Advances in Immunotherapy for Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Immune Subclass of Hepatocellular Carcinoma (Reported to Date)
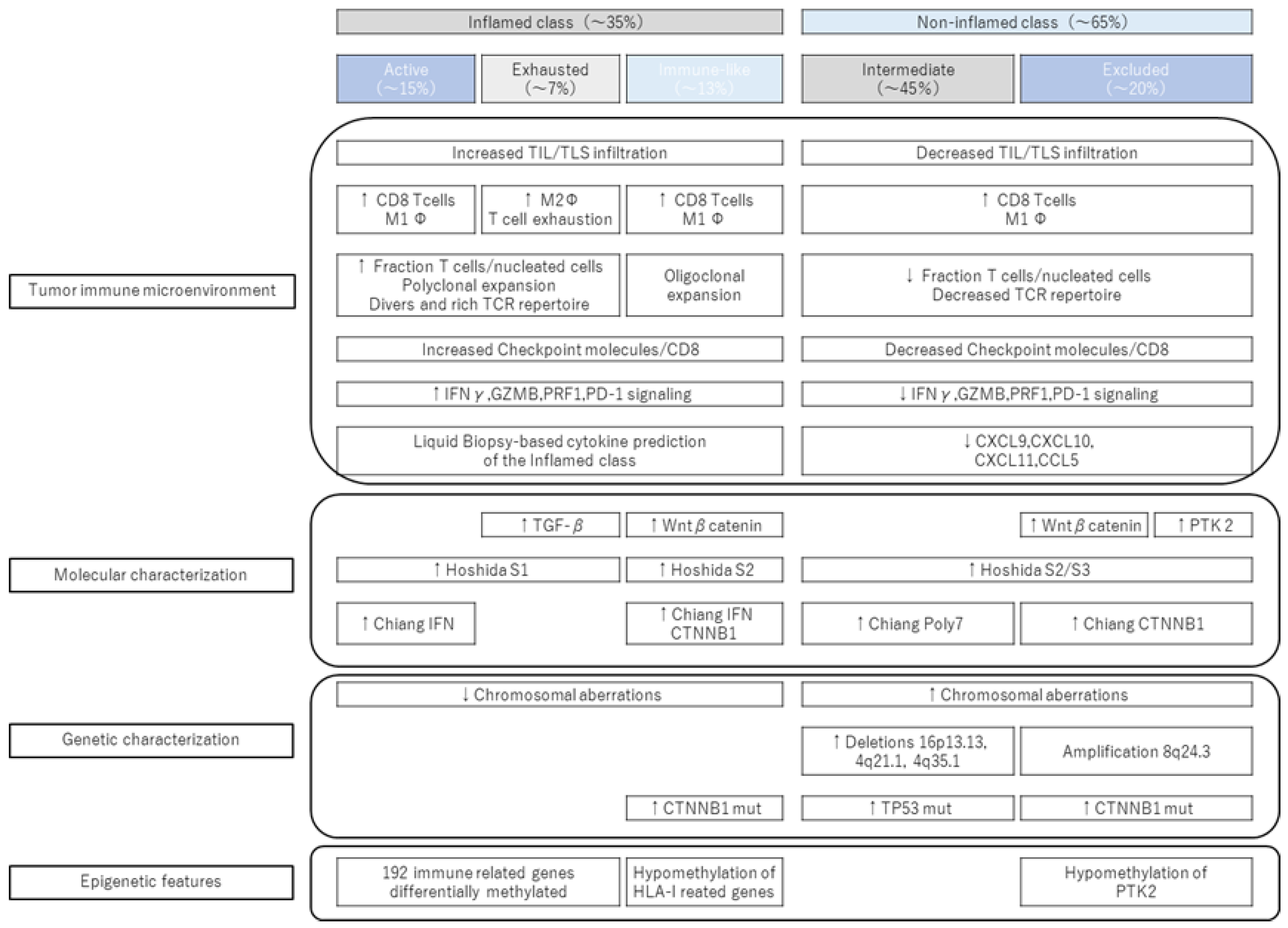
1.1.1. Inflamed Class and Noninflamed Class
1.1.2. CTNNB1 Mutation and Immune Exclusion
1.2. Proposing New Immune Subclasses
1.2.1. Inflamed Class
1.2.2. New Subclass of Inflamed Class (Proposal of Immune-like Subclass)
1.2.3. Noninflamed Class and Its Features
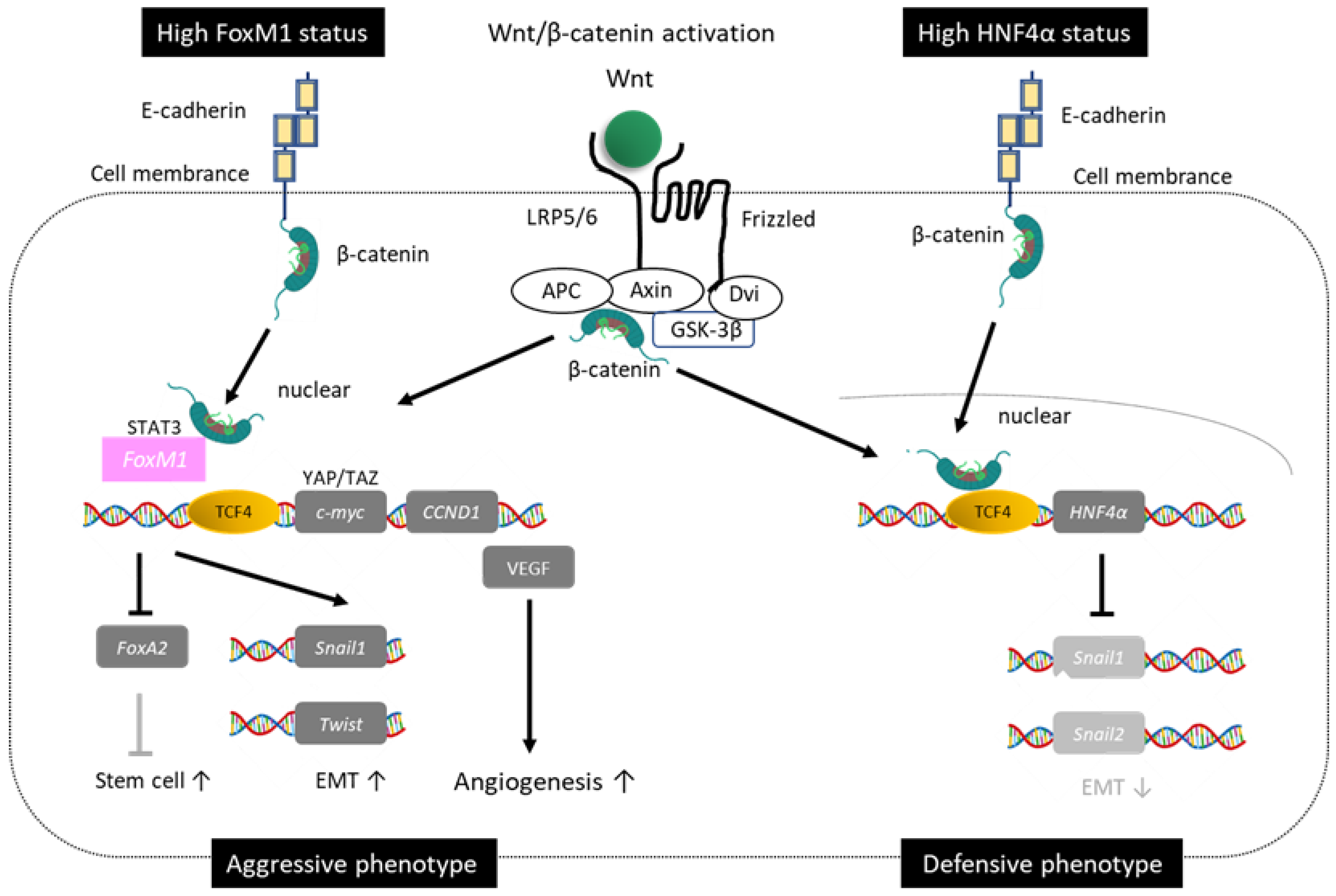
1.3. Duality of Wnt/β-Catenin Mutations
1.3.1. Good Prognosis Group for Wnt/β-Catenin
1.3.2. Poor Prognosis of Wnt/β-Catenin
1.4. Gadoxetic Acid-Enhanced Magnetic Resonance Imaging (EOB-MRI) as an Imaging Biomarker for Wnt/β-Catenin Mutations
1.5. Effects of Immunotherapy on Nonalcoholic Steatohepatitis (NASH)-HCC
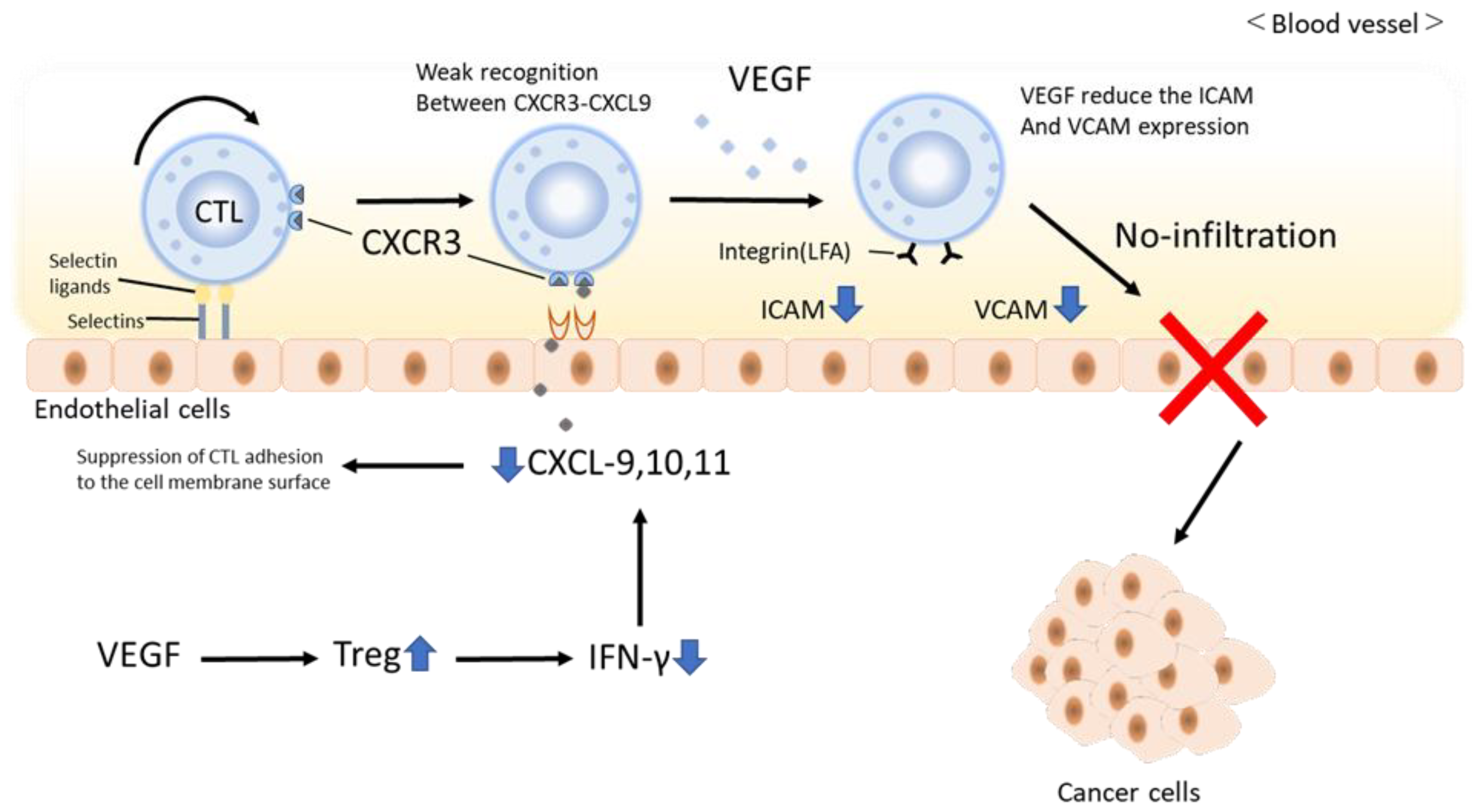
1.6. Does Atezolizumab and Bevacizumab Combination Therapy Transform Immune Cold Tumors into Immune Hot Tumors?
1.7. Onset Mechanism of Hyperprogression Disease in ICI Treatment
1.8. Hepatitis B (HBV) Reactivation by ICIs
1.9. The Latest Treatment Using ICIs: About Atezolizumab/Bevacizumab Curative (ABC) Conversion
- (1)
- Curative conversion after tumor shrinkage.
- (2)
- Curative conversion when tumor shrinkage is not obtained.
- (3)
- TACE-unsuitable nodules other than high tumor burden (multinodular, poorly differentiated HCC, amongst others).
- (4)
- Curative conversion during withdrawal due to AE.
- (5)
- Curative conversion for positron-emission tomography (PET)-positive HCC.
1.10. Mechanism of Immune-Related Adverse Effects (irAE) Onset by ICIs
1.11. Classifications of Tumor Microenvironment (TME) Factors
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Hagiwara, S.; Kudo, M.; Nagai, T.; Inoue, T.; Ueshima, K.; Nishida, N.; Watanabe, T.; Sakurai, T. Activation of JNK and high expression level of CD133 predict a poor response to sorafenib in hepatocellular carcinoma. Br. J. Cancer 2012, 106, 1997–2003. [Google Scholar] [CrossRef]
- Clarke, J.M.; Hurwitz, H.I. Targeted inhibition of VEGF receptor 2: An update on ramucirumab. Expert Opin. Biol. Ther. 2013, 13, 1187–1196. [Google Scholar] [CrossRef]
