehydrogenase (IDH) Inhibitors
2.3. Human Epidermal Growth Factor Receptor 2 (HER2) Inhibitors
3. Tumor-Agnostic Treatments
3.1. BRAF/MEK Inhibitors
3.2. Tropomyosin Receptor Kinase (TRK) Inhibitors
3.3. RET Inhibitors
| Target | Targeted Drug | Trial Name(s) | Cohort Size (n) | ORR | mPFS | mOS | % w/TRAE Grade ≥ 3 |
|---|---|---|---|---|---|---|---|
| FGFR2 | Pemigatinib [16] | FIGHT-202 | 146 | 35.5% | 6.9 months | 21.1 months | 64% |
| Futibatinib [18] | FOENIX-CCA2 | 203 | 41.7% | 8.9 months | 20 months | 73.1% | |
| RLY-4008 [21] | ReFocus | 38 | 63.2% | Not reported | Not reported | Not reported | |
| IDH1 | Ivosidenib [26,27] | CLARIDHY | 187 | 2.4% | 2.7 months | 10.3 months | 53% |
| HER2 | Trastuzumab/Pertuzumab [34] | MyPathway | 39 | 23% | 4 months | 10.9 months | 46% |
| Trastuzumab/Deruxtecan [37] | HERB | 22 | 36.4% | 4.4 months | 7.1 months | 81.3% | |
| BRAF/MEK | Dabrafenib/Trametinib [41] | ROAR | 43 | 51% | 9 months | 14 months | 56% |
| TRK | Entrectinib [52,53] | STARTRK-1/2 plus ALKA-372-002 | 150 (17 tumor types) | 61.3% | 13.8 months | 37.1 months | Not reported |
| Larotrectinib [49,50] | LOXO-TRK-14001 SCOUT NAVIGATE | 206 (25 tumor types) | 69% | 29.4 months | Not reached | 20% | |
| RET | Selpercatinib [60] | LIBRETTO-001 | 41 (two BTC) | 43.9% | 13.2 months | 18 months | 38% |
| Pralsetinib [62] | ARROW | 23 (three BTC) | 57% | 7.4 months | 13.6 months | 72% |
3.4. Multikinase Inhibitors
3.5. High Tumor Mutational Burden and Immunotherapy
3.6. Bifunctional Antibody Therapy
4. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Lee, S.; Azad, N.S.; Borad, M.J.; Kate Kelley, R.; Sivaraman, S.; Teschemaker, A.; Chopra, I.; Janjan, N.; Parasuraman, S.; et al. Temporal Changes in Cholangiocarcinoma Incidence and Mortality in the United States from 2001 to 2017. Oncologist 2022, 27, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lim, J.; Han, S.-S.; Park, H.M.; Kim, S.-W.; Lee, W.J.; Woo, S.M.; Kim, T.H.; Won, Y.-J.; Park, S.-J. Distinct Prognosis of Biliary Tract Cancer According to Tumor Location, Stage, and Treatment: A Population-Based Study. Sci. Rep. 2022, 12, 10206. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhao, S.; Zhu, H.; Ji, G.; Zhang, X. Primary Tumor Resection Improves Survival in Patients with Multifocal Intrahepatic Cholangiocarcinoma Based on a Population Study. Sci. Rep. 2021, 11, 12166. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, J.; Youssef, M.; Klimstra, D.; Blumgart, L.H. Staging, Resectability, and Outcome in 225 Patients with Hilar Cholangiocarcinoma. Ann. Surg. 2001, 234, 507–519. [Google Scholar] [CrossRef]
- Endo, I.; Gonen, M.; Yopp, A.C.; Dalal, K.M.; Zhou, Q.; Klimstra, D.; D’Angelica, M.; DeMatteo, R.P.; Fong, Y.; Schwartz, L.; et al. Intrahepatic Cholangiocarcinoma: Rising Frequency, Improved Survival, and Determinants of Outcome After Resection. Ann. Surg. 2008, 248, 84–96. [Google Scholar] [CrossRef]
- Koerkamp, B.G.; Wiggers, J.K.; Allen, P.J.; Besselink, M.G.; Blumgart, L.H.; Busch, O.R.C.; Coelen, R.J.; D’Angelica, M.I.; DeMatteo, R.P.; Gouma, D.J.; et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J. Am. Coll. Surg. 2015, 221, 1041–1049. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Beal, E.W.; Chakedis, J.; Chen, Q.; Lv, Y.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-Intent Surgery: A Multi-Institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J. Surg. 2018, 42, 2919–2929. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus Gemcitabine versus Gemcitabine for Biliary Tract Cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth He, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Ah Lee, M.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and Cisplatin plus Durvalumab with or without Tremelimumab in Chemotherapy-Naive Patients with Advanced Biliary Tract Cancer: An Open-Label, Single-Centre, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers Version 4. 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 31 December 2022).
