The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions
Abstract
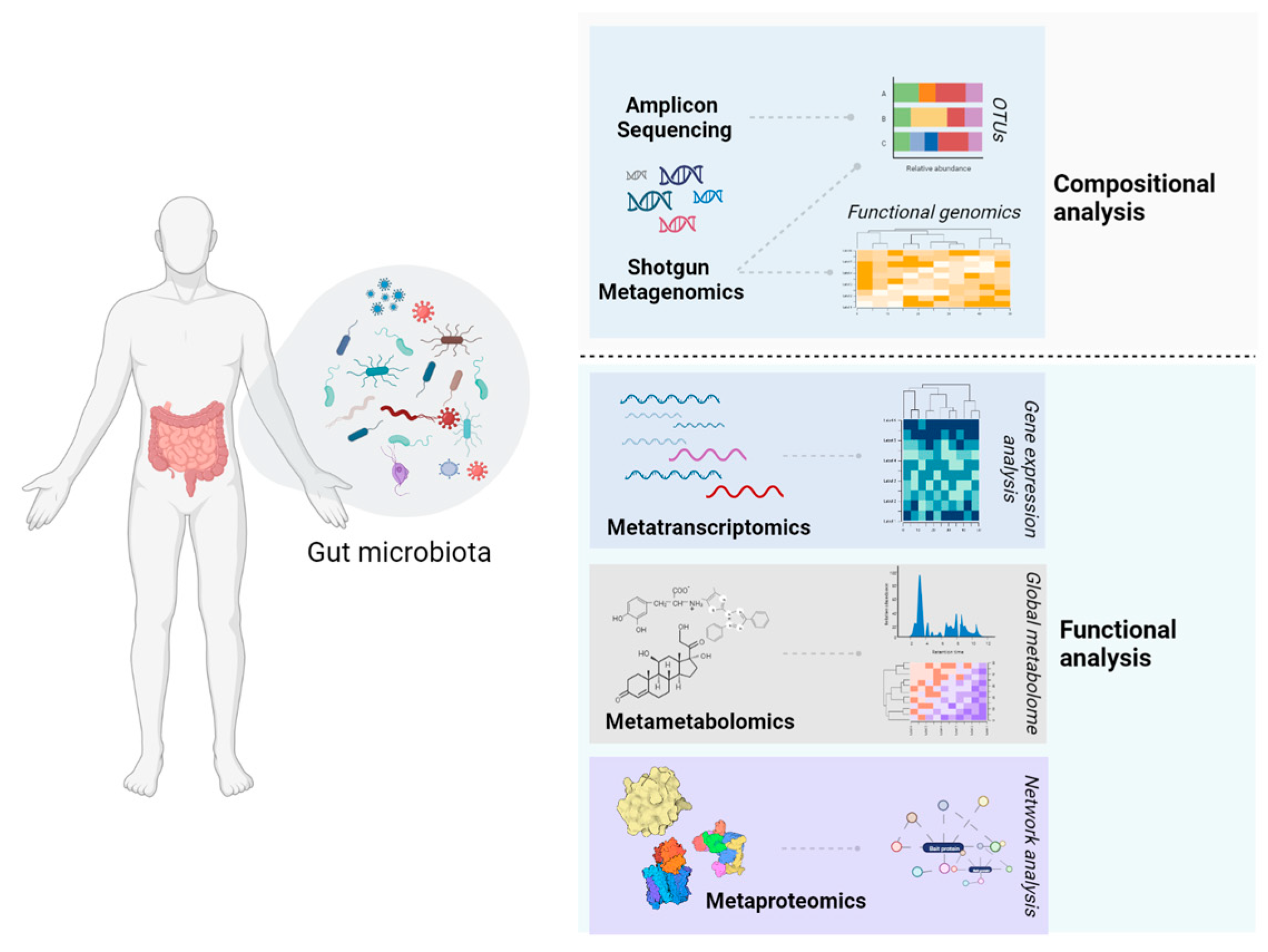
Simple Summary
Abstract
1. Introduction
2. Anti-Tumor Immune Response, Immunological Tolerance, and Resistance to ICI Therapy
3. The Gut Microbiota–Immune System Crosstalk and Anti-Tumor Immunity
4. The Gut Microbiome and Modulation of ICI Response
| Participants | Disease Stage | Immuno-Therapy type | Study Design | Samples | Analysis | Findings | Microbiota Diversity | Ref. |
|---|---|---|---|---|---|---|---|---|
| Melanoma | ||||||||
| Unresectable/ metastatic melanoma (n = 39, 30M/9F) | IV | Anti-PD-1 or anti-CTLA-4, or anti-PD-1/anti-CTLA-4 | Assessment of GM composition at baseline and before each ICIs infusion | Feces | MSS, UPLC-MS/MS | ↑B. caccae, F. prausnitzii, B. thetaiotamicron, and Holdemania filiformis, D. formicogenerans in R ↑Anacardic acid in R | No significant differences between R and NR | [48] |
| Unresectable cutaneous melanoma (n = 25, 10M/15F) | IIIc/IV | Anti-PD-1, or anti-PD-1/anti-CTLA-4 | Assessment of overall gut microbiome composition, relative microbial abundance, and differences in prevalence between responders and non-responders | Feces | MSS | ↑Ruminococcus gnavus, E. coli, E. biforme, Phascolarctobacterium succinatutens, and Streptococcus salivarius in R ↑B. longum, Prevotella copri, Coprococcus sp ART55-1, Eggerthella unclassified, and Eubacterium ramulus in NR ↑Streptococcus parasanguinis carriers → longer OS ↑B. massiliensis → longer PFS ↑Peptostreptococcaceae (unclassified species) carriers → shorter OS and PFS 17 microbial pathways differentially enriched between R and NR | No significant differences between R and NR | [49] |
| Metastatic melanoma (n = 26, 13M/13F) | IV | CTLA-4 blockade | Assessment of GM composition and blood-based biomarkers at baseline, before each ipilimumab infusion, at the end of the treatment, and at the time of colitis | Feces, Blood serum | 16S rRNA gene sequencing, Immunopheno-typing, Soluble immune markers analysis | Baseline GM enriched with Faecalibacterium spp. and other Firmicutes → longer PFS and OS Baseline GM enriched with Bacteroides spp. → No ipilimumab-induced colitis | No significant differences | [50] |
| Metastatic melanoma (n = 112) | IV | PD-1 blockade | Assessment of oral and gut microbiome composition at baseline | Buccal swabs, Feces, Tumor biopsies, Blood | 16S rRNA gene sequencing | ↑Clostridiales/Ruminococcaceae and Faecalibacterium spp. in R ↑Bacteroidales in NR ↑Faecalibacterium abundance → prolonged PFS | ↑α-diversity in R → prolonged PFS | [12] |
| Metastatic melanoma (n = 27, 21M/6F) | III (n = 9) IV (n = 18) | Anti-PD-1/ anti-CTLA-4 | Assessment of gut microbiome overall diversity and composition Correlation with PFS | Feces | 16S rRNA gene sequencing, MSS | ↑F. prausnitzii, Coprococcus eutactus, Prevotella stercorea, Streptococcus spp., and Lachnospiraceae bacterium → longer PFS ↑Bacteroides spp., Ruminococcus gnavus, and Blautia producta abundance → shorter PFS | Higher community diversity → longer PFS | [55] |
| Metastatic melanoma (n = 42, 20M/22F) | IV | Anti-PD-1/ anti-CTLA-4 | Assessment of GM composition before treatment | Feces | 16S rRNA gene sequencing, MSS | ↑E. faecium, Collinsella aerofaciens, B. adolescentis, Klebsiella pneumoniae, Veillonella parvula, Parabacteroides merdae, Lactobacillus sp., and B. longum in R ↑Ruminococcus obeum and Roseburia intestinalis in NR | ND | [63] |
| Non-small cell lung cancer | ||||||||
| NSCLC (n = 11, 8M/3F) | IV | PD-1 blockade | Assessment of gut microbiota composition at baseline and during immunotherapy Comparison between patients and healthy controls | Feces | 16S rRNA sequencing, Meta-metabolomics (GC–MS/SPME) | ↑A. muciniphila, B. longum, Faecalibacterium prausnitzii in R ↑Propionibacterium acnes, Veillonella, Staphylococcus aureus, Peptostreptococcus, Ruminococcus bromii, Dialister, and Sutterella in NR ↑Rikenellaceae, Prevotella, Streptococcus, Lactobacillus, Bacteroides plebeius, Oscillospira, and Enterobacteriaceae enriched in patients compared to HC | ND | [58] |
| Advanced ΝSCLC (n = 37, 29M/8F) | IIIB (n = 6) IV (n = 31) | PD-1 blockade | Assessment of gut microbiota composition at baseline and prior to infusion | Feces, Blood | 16S rRNA sequencing, Flow cytometry | ↑Μicrobiome diversity → Better response, prolonged PSF ↑Alistipes putredini, Prevotella copri, B. longum, Lachnobacterium sp, Lachnospiraceae, and Shigella in R ↑Ruminococcus_unclassified in NR | α-diversity; significantly higher in R vs. NR at baseline β-diversity; significant difference between R and NR | [59] |
| Advanced NSCLC (n = 17, 13M/4F) | III (n = 6) IV (n = 8) POR (n = 3) | PD-1 blockade | Assessment of gut microbiota composition during treatment along with clinical evaluations and response to immunotherapy | Feces | 16S rRNA sequencing | ↑Lactobacillus and Clostridium in patients with longer TTF ↓Bilophila and Sutterella in patients with prolonged TTF ↑Lactobacillus, Clostridium, and Syntrophococcus in R ↑Bilophila, Sutterella, and Parabacteroides in NR | α-diversity; No significant differences between R and NR | [69] |