- Boucher, J.M.; Clark, R.P.; Chong, D.C.; Citrin, K.M.; Wylie, L.A.; Bautch, V.L. Dynamic alterations in decoy VEGF receptor-1 stability regulate angiogenesis. Nat. Commun. 2017, 8, 15699. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Zheng, J.; Shao, M.; Yang, W.; Ren, J.; Chen, X.; Yang, H. Benefits of combination therapy with immune checkpoint inhibitors and predictive role of tumour mutation burden in hepatocellular carcinoma: A systematic review and meta-analysis. Int. Immunopharmacol. 2022, 112, 109244. [Google Scholar] [CrossRef]
- Spahn, S.; Roessler, D.; Pompilia, R.; Gabernet, G.; Gladstone, B.P.; Horger, M.; Biskup, S.; Feldhahn, M.; Nahnsen, S.; Hilke, F.J.; et al. Clinical and genetic tumor characteristics of responding and non-responding patients to PD-1 inhibition in hepatocellular carcinoma. Cancers 2020, 12, 3830. [Google Scholar] [CrossRef]
- Nishida, N.; Kudo, M. Immune phenotype and immune checkpoint inhibitors for the treatment of human hepatocellular carcinoma. Cancers 2020, 12, 1274. [Google Scholar] [CrossRef]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; Castro de Moura, M.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef]
- Pinyol, R.; Sia, D.; Llovet, J.M. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin. Cancer Res. 2019, 25, 2021–2023. [Google Scholar] [CrossRef]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. β-catenin Activation Promotes Immune Escape and Resistance to anti-PD-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Nishida, N.; Sakai, K.; Morita, M.; Aoki, T.; Takita, M.; Hagiwara, S.; Komeda, Y.; Takenaka, M.; Minami, Y.; Ida, H.; et al. Association between genetic and immunological background of hepatocellular carcinoma and expression of programmed cell Death-1. Liver Cancer 2020, 9, 426–439. [Google Scholar] [CrossRef]
- Morita, M.; Nishida, N.; Sakai, K.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; et al. Immunological microenvironment predicts the survival of the patients with hepatocellular carcinoma treated with anti-PD-1 antibody. Liver Cancer 2021, 10, 380–393. [Google Scholar] [CrossRef]