- Koeberle, D.; Fritsch, R. Targeting HER2 in Biliary Tract Carcinomas: Challenges and Opportunities. Oncol. Res. Treat. 2021, 44, 1–3. [Google Scholar] [CrossRef]
- Liu, G.; Chen, T.; Ding, Z.; Wang, Y.; Wei, Y.; Wei, X. Inhibition of FGF-FGFR and VEGF-VEGFR Signalling in Cancer Treatment. Cell. Prolif. 2021, 54, e13009. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Kongpetch, S.; Crolley, V.E.; Bridgewater, J. Targeting FGFR Inhibition in Cholangiocarcinoma. Cancer Treat. Rev. 2021, 95, 102170. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: FDA Grants Accelerated Approval to Futibatinib for Cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-futibatinib-cholangiocarcinoma (accessed on 31 December 2022).
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Morizane, C.; Valle, J.W.; Karasic, T.B.; Abrams, T.A.; Kelley, R.K.; Cassier, P.A.; Furuse, J.; et al. Updated Results of the FOENIX-CCA2 Trial: Efficacy and Safety of Futibatinib in Intrahepatic Cholangiocarcinoma (ICCA) Harboring FGFR2 Fusions/Rearrangements. J. Clin. Oncol. 2022, 40, 4009-4009. [Google Scholar] [CrossRef]
- European Society for Medical Oncology Withdrawal of Application for the EMA Marketing Authorisation of Infigratinib. Available online: https://www.esmo.org/oncology-news/withdrawal-of-application-for-the-ema-marketing-authorisation-of-infigratinib (accessed on 31 December 2022).
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in Previously Treated Patients with Advanced or Metastatic Cholangiocarcinoma with FGFR2 Fusions or Rearrangements: Mature Results from a Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef]
- Hollebecque, A.; Borad, M.; Goyal, L.; Schram, A.; Park, J.O.; Cassier, P.A.; Kamath, S.D.; Meng, D.T.W.; Dotan, E.; Kim, R.; et al. LBA12 Efficacy of RLY-4008, a Highly Selective FGFR2 Inhibitor in Patients (Pts) with an FGFR2-Fusion or Rearrangement (f/r), FGFR Inhibitor (FGFRi)-Naïve Cholangiocarcinoma (CCA): ReFocus Trial. Ann. Oncol. 2022, 33, S1381. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary Cancer: Utility of next-Generation Sequencing for Clinical Management: Genomic Profiling of Biliary Tract Cancer. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef]
- Borger, D.R.; Tanabe, K.K.; Fan, K.C.; Lopez, H.U.; Fantin, V.R.; Straley, K.S.; Schenkein, D.P.; Hezel, A.F.; Ancukiewicz, M.; Liebman, H.M.; et al. Frequent Mutation of Isocitrate Dehydrogenase (IDH)1 and IDH2 in Cholangiocarcinoma Identified Through Broad-Based Tumor Genotyping. Oncologist 2012, 17, 72–79. [Google Scholar] [CrossRef]
- Ward, P.S.; Patel, J.; Wise, D.R.; Abdel-Wahab, O.; Bennett, B.D.; Coller, H.A.; Cross, J.R.; Fantin, V.R.; Hedvat, C.V.; Perl, A.E.; et al. The Common Feature of Leukemia-Associated IDH1 and IDH2 Mutations Is a Neomorphic Enzyme Activity Converting α-Ketoglutarate to 2-Hydroxyglutarate. Cancer Cell. 2010, 17, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Salati, M.; Caputo, F.; Baldessari, C.; Galassi, B.; Grossi, F.; Dominici, M.; Ghidini, M. IDH Signalling Pathway in Cholangiocarcinoma: From Biological Rationale to Therapeutic Targeting. Cancers 2020, 12, 3310. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-Mutant, Chemotherapy-Refractory Cholangiocarcinoma (ClarIDHy): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669. [Google Scholar] [CrossRef]