| NSCLC (n = 63, 53M/10F) | III (n = 10) IV (n = 53) | PD-1 blockade | Assessment of overall gut microbiome composition prior to immunotherapy | Feces | MSS | ↑Methanobrevibacter and Parabacteroides in patients PFS ≥ 6 months ↑Veillonella, Selenomonadales, and Negativicutes in patients with PFS < 6 months | β-diversity; significant differences between patients with PFS ≥ 6 months and patients with PFS < 6 months | [73] |
| Other cancer types | ||||||||
| Advanced thoracic carcinoma (n = 42, 32M/10F) | IV | PD-1 blockade | Assessment of predictive potential of the gut microbiome prior to ICI therapy | Feces | 16S rRNA sequencing | ↑Akkermansiaceae, Enterococcaceae, Enterobacteriaceae, Carnobacteriaceae, and Clostridiales Family XI in the R group, correlated with longer PFS | α-diversity; β-diversity; No significant differences between R and NR | [57] |
| Advanced-stage GI (n = 74, 53M/21F) | III/IV | Anti-PD-1 or anti-PD-1/anti-CTLA-4 | Assessment of gut microbiota composition prior to and during immunotherapy, along with clinical evaluations | Feces | 16S rRNA sequencing, MSS | ↑Prevotellaceae, Ruminococcaceae, and Lachnospiraceae in R ↓Bacteroidaceae in R ↓Prevotella/Bacteroides ratio in R SCFA producers (Eubacterium, Lactobacillus, and Streptococcus) → positively associated with anti-PD-1/PD-L1 response | α-diversity; No significant differences between R and NR | [61] |
| Hepato-cellular carcinoma (n = 8) | BCLC Stage C | PD-1 blockade | Assessment of gut microbiota composition at baseline and during ICIs infusion | Feces | MSS | Higher taxa richness and more gene counts in R vs. NR ↑Proteobacteria in NR during therapy | Dissimilarity in β-diversity across patients | [62] |
5. Modulation of Gut Microbiota and Efficacy of Cancer Immunotherapy
6. Incorporating Gut Microbiome Research in the Clinic—Pitfalls and Opportunities
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; de Toni, E.; Schett, G.; Hundorfean, G.; Zimmer, L. Checkpoint Inhibitors: The Diagnosis and Treatment of Side Effects. Dtsch. Arztebl. Int. 2019, 116, 119. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, H.R.; Ha, S.J. Immune Checkpoint Inhibitors in 10 Years: Contribution of Basic Research and Clinical Application in Cancer Immunotherapy. Immune Netw. 2022, 22, e2. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum. Vaccin. Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Larroquette, M.; Domblides, C.; Lefort, F.; Lasserre, M.; Quivy, A.; Sionneau, B.; Bertolaso, P.; Gross-Goupil, M.; Ravaud, A.; Daste, A. Combining immune checkpoint inhibitors with chemotherapy in advanced solid tumours: A review. Eur. J. Cancer 2021, 158, 47–62. [Google Scholar] [CrossRef]
- Yu, C.; Liu, X.; Yang, J.; Zhang, M.; Jin, H.; Ma, X.; Shi, H. Combination of Immunotherapy with Targeted Therapy: Theory and Practice in Metastatic Melanoma. Front. Immunol. 2019, 10, 990. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M.D. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut Microbiome Modulates Response to Anti-PD-1 Immunotherapy in Melanoma Patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Poudel, A.; Kafle, S.; Thapa Magar, M.; Cancarevic, I. Influence of Microbiome and Antibiotics on the Efficacy of Immune Checkpoint Inhibitors. Cureus 2021, 13, e16829. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Concept of Immune Surveillance against Tumors: The First Theories. Oncotarget 2017, 8, 7175. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer Immunoediting from Immune Surveillance to Immune Escape. Immunology 2007, 121, 1. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef]
- Pio, R.; Ajona, D.; Ortiz-Espinosa, S.; Mantovani, A.; Lambris, J.D. Complementing the Cancer-Immunity Cycle. Front. Immunol. 2019, 10, 774. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Zitvogel, L.; Tesniere, A.; Kroemer, G. Cancer despite Immunosurveillance: Immunoselection and Immunosubversion. Nat. Rev. Immunol. 2006, 6, 715–727. [Google Scholar] [CrossRef]
- Borroni, E.M.; Grizzi, F. Cancer Immunoediting and beyond in 2021. Int. J. Mol. Sci. 2021, 22, 22. [Google Scholar] [CrossRef]
- Gu, D.; Ao, X.; Yang, Y.; Chen, Z.; Xu, X. Soluble Immune Checkpoints in Cancer: Production, Function and Biological Significance. J. Immunother. Cancer 2018, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Akinleye, A.; Rasool, Z. Immune Checkpoint Inhibitors of PD-L1 as Cancer Therapeutics. J. Hematol. Oncol. 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Aghamajidi, A.; Maleki Vareki, S. The Effect of the Gut Microbiota on Systemic and Anti-Tumor Immunity and Response to Systemic Therapy against Cancer. Cancers 2022, 14, 3563. [Google Scholar] [CrossRef]
- Oyewole-Said, D.; Konduri, V.; Vazquez-Perez, J.; Weldon, S.A.; Levitt, J.M.; Decker, W.K. Beyond T-Cells: Functional Characterization of CTLA-4 Expression in Immune and Non-Immune Cell Types. Front. Immunol. 2020, 11, 608024. [Google Scholar] [CrossRef]
- Cogdill, A.P.; Andrews, M.C.; Wargo, J.A. Hallmarks of Response to Immune Checkpoint Blockade. Br. J. Cancer 2017, 117, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune Checkpoint Inhibitors for Lung Cancer Treatment: A Review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef]