- Montironi, C.; Castet, F.; Haber, P.K.; Pinyol, R.; Torres-Martin, M.; Torrens, L.; Mesropian, A.; Wang, H.; Puigvehi, M.; Maeda, M.; et al. Inflamed and non-inflamed classes of HCC: A revised immunogenomic classification. Gut 2022, 72, 129–140. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Aoki, T.; Nishida, N.; Kudo, M. Clinical significance of the duality of Wnt/β-catenin signaling in human hepatocellular carcinoma. Cancers 2022, 14, 444. [Google Scholar] [CrossRef]
- Neth, P.; Ciccarella, M.; Egea, V.; Hoelters, J.; Jochum, M.; Ries, C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells 2006, 24, 1892–1903. [Google Scholar] [CrossRef]
- Narita, M.; Hatano, E.; Arizono, S.; Miyagawa-Hayashino, A.; Isoda, H.; Kitamura, K.; Taura, K.; Yasuchika, K.; Nitta, T.; Ikai, I.; et al. Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J. Gastroenterol. 2009, 44, 793–798. [Google Scholar] [CrossRef]
- Inagawa, S.; Itabashi, M.; Adachi, S.; Kawamoto, T.; Hori, M.; Shimazaki, J.; Yoshimi, F.; Fukao, K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: Correlation with tumor progression and postoperative survival. Clin. Cancer Res. 2002, 8, 450–456. [Google Scholar] [PubMed]
- Ueno, A.; Masugi, Y.; Yamazaki, K.; Komuta, M.; Effendi, K.; Tanami, Y.; Tsujikawa, H.; Tanimoto, A.; Okuda, S.; Itano, O.; et al. OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J. Hepatol. 2014, 61, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Kitao, A.; Matsui, O.; Yoneda, N.; Kozaka, K.; Kobayashi, S.; Sanada, J.; Koda, W.; Minami, T.; Inoue, D.; Yoshida, K.; et al. Hepatocellular Carcinoma with β-Catenin Mutation: Imaging and Pathologic Characteristics. Radiology 2015, 275, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Nishida, N.; Ueshima, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Ida, H.; Minami, Y.; Yamada, A.; et al. Higher Enhancement Intrahepatic Nodules on the Hepatobiliary Phase of Gd-EOB-DTPA-Enhanced MRI as a Poor Responsive Marker of Anti-PD-1/PD-L1 Monotherapy for Unresectable Hepatocellular Carcinoma. Liver Cancer 2021, 10, 615–628. [Google Scholar] [CrossRef]