- Cleary, J.M.; Rouaisnel, B.; Daina, A.; Raghavan, S.; Roller, L.A.; Huffman, B.M.; Singh, H.; Wen, P.Y.; Bardeesy, N.; Zoete, V.; et al. Secondary IDH1 Resistance Mutations and Oncogenic IDH2 Mutations Cause Acquired Resistance to Ivosidenib in Cholangiocarcinoma. NPJ Precis. Onc. 2022, 6, 61. [Google Scholar] [CrossRef]
- Jones, R.L.; Macarulla, T.; Charlson, J.A.; Van Tine, B.A.; Goyal, L.; Italiano, A.; Massard, C.; Rosenthal, M.; De La Fuente, M.I.; Roxburgh, P.; et al. A Phase Ib/II Study of Olutasidenib in Patients with Relapsed/Refractory IDH1 Mutant Solid Tumors: Safety and Efficacy as Single Agent. J. Clin. Oncol. 2020, 38, e16643-e16643. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Penas-Prado, M.; Peters, K.B.; Burris, H.A.; Maher, E.A.; Janku, F.; Cote, G.M.; de la Fuente, M.I.; Clarke, J.L.; Ellingson, B.M.; et al. Vorasidenib, a Dual Inhibitor of Mutant IDH1/2, in Recurrent or Progressive Glioma; Results of a First-in-Human Phase I Trial. Clin. Cancer Res. 2021, 27, 4491–4499. [Google Scholar] [CrossRef]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.-Y.; Zhu, A.X. Biliary Tract Cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB Signalling Network. Nat. Rev. Mol. Cell. Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.A.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results from MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and Trastuzumab for HER2-Positive, Metastatic Biliary Tract Cancer (MyPathway): A Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chon, H.J.; Cheon, J.; Lee, M.A.; Im, H.-S.; Jang, J.-S.; Kim, M.H.; Park, S.; Kang, B.; Hong, M.; et al. Trastuzumab plus FOLFOX for HER2-Positive Biliary Tract Cancer Refractory to Gemcitabine and Cisplatin: A Multi-Institutional Phase 2 Trial of the Korean Cancer Study Group (KCSG-HB19–14). Lancet Gastroenterol. Hepatol. 2023, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter Phase II Trial of Trastuzumab Deruxtecan for HER2-Positive Unresectable or Recurrent Biliary Tract Cancer: HERB Trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab Deruxtecan (T-DXd; DS-8201) in Patients (Pts) with HER2-Expressing Unresectable or Recurrent Biliary Tract Cancer (BTC): An Investigator-Initiated Multicenter Phase 2 Study (HERB Trial). J. Clin. Oncol. 2022, 40, 4006-4006. [Google Scholar] [CrossRef]
- Terrell, E.M.; Morrison, D.K. Ras-Mediated Activation of the Raf Family Kinases. Cold Spring Harb. Perspect. Med. 2019, 9, a033746. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF Gene in Human Cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Javle, M. Molecular Profiling of Biliary Tract Cancer: A Target Rich Disease. J. Gastrointest. Oncol. 2016, 7, 797–803. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus Trametinib in Patients with BRAFV600E-Mutated Biliary Tract Cancer (ROAR): A Phase 2, Open-Label, Single-Arm, Multicentre Basket Trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: FDA Grants Accelerated Approval to Dabrafenib in Combination with Trametinib for Unresectable or Metastatic Solid Tumors with BRAF V600E Mutation. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid (accessed on 31 December 2022).