- Loo, K.; Smithy, J.W.; Postow, M.A.; Betof Warner, A. Factors Determining Long-Term Antitumor Responses to Immune Checkpoint Blockade Therapy in Melanoma. Front. Immunol. 2022, 12, 810388. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, S.; Deng, Y.; Wang, P.; Hou, Q.; Xu, H. Prognostic Factors for Checkpoint Inhibitor Based Immunotherapy: An Update with New Evidences. Front. Pharmacol. 2018, 9, 1050. [Google Scholar] [CrossRef]
- Pérez-Ruiz, E.; Melero, I.; Kopecka, J.; Sarmento-Ribeiro, A.B.; García-Aranda, M.; de Las Rivas, J. Cancer Immunotherapy Resistance Based on Immune Checkpoints Inhibitors: Targets, Biomarkers, and Remedies. Drug Resist. Updat. 2020, 53, 100718. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, S.; Qiao, H. Understanding the Functional Inflammatory Factors Involved in Therapeutic Response to Immune Checkpoint Inhibitors for Pan-Cancer. Front. Pharmacol. 2022, 13, 990445. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef]
- Round, J.L.; Lee, S.M.; Li, J.; Tran, G.; Jabri, B.; Chatila, T.A.; Mazmanian, S.K. The Toll-like Receptor Pathway Establishes Commensal Gut Colonization. Science 2011, 332, 974–977. [Google Scholar] [CrossRef]
- Telesford, K.M.; Yan, W.; Ochoa-Reparaz, J.; Pant, A.; Kircher, C.; Christy, M.A.; Begum-Haque, S.; Kasper, D.L.; Kasper, L.H. A Commensal Symbiotic Factor Derived from Bacteroides Fragilis Promotes Human CD39+Foxp3+ T Cells and Treg Function. Gut Microbes 2015, 6, 234. [Google Scholar] [CrossRef]
- Schulz, O.; Pabst, O. Antigen sampling in the small intestine. Trends Immunol. 2013, 34, 155–161. [Google Scholar] [CrossRef]
- Rios, D.; Wood, M.B.; Li, J.; Chassaing, B.; Gewirtz, A.T.; Williams, I.R. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016, 9, 907–916. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1578. [Google Scholar] [CrossRef]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrate Conditions Human Dendritic Cells to Prime Type 1 Regulatory T Cells via Both Histone Deacetylase Inhibition and G Protein-Coupled Receptor 109A Signaling. Front. Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-Derived Inosine Modulates Response to Checkpoint Inhibitor Immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Lin, Y.; Ma, Y.; Li, X.; Liang, J.; Chen, Z.; Liu, K.; Huang, Y.; Luo, H.; Huang, R.; et al. Exploring the Emerging Role of the Gut Microbiota and Tumor Microenvironment in Cancer Immunotherapy. Front. Immunol. 2020, 11, 612202. [Google Scholar] [CrossRef] [PubMed]
- Zgouras, D.; Wächtershäuser, A.; Frings, D.; Stein, J. Butyrate Impairs Intestinal Tumor Cell-Induced Angiogenesis by Inhibiting HIF-1α Nuclear Translocation. Biochem. Biophys. Res. Commun. 2003, 300, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Geuking, M.B.; Cahenzli, J.; Lawson, M.A.; Ng, D.C.; Slack, E.; Hapfelmeier, S.; McCoy, K.D.; Macpherson, A.J. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 2011, 34, 794–806. [Google Scholar] [CrossRef]
- Chappert, P.; Bouladoux, N.; Naik, S.; Schwartz, R.H. Specific gut commensal flora locally alters T cell tuning to endogenous ligands. Immunity 2013, 38, 1198–1210. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kroemer, G. Cross-reactivity between cancer and microbial antigens. Oncoimmunology 2021, 10, 1877416. [Google Scholar] [CrossRef]
- Frankel, A.E.; Coughlin, L.A.; Kim, J.; Froehlich, T.W.; Xie, Y.; Frenkel, E.P.; Koh, A.Y. Metagenomic Shotgun Sequencing and Unbiased Metabolomic Profiling Identify Specific Human Gut Microbiota and Metabolites Associated with Immune Checkpoint Therapy Efficacy in Melanoma Patients. Neoplasia 2017, 19, 848–855. [Google Scholar] [CrossRef]
- Wind, T.T.; Gacesa, R.; Vich Vila, A.; de Haan, J.J.; Jalving, M.; Weersma, R.K.; Hospers, G.A.P. Gut Microbial Species and Metabolic Pathways Associated with Response to Treatment with Immune Checkpoint Inhibitors in Metastatic Melanoma. Melanoma Res. 2020, 30, 235–246. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Peters, B.A.; Wilson, M.; Moran, U.; Pavlick, A.; Izsak, A.; Wechter, T.; Weber, J.S.; Osman, I.; Ahn, J. Relating the Gut Metagenome and Metatranscriptome to Immunotherapy Responses in Melanoma Patients. Genome Med. 2019, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Olekhnovich, E.I.; Ivanov, A.B.; Babkina, A.A.; Sokolov, A.A.; Ulyantsev, V.I.; Fedorov, D.E.; Ilina, E.N. Consistent Stool Metagenomic Biomarkers Associated with the Response to Melanoma Immunotherapy. mSystems 2023, e0102322. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Yang, Y.; Wang, W. The Role of Intestinal Bifidobacteria on Immune System Development in Young Rats. Early Hum. Dev. 2010, 86, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res 2020, 8, 49. [Google Scholar] [CrossRef]
- Steven, A.; Fisher, S.A.; Robinson, B.W. Immunotherapy for Lung Cancer. Respirology 2016, 21, 821–833. [Google Scholar] [CrossRef]
- Yin, H.; Yang, L.; Peng, G.; Yang, K.; Mi, Y.; Hu, X.; Hao, X.; Jiao, Y.; Wang, X.; Wang, Y. The Commensal Consortium of the Gut Microbiome Is Associated with Favorable Responses to Anti-Programmed Death Protein 1 (PD-1) Therapy in Thoracic Neoplasms. Cancer Biol. Med. 2021, 18, 1040. [Google Scholar] [CrossRef]