- Kudo, M. Gd-EOB-DTPA-MRI Could Predict WNT/β-Catenin Mutation and Resistance to Immune Checkpoint Inhibitor Therapy in Hepatocellular Carcinoma. Liver Cancer 2020, 9, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kitao, A.; Matsui, O.; Hayashi, T.; Nio, K.; Kondo, M.; Ohno, N.; Miyati, T.; Okada, H.; Yamashita, T.; et al. Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology 2014, 60, 1674–1685. [Google Scholar] [CrossRef]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Pinyol, R.; Torrecilla, S.; Wang, H.; Montironi, C.; Piqué-Gili, M.; Torres-Martin, M.; Wei-Qiang, L.; Willoughby, C.E.; Ramadori, P.; Andreu-Oller, C.; et al. Molecular characterisation of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J. Hepatol. 2021, 75, 865–878. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A.C.; Donovan, D.J.; Thung, S.N.; Solé, M.; et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008, 68, 6779–6788. [Google Scholar] [CrossRef]
- Osada, T.; Chong, G.; Tansik, R.; Hong, T.; Spector, N.; Kumar, R.; Hurwitz, H.I.; Dev, I.; Nixon, A.B.; Lyerly, H.K.; et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol. Immunother. 2008, 57, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FASL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef] [PubMed]
- Voron, T.; Marcheteau, E.; Pernot, S.; Colussi, O.; Tartour, E.; Taieb, J.; Terme, M. Control of the immune response by pro-angiogenic factors. Front. Oncol. 2014, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-angiogenic agents-overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Sugimoto, M.; Patil, N.S.; Bower, D.; Suzuki, M.; Kato, C.; Yorozu, K.; Kurasawa, M.; Shames, D.S.; Kondoh, O. Both T cell priming in lymph node and CXCR3-dependent migration are the key events for predicting the response of atezolizumab. Sci. Rep. 2021, 11, 13912. [Google Scholar] [CrossRef]
- Ishikura, N.; Sugimoto, M.; Yorozu, K.; Kurasawa, M.; Kondoh, O. Anti-VEGF antibody triggers the effect of anti-PD-L1 antibody in PD-L1low and immune desert-like mouse tumors. Oncol. Rep. 2022, 47, 36. [Google Scholar] [CrossRef]
- Roman, J.; Rangasamy, T.; Guo, J.; Sugunan, S.; Meednu, N.; Packirisamy, G.; Shimoda, L.A.; Golding, A.; Semenza, G.; Georas, S.N. T-cell activation under hypoxic conditions enhances IFN-gamma secretion. Am. J. Respir. Cell Mol. Biol. 2010, 42, 123–128. [Google Scholar] [CrossRef]
- de Almeida, P.E.; Mak, J.; Hernandez, G.; Jesudason, R.; Herault, A.; Javinal, V.; Borneo, J.; Kim, J.M.; Walsh, K.B. Anti-VEGF treatment enhances CD8+ T-cell antitumor activity by amplifying hypoxia. Cancer Immunol. Res. 2020, 8, 806–818. [Google Scholar] [CrossRef]