- Rajkumar, S.; Berry, D.; Heney, K.A.; Strong, C.; Ramsay, L.; Lajoie, M.; Alkallas, R.; Nguyen, T.-T.; Thomson, C.; Ahanfeshar-Adams, M.; et al. Melanomas with Concurrent BRAF Non-p.V600 and NF1 Loss-of-Function Mutations Are Targetable by BRAF/MEK Inhibitor Combination Therapy. Cell Rep. 2022, 39, 110634. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin Receptor Kinase (TRK) Biology and the Role of NTRK Gene Fusions in Cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK Fusion-Positive Cancers and TRK Inhibitor Therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Tognon, C.; Knezevich, S.R.; Huntsman, D.; Roskelley, C.D.; Melnyk, N.; Mathers, J.A.; Becker, L.; Carneiro, F.; MacPherson, N.; Horsman, D.; et al. Expression of the ETV6-NTRK3 Gene Fusion as a Primary Event in Human Secretory Breast Carcinoma. Cancer Cell 2002, 2, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Linkov, I.; Rosado, A.; Mullaney, K.; Rosen, E.Y.; Frosina, D.; Jungbluth, A.A.; Zehir, A.; Benayed, R.; Drilon, A.; et al. NTRK Fusion Detection across Multiple Assays and 33,997 Cases: Diagnostic Implications and Pitfalls. Mod. Pathol. 2020, 33, 38–46. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration: FDA Approves Larotrectinib for Solid Tumors with NTRK Gene Fusions. Available online: https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions (accessed on 31 December 2022).
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.E.; Hong, D.S.; van Tilburg, C.M.; Doz, F.; Tan, D.S.W.; Kummar, S.; Lin, J.J.; McDermott, R.S.; Zwaan, C.M.; Norenberg, R.; et al. Long-Term Efficacy and Safety of Larotrectinib in a Pooled Analysis of Patients with Tropomyosin Receptor Kinase (TRK) Fusion Cancer. J. Clin. Oncol. 2022, 40 (Suppl. 16), 3100-3100. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: FDA Approves Entrectinib for NTRK Tumors and ROS-1 NSCLC. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (accessed on 31 December 2022).
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1–2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Krzakowski, M.J.; Lu, S.; Cousin, S.; Smit, E.F.; Springfeld, C.; Goto, K.; Garrido, P.; Chung, C.H.; Lin, J.J.; Bray, V.J.; et al. Updated Analysis of the Efficacy and Safety of Entrectinib in Patients (Pts) with Locally Advanced/Metastatic NTRK Fusion-Positive (NTRK-Fp) Solid Tumors. J. Clin. Oncol. 2022, 40 (Suppl. 16), 3099-3099. [Google Scholar] [CrossRef]
- Drilon, A.; Ou, S.-H.I.; Cho, B.C.; Kim, D.-W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.-J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef]
- Drilon, A.; Nagasubramanian, R.; Blake, J.F.; Ku, N.; Tuch, B.B.; Ebata, K.; Smith, S.; Lauriault, V.; Kolakowski, G.R.; Brandhuber, B.J.; et al. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion–Positive Solid Tumors. Cancer Discov. 2017, 7, 963–972. [Google Scholar] [CrossRef]
- Qin, H.; Patel, M. The Challenge and Opportunity of NTRK Inhibitors in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 2916. [Google Scholar] [CrossRef]
- Kurzrock, R. Selpercatinib Aimed at RET-Altered Cancers. N. Engl. J. Med. 2020, 383, 868–869. [Google Scholar] [CrossRef]
- Kato, S.; Subbiah, V.; Marchlik, E.; Elkin, S.K.; Carter, J.L.; Kurzrock, R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin. Cancer Res. 2017, 23, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration: FDA Approves Selpercatinib for Locally Advanced or Metastatic RET Fusion-Positive Solid Tumors. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-solid-tumors (accessed on 31 December 2022).