- Botticelli, A.; Vernocchi, P.; Marini, F.; Quagliariello, A.; Cerbelli, B.; Reddel, S.; del Chierico, F.; di Pietro, F.; Giusti, R.; Tomassini, A.; et al. Gut Metabolomics Profiling of Non-Small Cell Lung Cancer (NSCLC) Patients under Immunotherapy Treatment. J. Transl. Med. 2020, 18, 49. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome Is Associated with Favorable Responses to Anti–Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef]
- Grenda, A.; Iwan, E.; Chmielewska, I.; Krawczyk, P.; Giza, A.; Bomba, A.; Frąk, M.; Rolska, A.; Szczyrek, M.; Kieszko, R.; et al. Presence of Akkermansiaceae in gut microbiome and immunotherapy effectiveness in patients with advanced non-small cell lung cancer. AMB Express 2022, 12, 86. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.F.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti-PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut Microbiome Affects the Response to Anti-PD-1 Immunotherapy in Patients with Hepatocellular Carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Gopalakrishnan, V.; Daillère, R.; Zitvogel, L.; Wargo, J.A.; Kroemer, G. The Gut Microbiota Influences Anticancer Immunosurveillance and General Health. Nat. Rev. Clin. Oncol. 2018, 15, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.W.; Xia, K.; Liu, Y.W.; Liu, J.H.; Rao, S.S.; Hu, X.K.; Chen, C.Y.; Xu, R.; Wang, Z.X.; Xie, H. Extracellular Vesicles from Akkermansia Muciniphila Elicit Antitumor Immunity against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. Int. J. Nanomed. 2021, 16, 2949–2963. [Google Scholar] [CrossRef]
- Bae, M.; Cassilly, C.D.; Liu, X.; Park, S.M.; Tusi, B.K.; Chen, X.; Kwon, J.; Filipčík, P.; Bolze, A.S.; Liu, Z.; et al. Akkermansia Muciniphila Phospholipid Induces Homeostatic Immune Responses. Nature 2022, 608, 168–173. [Google Scholar] [CrossRef]
- Xu, X.; Lv, J.; Guo, F.; Li, J.; Jia, Y.; Jiang, D.; Wang, N.; Zhang, C.; Kong, L.; Liu, Y.; et al. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front. Microbiol. 2020, 11, 814. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, X.; Chen, S.; Fu, X.; Ma, G.; Zhang, A. Akkermansia Muciniphila Enhances the Antitumor Effect of Cisplatin in Lewis Lung Cancer Mice. J. Immunol. Res. 2020, 2020, 2969287. [Google Scholar] [CrossRef]
- Katayama, Y.; Yamada, T.; Shimamoto, T.; Iwasaku, M.; Kaneko, Y.; Uchino, J.; Takayama, K. The Role of the Gut Microbiome on the Efficacy of Immune Checkpoint Inhibitors in Japanese Responder Patients with Advanced Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2019, 8, 847–853. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef]
- Saxami, G.; Karapetsas, A.; Chondrou, P.; Vasiliadis, S.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Botaitis, S.; Simopoulos, C.; Galanis, A. Potentially Probiotic Lactobacillus Strains with Anti-Proliferative Activity Induce Cytokine/Chemokine Production and Neutrophil Recruitment in Mice. Benef. Microbes 2017, 8, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Chondrou, P.; Karapetsas, A.; Kiousi, D.E.; Vasileiadis, S.; Ypsilantis, P.; Botaitis, S.; Alexopoulos, A.; Plessas, S.; Bezirtzoglou, E.; Galanis, A. Assessment of the Immunomodulatory Properties of the Probiotic Strain Lactobacillus Paracasei K5 In Vitro and In Vivo. Microorganisms 2020, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Yang, D.; Wang, H.; Cui, X.; Si, X.; Zhang, X.; Zhang, L. Relationship between the Efficacy of Immunotherapy and Characteristics of Specific Tumor Mutation Genes in Non-Small Cell Lung Cancer Patients. Thorac. Cancer 2020, 11, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Boyle, F.; Pavlakis, N.; Clarke, S.; Eade, T.; Hruby, G.; Lamoury, G.; Carroll, S.; Morgia, M.; Kneebone, A.; et al. The Gut Microbiome and Cancer Immunotherapy: Can We Use the Gut Microbiome as a Predictive Biomarker for Clinical Response in Cancer Immunotherapy? Cancers 2021, 13, 4824. [Google Scholar] [CrossRef] [PubMed]
- Heshiki, Y.; Vazquez-Uribe, R.; Li, J.; Ni, Y.; Quainoo, S.; Imamovic, L.; Li, J.; Sørensen, M.; Chow, B.K.C.; Weiss, G.J.; et al. Predictable Modulation of Cancer Treatment Outcomes by the Gut Microbiota. Microbiome 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Ho, K.; Hwang, E.K.; Peña, C.; Brouwer, J.; Hoffman, K.; Betel, D.; Sonnenberg, G.F.; Faltas, B.; Saxena, A.; et al. Impact of Use of Antibiotics on Response to Immune Checkpoint Inhibitors and Tumor Microenvironment. Am. J. Clin. Oncol. 2021, 44, 247–253. [Google Scholar] [CrossRef]
- Jiang, S.; Geng, S.; Chen, Q.; Zhang, C.; Cheng, M.; Yu, Y.; Zhang, S.; Shi, N.; Dong, M. Effects of Concomitant Antibiotics Use on Immune Checkpoint Inhibitor Efficacy in Cancer Patients. Front. Oncol. 2022, 12, 823705. [Google Scholar] [CrossRef]
- Serpas Higbie, V.; Rogers, J.; Hwang, H.; Qiao, W.; Xiao, L.; Dasari, A.; Mola-Rudd, K.; Morris, V.K.; Wolff, R.A.; Raghav, K.; et al. Antibiotic Exposure Does Not Impact Immune Checkpoint Blockade Response in MSI-H/dMMR Metastatic Colorectal Cancer: A Single-Center Experience. Oncologist 2022, 27, 952–957. [Google Scholar] [CrossRef]
- Kaderbhai, C.; Richard, C.; Fumet, J.D.; Aarnink, A.; Foucher, P.; Coudert, B.; Favier, L.; Lagrange, A.; Limagne, E.; Boidot, R.; et al. Antibiotic Use Does Not Appear to Influence Response to Nivolumab. Anticancer Res. 2017, 37, 3195–3200. [Google Scholar] [CrossRef]