- Gropper, Y.; Feferman, T.; Shalit, T.; Salame, T.M.; Porat, Z.; Shakhar, G. Culturing CTLs under hypoxic conditions enhances their cytolysis and improves their anti-tumor function. Cell Rep. 2017, 20, 2547–2555. [Google Scholar] [CrossRef]
- Kudo, M. Combination Immunotherapy with Anti-PD-1/PD-L1 Antibody plus Anti-VEGF Antibody May Promote Cytotoxic T Lymphocyte Infiltration in Hepatocellular Carcinoma, Including in the Noninflamed Subclass. Liver Cancer 2022, 11, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression-implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Koyama, S.; Itahashi, K.; Tanegashima, T.; Lin, Y.T.; Togashi, Y.; Kamada, T.; Irie, T.; Okumura, G.; Kono, H.; et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell 2022, 40, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Nishida, N.; Ida, H.; Ueshima, K.; Minami, Y.; Takita, M.; Aoki, T.; Morita, M.; Chishina, H.; Komeda, Y.; et al. Clinical implication of immune checkpoint inhibitor on the chronic hepatitis B virus infection. Hepatol. Res. 2022, 52, 754–761. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Chen, C.; Fang, W.; Cai, X.; Zhang, X.; Zhao, M.; Zhang, B.; Jiang, W.; Lin, Z.; et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J. Immunother. Cancer 2019, 7, 322. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, D.; Shim, J.H.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; Yoo, C.; Ryoo, B.Y.; Choi, J. Risk of Hepatitis B Virus Reactivation in Patients Treated With Immunotherapy for Anti-cancer Treatment. Clin. Gastroenterol. Hepatol. 2022, 20, 898–907. [Google Scholar] [CrossRef]
- Gane, E.; Verdon, D.J.; Brooks, A.E.; Gaggar, A.; Nguyen, A.H.; Subramanian, G.M.; Schwabe, C.; Dunbar, P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019, 71, 900–907. [Google Scholar] [CrossRef]
- Kudo, M.; Aoki, T.; Ueshima, K.; Tsuchiya, K.; Morita, M.; Chishina, H.; Takita, M.; Hagiwara, S.; Minami, Y.; Ida, H.; et al. Achievement of Complete Response and Drug-free Status by Atezolizumab Plus Bevacizumab Combined with or without Curative Conversion in Patients with Transarterial Chemoembolization-Unsuitable, Intermediate-stage Hepatocellular Carcinoma: A Multicenter Proof-of-Concept Study. Liver Cancer, 2023; in press. [Google Scholar]