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-Agnostic Efficacy and Safety of Selpercatinib in Patients with RET Fusion-Positive Solid Tumours Other than Lung or Thyroid Tumours (LIBRETTO-001): A Phase 1/2, Open-Label, Basket Trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bradford, D.; Larkins, E.; Pai-Scherf, L.H.; Chatterjee, S.; Mishra-Kalyani, P.S.; Wearne, E.; Helms, W.S.; Ayyoub, A.; Bi, Y.; et al. FDA Approval Summary: Pralsetinib for the Treatment of Lung and Thyroid Cancers with RET Gene Mutations or Fusions. Clin. Cancer Res. 2021, 27, 5452–5456. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-Cancer Efficacy of Pralsetinib in Patients with RET Fusion–Positive Solid Tumors from the Phase 1/2 ARROW Trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 Pathway: Current Researches in Cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Motta, R.; Cabezas-Camarero, S.; Torres-Mattos, C.; Riquelme, A.; Calle, A.; Figueroa, A.; Sotelo, M.J. Immunotherapy in Microsatellite Instability Metastatic Colorectal Cancer: Current Status and Future Perspectives. J. Clin. Transl. Res. 2021, 7, 511–522. [Google Scholar]
- Dienstmann, R.; Salazar, R.; Tabernero, J. Molecular Subtypes and the Evolution of Treatment Decisions in Metastatic Colorectal Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 231–238. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration: FDA Grants Accelerated Approval to Pembrolizumab for First Tissue/Site Agnostic Indication. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (accessed on 31 December 2022).
- Klempner, S.J.; Fabrizio, D.; Bane, S.; Reinhart, M.; Peoples, T.; Ali, S.M.; Sokol, E.S.; Frampton, G.; Schrock, A.B.; Anhorn, R.; et al. Tumor Mutational Burden as a Predictive Biomarker for Response to Immune Checkpoint Inhibitors: A Review of Current Evidence. Oncologist 2020, 25, e147–e159. [Google Scholar] [CrossRef]
- Winkelmann, R.; Schneider, M.; Hartmann, S.; Schnitzbauer, A.; Zeuzem, S.; Peveling-Oberhag, J.; Hansmann, M.; Walter, D. Microsatellite Instability Occurs Rarely in Patients with Cholangiocarcinoma: A Retrospective Study from a German Tertiary Care Hospital. Int. J. Mol. Sci. 2018, 19, 1421. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Xiu, J.; Lindberg, M.R.; Shields, A.F.; Hwang, J.J.; Poorman, K.; Salem, M.E.; Pishvaian, M.J.; Holcombe, R.F.; Marshall, J.L.; et al. Molecular Profiling of Biliary Cancers Reveals Distinct Molecular Alterations and Potential Therapeutic Targets. J. Gastrointest. Oncol. 2019, 10, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Sokol, E.S.; Frampton, G.M.; Lippman, S.M.; Kurzrock, R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Lee, M.; Hoen, D.; Zehir, A.; Berger, M.F.; Seshan, V.E.; Chan, T.A.; Morris, L.G.T. Response Rates to Anti–PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors with 10 or More Mutations per Megabase. JAMA Oncol. 2021, 7, 739. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration: FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (accessed on 31 December 2022).