- Lau, H.C.H.; Sung, J.J.Y.; Yu, J. Gut Microbiota: Impacts on Gastrointestinal Cancer Immunotherapy. Gut Microbes 2021, 13, 1869504. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Q.; Zhu, H.; Xu, H.; Long, W.; Yu, B.; Zhou, L.; Xu, H.; Wu, Y.; Su, Z. Resveratrol Ameliorates Lewis Lung Carcinoma-Bearing Mice Development, Decreases Granulocytic Myeloid-Derived Suppressor Cell Accumulation and Impairs Its Suppressive Ability. Cancer Sci. 2018, 109, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Nannini, G.; Dinu, M.; Pagliai, G.; Sofi, F.; Amedei, A. World Journal of Gastroenterology Exploring the Food-Gut Axis in Immunotherapy Response of Cancer Patients Conflict-of-Interest Statement. FCR 2017, 26, 4919–4932. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Alemao, C.A.; Budden, K.F.; Gomez, H.M.; Rehman, S.F.; Marshall, J.E.; Shukla, S.D.; Donovan, C.; Forster, S.C.; Yang, I.A.; Keely, S.; et al. Impact of Diet and the Bacterial Microbiome on the Mucous Barrier and Immune Disorders. Allergy Eur. J. Allergy Clin. Immunol. 2021, 76, 714–734. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Wadud Khan, M.A.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary Fiber and Probiotics Influence the Gut Microbiome and Melanoma Immunotherapy Response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Recent Advances in Understanding the Structure and Function of the Human Microbiome. Front. Microbiol. 2022, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Zilberman-Schapira, G.; Suez, J.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Kotler, E.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.Z.; et al. Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 2018, 174, 1388–1405.e21. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49. [Google Scholar] [CrossRef]
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M.; Yamada, T.; Miki, T.; Sakai, T. Clostridium Butyricum MIYAIRI 588 Shows Antitumor Effects by Enhancing the Release of TRAIL from Neutrophils through MMP-8. Int. J. Oncol. 2013, 42, 903–911. [Google Scholar] [CrossRef]
- FDA Approves First Fecal Microbiota Product|FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-fecal-microbiota-product (accessed on 12 February 2023).
- Sbahi, H.; di Palma, J.A. Faecal Microbiota Transplantation: Applications and Limitations in Treating Gastrointestinal Disorders. BMJ Open Gastroenterol. 2016, 3, 87. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal Microbiota Transplant Overcomes Resistance to Anti-PD-1 Therapy in Melanoma Patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Problems with the Concept of Gut Microbiota Dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic-Cvijin, I.; Sklar, J.; Jiang, L.; Natarajan, L.; Knight, R.; Belkaid, Y. Host Variables Confound Gut Microbiota Studies of Human Disease. Nature 2020, 587, 448–454. [Google Scholar] [CrossRef]
- Fessler, J.; Matson, V.; Gajewski, T.F. Exploring the Emerging Role of the Microbiome in Cancer Immunotherapy. J. Immunother. Cancer 2019, 7, 108. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Xiao, X.; Zheng, J.; Zhou, H. Metaproteomics: A Strategy to Study the Taxonomy and Functionality of the Gut Microbiota. J. Proteom. 2020, 219, 103737. [Google Scholar] [CrossRef]
- Issa Isaac, N.; Philippe, D.; Nicholas, A.; Raoult, D.; Eric, C. Metaproteomics of the Human Gut Microbiota: Challenges and Contributions to Other OMICS. Clin. Mass Spectrom. 2019, 14, 18–30. [Google Scholar] [CrossRef]
- Karaduta, O.; Dvanajscak, Z.; Zybailov, B.; Donovan, M. Metaproteomics-An Advantageous Option in Studies of Host-Microbiota Interaction. Microorganisms 2021, 9, 980. [Google Scholar] [CrossRef]
- Vuik, F.; Dicksved, J.; Lam, S.Y.; Fuhler, G.M.; van der Laan, L.; van de Winkel, A.; Konstantinov, S.R.; Spaander, M.; Peppelenbosch, M.P.; Engstrand, L.; et al. Composition of the Mucosa-Associated Microbiota along the Entire Gastrointestinal Tract of Human Individuals. United Eur. Gastroenterol. J. 2019, 7, 897–907. [Google Scholar] [CrossRef]
- Clavel, T.; Horz, H.P.; Segata, N.; Vehreschild, M. Next Steps after 15 Stimulating Years of Human Gut Microbiome Research. Microb. Biotechnol. 2022, 15, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jin, G.; Wang, G.; Liu, T.; Liu, X.; Wang, B.; Cao, H. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front. Cell Infect. Microbiol. 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.M.; de Goffau, M.C.; Elisa Perez-Muñoz, M.; Arrieta, M.-C.; Bäckhed, F.; Braun, T.; Bushman, F.D.; Dore, J.; de Vos, W.M.; Earl, A.M.; et al. Questioning the Fetal Microbiome Illustrates Pitfalls of Low-Biomass Microbial Studies Check for Updates. Nature 2023, 613, 61. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Liu, L.; Gong, T.; Tao, W.; Lin, B.; Li, C.; Zheng, X.; Zhu, S.; Jiang, W.; Zhou, R. Commensal Viruses Maintain Intestinal Intraepithelial Lymphocytes via Noncanonical RIG-I Signaling. Nat. Immunol. 2019, 20, 1681–1691. [Google Scholar] [CrossRef]
- Pérez, J.C. Fungi of the Human Gut Microbiota: Roles and Significance. Int. J. Med. Microbiol. 2021, 311, 151490. [Google Scholar] [CrossRef]