- Kudo, M. A Novel Treatment Strategy for Patients with Intermediate-Stage HCC Who Are Not Suitable for TACE: Upfront Systemic Therapy Followed by Curative Conversion. Liver Cancer 2021, 10, 539–544. [Google Scholar] [CrossRef]
- Kudo, M. Atezolizumab plus Bevacizumab Followed by Curative Conversion (ABC Conversion) in Patients with Unresectable, TACE-Unsuitable Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2022, 11, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. New treatment paradigm with systemic therapy in intermediate-stage hepatocellular carcinoma. Int. J. Clin. Oncol. 2022, 27, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Ryoo, B.Y.; Hsu, C.H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. GO30140 investigators. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Han, K.H.; Ye, S.L.; Zhou, J.; Huang, Y.H.; Lin, S.M.; Wang, C.K.; Ikeda, M.; Chan, S.L.; Choo, S.P. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020, 9, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Kawamura, Y.; Hasegawa, K.; Tateishi, R.; Kariyama, K.; Shiina, S.; Toyoda, H.; Imai, Y.; Hiraoka, A.; Ikeda, M.; et al. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 2021, 10, 181–223. [Google Scholar] [CrossRef]
- Nakashima, Y.; Nakashima, O.; Tanaka, M.; Okuda, K.; Nakashima, M.; Kojiro, M. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol. Res. 2003, 26, 142–147. [Google Scholar] [CrossRef]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef]
- Rigo, P.; Paulus, P.; Kaschten, B.J.; Hustinx, R.; Bury, T.; Jerusalem, G.; Benoit, T.; Foidart-Willems, J. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur. J. Nucl. Med. 1996, 23, 1641–1674. [Google Scholar] [CrossRef]
- Seo, S.; Hatano, E.; Higashi, T.; Hara, T.; Tada, M.; Tamaki, N.; Iwaisako, K.; Ikai, I.; Uemoto, S. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin. Cancer Res. 2007, 13, 427–433. [Google Scholar] [CrossRef]