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Addeo, A. The FDA Approval of Pembrolizumab for Patients with TMB >10 Mut/Mb: Was It a Wise Decision? No. Ann. Oncol. 2020, 31, 1112–1114. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor Mutational Load Predicts Survival after Immunotherapy across Multiple Cancer Types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High Tumor Mutation Burden Fails to Predict Immune Checkpoint Blockade Response across All Cancer Types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef]
- Subbiah, V.; Solit, D.B.; Chan, T.A.; Kurzrock, R. The FDA Approval of Pembrolizumab for Adult and Pediatric Patients with Tumor Mutational Burden (TMB) ≥ 10: A Decision Centered on Empowering Patients and Their Physicians. Ann. Oncol. 2020, 31, 1115–1118. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernández, V.M.; et al. Enhanced Preclinical Antitumor Activity of M7824, a Bifunctional Fusion Protein Simultaneously Targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 10, eaan5488. [Google Scholar] [CrossRef]
- Yoo, C.; Oh, D.-Y.; Choi, H.J.; Kudo, M.; Ueno, M.; Kondo, S.; Chen, L.-T.; Osada, M.; Helwig, C.; Dussault, I.; et al. Phase I Study of Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Patients with Pretreated Biliary Tract Cancer. J. Immunother. Cancer 2020, 8, e000564. [Google Scholar] [CrossRef] [PubMed]
- Merck KGaA, Darmstadt, Germany, Reports Topline Data for Brntrafusp Alfa as Second-Line Monotherapy Treatment in Biliary Tract Cancer. Available online: https://www.emdgroup.com/en/news/bintrafusp-topline-data-biliary-tract-cancer-16-03-2021.html (accessed on 31 December 2022).
- Giraldo, N.A.; Drill, E.; Satravada, B.A.; Dika, I.E.; Brannon, A.R.; Dermawan, J.; Mohanty, A.; Ozcan, K.; Chakravarty, D.; Benayed, R.; et al. Comprehensive Molecular Characterization of Gallbladder Carcinoma and Potential Targets for Intervention. Clin. Cancer Res. 2022, 28, 5359–5367. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Dong, K.; Bai, Y.; Zhao, S.; Dong, Y.; Shi, J.; Shi, W.; Long, J.; Yang, X.; Wang, D.; et al. Precision Oncology for Gallbladder Cancer: Insights from Genetic Alterations and Clinical Practice. Ann. Transl. Med. 2019, 7, 467. [Google Scholar] [CrossRef] [PubMed]
- de Bitter, T.J.J.; de Reuver, P.R.; de Savornin Lohman, E.A.J.; Kroeze, L.I.; Vink-Börger, M.E.; van Vliet, S.; Simmer, F.; von Rhein, D.; Jansen, E.A.M.; Verheij, J.; et al. Comprehensive Clinicopathological and Genomic Profiling of Gallbladder Cancer Reveals Actionable Targets in Half of Patients. NPJ Precis. Onc. 2022, 6, 83. [Google Scholar] [CrossRef]
- Goldenberg, D.; Rosenbaum, E.; Argani, P.; Wistuba, I.I.; Sidransky, D.; Thuluvath, P.J.; Hidalgo, M.; Califano, J.; Maitra, A. The V599E BRAF Mutation Is Uncommon in Biliary Tract Cancers. Mod. Pathol. 2004, 17, 1386–1391. [Google Scholar] [CrossRef]
- Kuipers, H.; de Bitter, T.J.J.; de Boer, M.T.; van der Post, R.S.; Nijkamp, M.W.; de Reuver, P.R.; Fehrmann, R.S.N.; Hoogwater, F.J.H. Gallbladder Cancer: Current Insights in Genetic Alterations and Their Possible Therapeutic Implications. Cancers 2021, 13, 5257. [Google Scholar] [CrossRef]