- Wang, W.L.; Xu, S.Y.; Ren, Z.G.; Tao, L.; Jiang, J.W.; Zheng, S.S. Application of metagenomics in the human gut microbiome. World J. Gastroenterol. 2015, 21, 803–814. [Google Scholar] [CrossRef]
- Sorbara, M.T.; Pamer, E.G. Microbiome-Based Therapeutics. Nat. Rev. Microbiol. 2022, 20, 365–380. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [PubMed]
- EFSA Statement on the Requirements for Whole Genome Sequence Analysis of Microorganisms Intentionally Used in the Food Chain. EFSA J. 2021, 19, e06506. [CrossRef]
- Stergiou, O.S.; Tegopoulos, K.; Kiousi, D.E.; Tsifintaris, M.; Papageorgiou, A.C.; Tassou, C.C.; Chorianopoulos, N.; Kolovos, P.; Galanis, A. Whole-Genome Sequencing, Phylogenetic and Genomic Analysis of Lactiplantibacillus Pentosus L33, a Potential Probiotic Strain Isolated from Fermented Sausages. Front. Microbiol. 2021, 12, 746659. [Google Scholar] [CrossRef] [PubMed]
- Tegopoulos, K.; Stergiou, O.S.; Kiousi, D.E.; Tsifintaris, M.; Koletsou, E.; Papageorgiou, A.C.; Argyri, A.A.; Chorianopoulos, N.; Galanis, A.; Kolovos, P. Genomic and Phylogenetic Analysis of Lactiplantibacillus Plantarum L125, and Evaluation of Its Anti-Proliferative and Cytotoxic Activity in Cancer Cells. Biomedicines 2021, 9, 1718. [Google Scholar] [CrossRef]
- Kiousi, D.E.; Efstathiou, C.; Tegopoulos, K.; Mantzourani, I.; Alexopoulos, A.; Plessas, S.; Kolovos, P.; Koffa, M.; Galanis, A. Genomic Insight into Lacticaseibacillus Paracasei SP5, Reveals Genes and Gene Clusters of Probiotic Interest and Biotechnological Potential. Front. Microbiol. 2022, 13, 922689. [Google Scholar] [CrossRef] [PubMed]
- Mirji, G.; Worth, A.; Bhat, S.A.; el Sayed, M.; Kannan, T.; Goldman, A.R.; Tang, H.Y.; Liu, Q.; Auslander, N.; Dang, C.V.; et al. The Microbiome-Derived Metabolite TMAO Drives Immune Activation and Boosts Responses to Immune Checkpoint Blockade in Pancreatic Cancer. Sci. Immunol. 2022, 7, eabn0704. [Google Scholar] [CrossRef]
- Kawanabe-Matsuda, H.; Takeda, K.; Nakamura, M.; Makino, S.; Karasaki, T.; Kakimi, K.; Nishimukai, M.; Ohno, T.; Omi, J.; Kano, K.; et al. Dietary Lactobacillus-Derived Exopolysaccharide Enhances Immune-Checkpoint Blockade Therapy. Cancer Discov. 2022, 12, 1336. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Masirah, N.; Zain, M.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, Risk and Safety of Faecal Microbiota Transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Ng, S.C.; Kamm, M.A.; Yeoh, Y.K.; Chan, P.K.S.; Zuo, T.; Tang, W.; Sood, A.; Andoh, A.; Ohmiya, N.; Zhou, Y.; et al. Scientific Frontiers in Faecal Microbiota Transplantation: Joint Document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 2020, 69, 83. [Google Scholar] [CrossRef]
- Bibbò, S.; Settanni, C.R.; Porcari, S.; Bocchino, E.; Ianiro, G.; Cammarota, G.; Gasbarrini, A. Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J. Clin. Med. 2020, 9, 1757. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-Generation Probiotics: The Spectrum from Probiotics to Live Biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Jo, J.H.; Zhang, Z.; MacGibeny, M.A.; Han, J.; Proctor, D.M.; Taylor, M.E.; Che, Y.; Juneau, P.; Apolo, A.B.; et al. Predicting Cancer Immunotherapy Response from Gut Microbiomes Using Machine Learning Models. Oncotarget 2022, 13, 876. [Google Scholar] [CrossRef] [PubMed]
- Limeta, A.; Ji, B.; Levin, M.; Gatto, F.; Nielsen, J. Meta-Analysis of the Gut Microbiota in Predicting Response to Cancer Immunotherapy in Metastatic Melanoma. JCI Insight 2020, 5, e140940. [Google Scholar] [CrossRef] [PubMed]
| NCT Number | Cancer Type—Disease Stage | Sample Size | Immunotherapy Type | Samples | Purpose—Expected Findings |
|---|---|---|---|---|---|
| NCT04136470 | NSCLC Melanoma | 130 | ICIs (anti-PD-1, anti-PD-L1 or anti-CTLA-4) | Feces, Blood, Biopsy | Detection of differences in GM between ICI responders and non-responders. |
| NCT04957511 | Gynecologic (advanced or recurrent) | 30 | ICIs (Not specified) | Feces, Blood, Saliva, Vaginal swab | Inter- and intra-patient microbiome changes related to immunotherapy. Association with the response to treatment. |
| NCT04636775 | NSCLC (I-IV) | 46 | PD-1 blockade | Feces, Nasal and Buccal swabs | Association between GM and prediction of the effectiveness of immunotherapy treatment. |
| NCT03643289 | Melanoma (III/IV) | 450 | ICIs (Not specified) | Feces, Blood | Assessment of the impact of the GM on treatment response rates and side effects induced by immunotherapy. |
| NCT04107168 | Melanoma, Renal, Lung (III/IV) | 1800 | ICIs (anti-PD-1, anti-PD-L1 or anti-CTLA-4) | Feces, Saliva | GM correlations with efficacy and toxicity of ICIs in patients with advanced cancer. |
| NCT05037825 | NSCLC, Melanoma, RCC, Triple-Negative Breast | 800 | ICIs (anti-PD-1, anti-PD-L1 or anti-CTLA-4) | Feces, Blood | Associations between the gut microbiota (composition and function), host immune system, and ICI treatment efficacy. |
| NCT04954885 | Lung (III-IV), NSCLC (IV) | 150 | ICIs (PD-1 blockade) | Feces | Estimation of the extent to which future interventions that seek to rationally modify the gut microbiome and/or functional status can improve outcomes. |