- Morio, K.; Kawaoka, T.; Aikata, H.; Namba, M.; Uchikawa, S.; Kodama, K.; Ohya, K.; Fujino, H.; Nakahara, T.; Murakami, E.; et al. Preoperative PET-CT is useful for predicting recurrent extrahepatic metastasis of hepatocellular carcinoma after resection. Eur. J. Radiol. 2020, 124, 108828. [Google Scholar] [CrossRef]
- Yaprak, O.; Acar, O.; Ertugrul, G.; Dayangac, M. Role of pre-transplant 18F-FDG PET/CT in predicting hepatocellular carcinoma recurrence after liver transplantation. World J. Gastrointest. Oncol. 2018, 10, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.J.; Mandaliya, R.; Nakshabandi, A.; Lewis, J.H. Hepatotoxicity induced by immune checkpoint inhibitors: A comprehensive review including current and alternative management strategies. Expert Opin. Drug Metab. Toxicol. 2019, 15, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, L.; Forschner, A.; Loquai, C.; Goldinger, S.M.; Zimmer, L.; Ugurel, S.; Schmidgen, M.I.; Gutzmer, R.; Utikal, J.S.; Göppner, D.; et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 2016, 60, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Watanabe, T.; Kudo, M.; Minaga, K.; Komeda, Y.; Kamata, K.; Kimura, M.; Hayashi, H.; Nakagawa, K.; Ueshima, K.; et al. Clinicopathological analysis of hepatic immune-related adverse events in comparison with autoimmune hepatitis and graft-versus host disease. Sci. Rep. 2021, 11, 9242. [Google Scholar] [CrossRef]
- Asano, T.; Meguri, Y.; Yoshioka, T.; Kishi, Y.; Iwamoto, M.; Nakamura, M.; Sando, Y.; Yagita, H.; Koreth, J.; Kim, H.T.; et al. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood 2017, 129, 2186–2197. [Google Scholar] [CrossRef]
- Kido, M.; Watanabe, N.; Okazaki, T.; Akamatsu, T.; Tanaka, J.; Saga, K.; Nishio, A.; Honjo, T.; Chiba, T. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology 2008, 135, 1333–1343. [Google Scholar] [CrossRef]
- Millian, D.E.; Saldarriaga, O.A.; Wanninger, T.; Burks, J.K.; Rafati, Y.N.; Gosnell, J.; Stevenson, H.L. Cutting-Edge Platforms for Analysis of Immune Cells in the Hepatic Microenvironment-Focus on Tumor-Associated Macrophages in Hepatocellular Carcinoma. Cancers 2022, 14, 1861. [Google Scholar] [CrossRef]
- Castella, B.; Mina, R.; Gay, F. CyTOF®: A New Tool to Decipher the Immunomodulatory Activity of Daratumumab. Cytom. A 2019, 95, 416–418. [Google Scholar] [CrossRef]