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision Medicine in Cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Brandi, G. Detecting and Targeting NTRK Gene Fusions in Cholangiocarcinoma: News and Perspectives. Expert Rev. Precis. Med. Drug Dev. 2021, 6, 225–227. [Google Scholar] [CrossRef]
- Silverman, I.M.; Hollebecque, A.; Friboulet, L.; Owens, S.; Newton, R.C.; Zhen, H.; Féliz, L.; Zecchetto, C.; Melisi, D.; Burn, T.C. Clinicogenomic Analysis of FGFR2 -Rearranged Cholangiocarcinoma Identifies Correlates of Response and Mechanisms of Resistance to Pemigatinib. Cancer Discov. 2021, 11, 326–339. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Corti, F.; Nichetti, F.; Raimondi, A.; Niger, M.; Prinzi, N.; Torchio, M.; Tamborini, E.; Perrone, F.; Pruneri, G.; Di Bartolomeo, M.; et al. Targeting the PI3K/AKT/MTOR Pathway in Biliary Tract Cancers: A Review of Current Evidences and Future Perspectives. Cancer Treat. Rev. 2019, 72, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Bridgewater, J.; Normanno, N. Practical Considerations in Screening for Genetic Alterations in Cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am. Soc. Clin. Oncol. Educ. Book. 2016, 35, e194–e203. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine Compared with Observation in Resected Biliary Tract Cancer (BILCAP): A Randomised, Controlled, Multicentre, Phase 3 Study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus Placebo in Radioiodine-Refractory Thyroid Cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Sahin, I.H.; Tan, E.; Kim, R. Regorafenib, an Investigational Agent for the Treatment of Cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 333–341. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Wu, S.; Robert, C.; Atkins, M.B.; Kong, H.H.; Guitart, J.; Garbe, C.; Hauschild, A.; Puzanov, I.; Alexandrescu, D.T.; et al. Evolving Strategies for the Management of Hand–Foot Skin Reaction Associated with the Multitargeted Kinase Inhibitors Sorafenib and Sunitinib. Oncologist 2008, 13, 1001–1011. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A Phase 2 Trial of Regorafenib as a Single Agent in Patients with Chemotherapy-refractory, Advanced, and Metastatic Biliary Tract Adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Sacco, R. Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis. Cancers 2019, 12, 36. [Google Scholar] [CrossRef]
- Morizane, C.; Ueno, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Hayata, N.; Ikezawa, H.; Suzuki, T.; Nakajima, R.; et al. Interim Analysis of a Phase 2 Study of Lenvatinib (LEN) Monotherapy as Second-Line Treatment in Unresectable Biliary Tract Cancer (BTC). J. Clin. Oncol. 2017, 35, 310-310. [Google Scholar] [CrossRef]
- Ueno, M.; Ikeda, M.; Sasaki, T.; Nagashima, F.; Mizuno, N.; Shimizu, S.; Ikezawa, H.; Hayata, N.; Nakajima, R.; Morizane, C. Phase 2 Study of Lenvatinib Monotherapy as Second-Line Treatment in Unresectable Biliary Tract Cancer: Primary Analysis Results. BMC Cancer 2020, 20, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lwin, Z.; Gomez-Roca, C.; Saada-Bouzid, E.; Yanez, E.; Muñoz, F.L.; Im, S.-A.; Castanon, E.; Senellart, H.; Graham, D.; Voss, M.; et al. LBA41 LEAP-005: Phase II Study of Lenvatinib (Len) plus Pembrolizumab (Pembro) in Patients (Pts) with Previously Treated Advanced Solid Tumours. Ann. Oncol. 2020, 31, S1170. [Google Scholar] [CrossRef]