| NCT04579978 | Advanced solid tumor | 60 | ICIs (Not specified) | Feces, Blood | Characterization of the diversity of gut bacteria and assessment of the potential mechanisms by which gut bacteria impact the immune response. |
| NCT04435964 | Melanoma, Lung, Head and Neck, Urogenital Neoplasms, Breast | 100 | ICIs (Not specified) | Feces, Blood | Investigation of sex differences in irAEs in relation to clinical factors and genetic, immunological, and hormonal profiles. |
| NCT04243720 | Solid tumors, Metastatic cancers | 100 | Not specified | Feces, Blood, Tumor sample | Investigation of resistance to immunotherapy and its correlation with different genomic, transcriptomic, immunophenotypic, and/or epigenetic profiles. |
| NCT04204434 | Advanced-stage cancer | 150 | ICIs (Not specified) | Feces, Tissue, Blood, Plasma | Characterization of serum and microbial predictors of response to response and toxicity. |
| NCT04913311 | NSCLC | 150 | ICIs (Not specified) and chemotherapy | Feces, Blood, Saliva | Creation of database by correlating blood, stool and saliva biomarkers, and data from lung function tests with treatment outcomes and side effects. |
| NCT Number | Status | Cancer Type (Disease Stage) | Sample Size | Immunotherapy Type | Intervention | Purpose—Outcomes | Type of Study | Phase |
|---|---|---|---|---|---|---|---|---|
| NCT04645680 | Recruiting | Cutaneous melanoma (III–IV), MM, UM | 42 | PD-1 blockade | Dietary intervention | Changes in systemic and tumor immunity, microbiome, and metabolic profile of patients, QoL, symptom profile, incidence of AE | Randomized, parallel assignment, double blind | Phase II |
| NCT04866810 | Recruiting | UM | 80 | Anti-PD-1/PD-L1 monotherapy | Dietary intervention | PFS, QoL, ORR | Randomized, parallel assignment, open label | N/A |
| NCT05384873 | Not yet recruiting | NSCLC | 180 | Not specified | Dietary intervention | PFS, QoL, DoR, incidence of AE, physical activity level | Randomized, parallel assignment, open label | N/A |
| NCT04636775 | Recruiting | NSCLC (IV), recurrent NSCLC | 46 | PD-1/PD-L1 blockade | Observational | Correlation of gut microbiota with response, adverse effects incidence, tumor tissue PD-L1 expression and diet | Observational, cohort prospective study | N/A |
| NCT05083416 | Recruiting | Head and neck | 62 | Not specified | Dietary intervention (Fasting) | Compliance, correlation of gut microbiome and microbial metabolites | Non-randomized, parallel assignment, open label | N/A |
| NCT04009122 | Active, not recruiting | NSCLC | 206 | Not specified | Dietary intervention | QoL, changes in microbiota, interleukin, and cytokine levels | Randomized, parallel assignment, quadruple masking | N/A |
| NCT05119010 | Not yet recruiting | Metastatic RCC (IV) | 60 | Anti-PD-1/anti-CTLA-4 combinatory treatment | Dietary intervention | QoL, OS, ORR, safety assessment, PFS | Non-randomized, parallel assignment, open label | N/A |
| NCT05032014 | Recruiting | Liver | 46 | PD-1 blockade | Dietary intervention L. rhamnosus Probio-M9 | Objective remission rate, PFS, OS | Randomized, parallel assignment, quadruple masking | N/A |
| NCT05094167 | Recruiting | NSCLC | 46 | PD-1 blockade | Dietary intervention L. Bifidobacterium V9 (Kex02) | Objective remission rate, PFS, OS | Randomized, parallel assignment, quadruple masking | N/A |
| NCT04699721 | Recruiting | NSCLC (III) | 40 | PD-1 blockade and chemotherapy | Dietary intervention—BiFico powder | Adverse effects, ORR, DFS, OS | Single group assignment, open label | Phase I |
| NCT03829111 | Active, not recruiting | RCC (III–IV), Unresectable RCC | 30 | Anti-PD-1/anti-CTLA-4 | Dietary intervention—Clostridium butyricum CBM 588 | OS, PFS, change in feces bifidobacterial count, change in Shannon index | Randomized, parallel assignment, open label | Phase I |
| NCT05220124 | Recruiting | Bladder Urothelial Carcinoma | 190 | Not specified | Dietary intervention Bifidobacterium, Lactobacillus and Enterococcus capsules | PFS, DoR, OS, ORR, SAE | Randomized, parallel assignment, open label | Phase IV |
| NCT05122546 | Recruiting | RCC (III–IV), Unresectable RCC, Metastatic RCC | 30 | Not specified | Dietary intervention—Clostridium butyricum CBM 588 | OS, PFS, change in feces bifidobacterial count, change in Shannon index, immunomodulation | Randomized, parallel assignment, open label | Phase I |
| NCT04163289 | Recruiting | RCC (III–IV) | 20 | PD-1 blockade | FMT Donors: HC | Safety of FMT combination treatment Changes in the immune populations, microbiome profile of patients, response to treatment, and OS | Single group, open Label | Phase I |
| NCT04264975 | Recruiting | Solid Carcinoma | 60 | Not specified | FMT (via colonoscopy) Donors: Patients with CR or PR | Prospects of utilization of microbiome as biomarkers and therapeutics in immuno-oncology | Single group, open label | N/A |
| NCT03353402 | Unknown | MM (IV) Unresectable Melanoma (III) | 40 | Not specified | FMT (via colonoscopy and oral capsules) Donors: Patients with MM who responded to immuno-therapy | Safety of FMT treatment. Changes in the composition and activity of immune populations, response to treatment. | Single group, open label | Phase I |