- Han, G.; Chen, S.Y.; Gonzalez, V.D.; Zunder, E.R.; Fantl, W.J.; Nolan, G.P. Atomic mass tag of bismuth-209 for increasing the immunoassay multiplexing capacity of mass cytometry. Cytom. A 2017, 91, 1150–1163. [Google Scholar] [CrossRef]
- Han, G.; Spitzer, M.H.; Bendall, S.C.; Fantl, W.J.; Nolan, G.P. Metal-isotope-tagged monoclonal antibodies for high-dimensional mass cytometry. Nat. Protoc. 2018, 13, 2121–2148. [Google Scholar] [CrossRef]
- Zhang, Q.; Lou, Y.; Yang, J.; Wang, J.; Feng, J.; Zhao, Y.; Wang, L.; Huang, X.; Fu, Q.; Ye, M.; et al. Integrated multiomic analysis reveals comprehensive tumour heterogeneity and novel immunophenotypic classification in hepatocellular carcinomas. Gut 2019, 68, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, R.; Nault, J.C.; Roberts, L.R.; Rossi, J.Z. Genomic Medicine and Implications for Hepatocellular Carcinoma Prevention and Therapy. Gastroenterology 2019, 156, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Furuta, M.; Totoki, Y.; Tsunoda, T.; Kato, M.; Shiraishi, Y.; Tanaka, H.; Taniguchi, H.; Kawakami, Y.; Ueno, M.; et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016, 48, 500–509. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, A.; Zhao, Y.; Ying, W.; Sun, H.; Yang, X.; Xing, B.; Sun, W.; Ren, L.; Hu, B.; et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 2019, 567, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Shen, Y.; Zhang, L.; Guo, X.; Wu, J. Understanding initiation and progression of hepatocellular carcinoma through single cell sequencing. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiwara, S.; Nishida, N.; Kudo, M. Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers 2023, 15, 2070. https://doi.org/10.3390/cancers15072070
Hagiwara S, Nishida N, Kudo M. Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers. 2023; 15(7):2070. https://doi.org/10.3390/cancers15072070
Chicago/Turabian StyleHagiwara, Satoru, Naoshi Nishida, and Masatoshi Kudo. 2023. "Advances in Immunotherapy for Hepatocellular Carcinoma" Cancers 15, no. 7: 2070. https://doi.org/10.3390/cancers15072070
APA StyleHagiwara, S., Nishida, N., & Kudo, M. (2023). Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers, 15(7), 2070. https://doi.org/10.3390/cancers15072070