- Buzzoni, R.; Pusceddu, S.; Bajetta, E.; De Braud, F.; Platania, M.; Iannacone, C.; Cantore, M.; Mambrini, A.; Bertolini, A.; Alabiso, O.; et al. Activity and Safety of RAD001 (Everolimus) in Patients Affected by Biliary Tract Cancer Progressing after Prior Chemotherapy: A Phase II ITMO Study. Ann. Oncol. 2014, 25, 1597–1603. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Iwahashi, S.; Saito, Y.; Yamada, S.; Wada, Y.; Yamashita, S.; et al. Carbohydrate Antigen 19-9 Is a Prognostic Factor Which Correlates with HDAC1 and HIF-1α for Intrahepatic Cholangiocarcinoma. Anticancer. Res. 2019, 39, 6025–6033. [Google Scholar] [CrossRef]
- Morine, Y.; Shimada, M.; Utsunomiya, T.; Imura, S.; Ikemoto, T.; Mori, H.; Hanaoka, J.; Kanamoto, M.; Iwahashi, S.; Miyake, H. Hypoxia Inducible Factor Expression in Intrahepatic Cholangiocarcinoma. HGE 2011, 58, 110–111. [Google Scholar] [CrossRef]
- Demols, A.; Borbath, I.; Van den Eynde, M.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.-C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after Failure of Gemcitabine and Platinum-Based Chemotherapy for Locally Advanced/Metastatic Biliary Tumors: REACHIN, a Randomized, Double-Blind, Phase II Trial. Ann. Oncol. 2020, 31, 1169–1177. [Google Scholar] [CrossRef]
- Koustas, E.; Trifylli, E.-M.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. Role of Autophagy in Cholangiocarcinoma: An Autophagy-Based Treatment Strategy. World J. Gastrointest. Oncol. 2021, 13, 1229–1243. [Google Scholar] [CrossRef]
- Aredia, F.; Giansanti, V.; Mazzini, G.; Savio, M.; Ortiz, L.M.G.; Jaadane, I.; Zaffaroni, N.; Forlino, A.; Torriglia, A.; Scovassi, A.I. Multiple Effects of the Na+/H+ Antiporter Inhibitor HMA on Cancer Cells. Apoptosis 2013, 18, 1586–1598. [Google Scholar] [CrossRef]
- Su, Y.; Yu, T.; Wang, Y.; Huang, X.; Wei, X. Circular RNA CircDNM3OS Functions as a MiR-145-5p Sponge to Accelerate Cholangiocarcinoma Growth and Glutamine Metabolism by Upregulating MORC2. Onco Targets Ther. 2021, 14, 1117–1129. [Google Scholar] [CrossRef]
- Yang, W.-H.; Qiu, Y.; Stamatatos, O.; Janowitz, T.; Lukey, M.J. Enhancing the Efficacy of Glutamine Metabolism Inhibitors in Cancer Therapy. Trends Cancer 2021, 7, 790–804. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, C.; Jiang, G.; Jin, S.; Gao, Z.; Wang, Q.; Yu, D.; Ke, A.; Fan, Y.; Li, D.; et al. Expression of GLS1 in Intrahepatic Cholangiocarcinoma and Its Clinical Significance. Mol. Med. Rep. 2019, 20, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farha, N.; Dima, D.; Ullah, F.; Kamath, S. Precision Oncology Targets in Biliary Tract Cancer. Cancers 2023, 15, 2105. https://doi.org/10.3390/cancers15072105
Farha N, Dima D, Ullah F, Kamath S. Precision Oncology Targets in Biliary Tract Cancer. Cancers. 2023; 15(7):2105. https://doi.org/10.3390/cancers15072105
Chicago/Turabian StyleFarha, Nicole, Danai Dima, Fauzia Ullah, and Suneel Kamath. 2023. "Precision Oncology Targets in Biliary Tract Cancer" Cancers 15, no. 7: 2105. https://doi.org/10.3390/cancers15072105
APA StyleFarha, N., Dima, D., Ullah, F., & Kamath, S. (2023). Precision Oncology Targets in Biliary Tract Cancer. Cancers, 15(7), 2105. https://doi.org/10.3390/cancers15072105