| NCT04521075 | Recruiting | MM (IV) Unresectable Melanoma (III) NSCLC (IV) | 42 | PD-1 blockade | FMT (oral capsules) Donors: Patients with DR, CR | FMT-related AE, ORR, PFS, OS, DoR, irAEs, immune activation markers | Single group, open label | Phase I and II |
| NCT04577729 | Recruiting | Melanoma (III–IV) | 60 | Not specified | FMT (oral capsules) Fecal implant donors; Prior malignant melanoma patients in remission for at least 1 year after ICIs | PFS, gut microbiota analysis, adverse effects, neutrophil-to-lymphocyte ratio | Randomized parallel assignment, double blind | N/A |
| NCT03341143 | Active, not recruiting | Melanoma | 18 | PD-1 blockade | FMT (via colonoscopy) Donors: Patients treated with a PD-1 inhibitor, rendered disease-free as a result | ORR, OS, immune parameters, frequency of grade III/IV toxicities | Single group, open label | Phase I |
| NCT03772899 | Active, not recruiting | Melanoma (advanced stage) | 20 | PD-1 blockade | FMT (oral capsules) Donors: HC | Safety assessment, ORR | Single group, open label | Phase I |
| NCT04116775 | Recruiting | mCRPC | 32 | PD-1 blockade | FMT (via endoscopy) Donors: Patients who respond to treatment at an earlier stage | Anticancer effect of FMT | Single group, open label | Phase II |
| NCT04988841 | Recruiting | Unresectable or MM (III/IV) | 60 | ICIs (anti-PD-1 or anti-CTLA-4) | FMT-pooled donor | Assessment of the tolerance and clinical benefit of FMT | Randomized parallel assignment, double blind | Phase II |
| NCT04951583 | Recruiting | NSCLC, Advanced Melanoma (IV) | 82 | PD-1 blockade | FMT (Investigational capsules) | Assessment of the impact of FMT on ICI response and survival Evaluation of the changes in patient’s GM composition and tumor microenvironment contexture following the combination treatment of ICI and FMT | Single group, open label | Phase II |
| NCT05502913 | Not yet recruiting | Metastatic Lung Cancer | 80 | PD-1 blockade | FMT (Oral capsules) Donors: CR | PFS, OS, ORR, microbiome analysis, safety, feasibility, immunomodulation | Randomized, parallel assignment, quadruple masking | Phase II |
| NCT05286294 | Recruiting | Melanoma (IV), Head and Neck Squamous Cell Carcinoma, Cutaneous Squamous Cell Carcinoma, Clear Cell Renal Cell Carcinoma | 20 | Not specified | FMT (Oral capsules) Donors: ICI R | PFS, OS, ORR, microbiome analysis, safety, feasibility, immunomodulation, QoL | Single group, open label | Phase II |
| NCT05008861 | Not yet recruiting | Advanced or Metastatic NSCLC | 20 | PD-1/PD-L1 blockade | FMT (Oral capsules) | ORR, microbiome analysis, safety, FMT-related adverse effects or treatment-related adverse effects, immunomodulation | Single group, open label | Phase I |
| NCT05251389 | Recruiting | Melanoma (III–IV) | 24 | Not specified | FMT from responders or non-responders to ICI treatment | Efficacy (SD, PR, CR), microbiome analysis, safety, immunomodulation, changes in metabolome | Randomized, parallel assignment, quadruple masking | Phase I/II |
| NCT04924374 | Recruiting | NSCLC (III–IV) | 20 | PD-1 blockade | FMT (Oral capsules) Pooled fecal microbiota capsules from 1 donor based on composition | Safety and efficacy (iRECIST criteria) | Randomized, parallel assignment, open label | N/A |
| NCT04729322 | Recruiting | CRC (IV), Metastatic CRC, Small intestinal adenocarcinoma (IV), Metastatic small intestinal adenocarcinoma | 15 | PD-1 blockade | FMT (via colonoscopy) Donors: PD-1 responding CRC patients | ORR | Non-randomized, parallel assignment, open label | Phase II |
| NCT04758507 | Recruiting | RCC | 50 | Not specified | FMT (via colonoscopy and frozen capsules) Donors: ICI R | PFS, PR or CR, OS, AE, gut microbiota diversity | Randomized, parallel assignment, quadruple masking | Phase I/II |
| NCT03686202 | Active, not recruiting | Any Solid Tumor | 65 | PD-1/PD-L1 and/or anti-CTLA4 blockade | Microbial Ecocystem Therapeutics (MET, Oral administration) Donor: HC | Immunotherapy response, bacterial taxonomic diversity | Randomized, single group—open label | Phase II/III |
| NCT05273255 | Recruiting | Any Solid Tumor (IV) | 30 | Not specified | FMT (Colonoscopic)—Donors: ICI R with stage III or IV solid cancers | PFS, OS, ORR, AE, QoL, gut microbiome profiling, immunomodulation | Single group—open label | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiousi, D.E.; Kouroutzidou, A.Z.; Neanidis, K.; Karavanis, E.; Matthaios, D.; Pappa, A.; Galanis, A. The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions. Cancers 2023, 15, 2101. https://doi.org/10.3390/cancers15072101
Kiousi DE, Kouroutzidou AZ, Neanidis K, Karavanis E, Matthaios D, Pappa A, Galanis A. The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions. Cancers. 2023; 15(7):2101. https://doi.org/10.3390/cancers15072101
Chicago/Turabian StyleKiousi, Despoina E., Antonia Z. Kouroutzidou, Konstantinos Neanidis, Emmanuel Karavanis, Dimitrios Matthaios, Aglaia Pappa, and Alex Galanis. 2023. "The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions" Cancers 15, no. 7: 2101. https://doi.org/10.3390/cancers15072101
APA StyleKiousi, D. E., Kouroutzidou, A. Z., Neanidis, K., Karavanis, E., Matthaios, D., Pappa, A., & Galanis, A. (2023). The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions. Cancers, 15(7), 2101. https://doi.org/10.3390/cancers15072101












