Role of β-Catenin Activation in the Tumor Immune Microenvironment and Immunotherapy of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
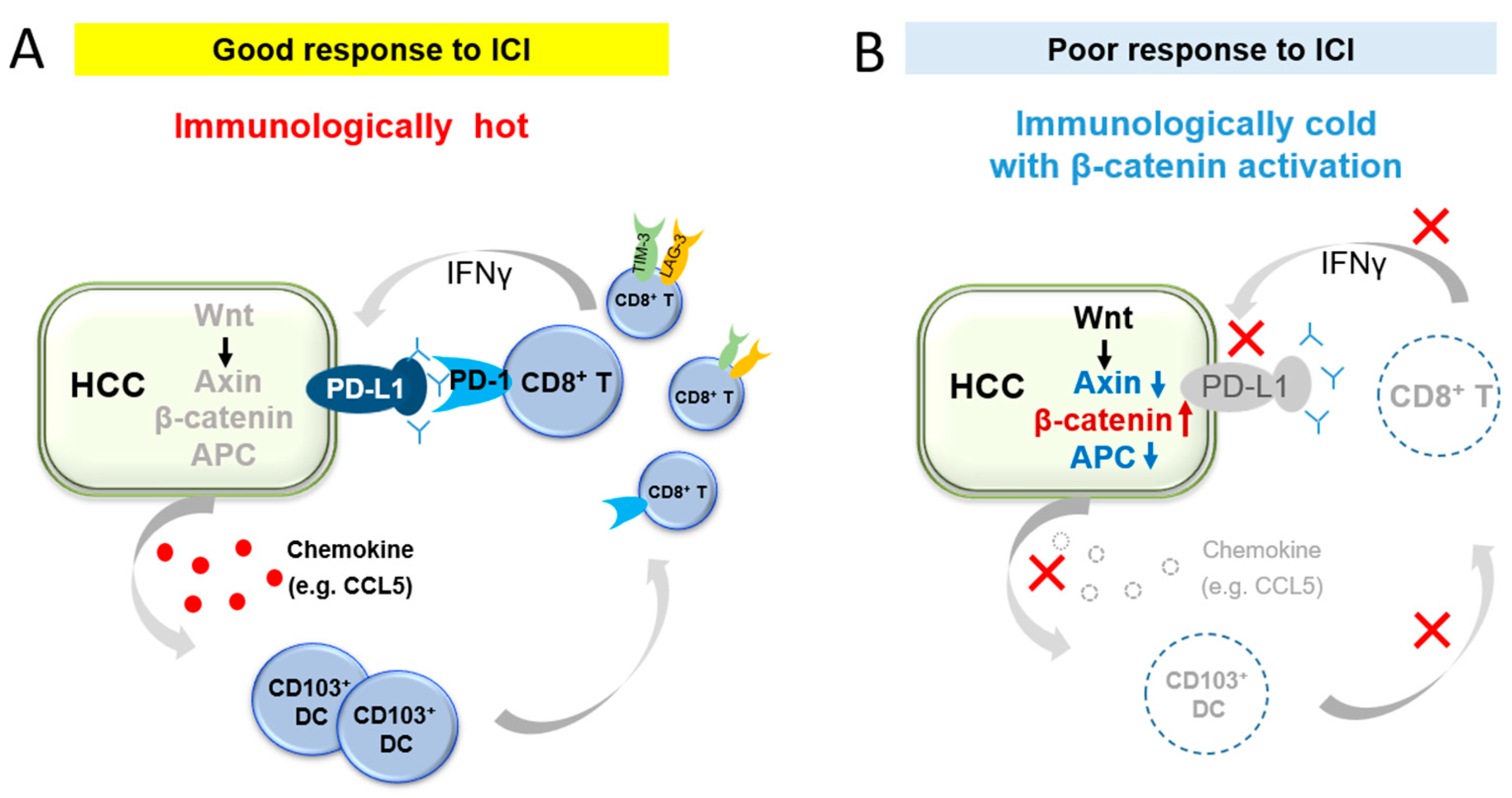
2. Immune Exclusion Associated with Activation of the β-Catenin Pathway according to Basic Studies
3. Relationship between β-Catenin Activation and ICI Effects in Human HCC
4. Relationship between β-Catenin Mutation and Immune-Related Gene Expression
5. Role of β-Catenin Mutation in the Classification of Immune Subclasses
5.1. Immune Subclass of HCC According to Pan-Cancer Analysis
5.2. HCC Immune Subclasses and β-Catenin Mutation
5.3. New Inflamed Subclass: Relationship between Immune-Like Subclass and CTNNB1 Mutation
5.4. Relationship between Nonalcoholic Steatohepatitis (NASH)/Nonalcoholic Fatty Liver Disease (NAFLD) HCC and β-Catenin Mutation
6. Factors Affecting the Wnt/β-Catenin Pathway
6.1. Molecules Acting on Wnt-Frizzled Complex and LPR5/6
6.2. Molecules Acting on GSK3β
6.3. Molecules Acting on Axin
6.4. Molecules Acting on Dvl
6.5. Molecules Acting on Adenomatous Polyposis coli (APC)
6.6. Molecules Acting on βcatenin and T-Cell Factor (TCF)/Lymphoid Enhancer Factor (LEF)
7. Development of Therapeutic Agents Targeting the β-Catenin Pathway
7.1. Small Molecule Wnt Pathway Inhibitor
7.2. The Potential of Kinase Inhibitors to Improve the Tumor Immune Microenvironment from Immunologically Cold to Hot
8. Potential Synergistic Effects of Combining Kinase Inhibitors and Wnt/β-Catenin Inhibitors with ICIs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altekruse, S.F.; Henley, S.J.; Cucinelli, J.E.; McGlynn, K.A. Changing Hepatocellular Carcinoma Incidence and Liver Cancer Mortality Rates in the United States. Am. J. Gastroenterol. 2014, 109, 542–553. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular Therapies and Precision Medicine for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in Patients with Advanced Hepatocellular Carcinoma (Checkmate 040): An Open-Label, Non-Comparative, Phase 1/2 Dose Escalation and Expansion Trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib (Keynote-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in Keynote-240: A Randomized, Double-Blind, Phase Iii Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab Versus Sorafenib in Advanced Hepatocellular Carcinoma (Checkmate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef]
- Harding, J.J.; Nandakumar, S.; Armenia, J.; Khalil, D.N.; Albano, M.; Ly, M.; Shia, J.; Hechtman, J.F.; Kundra, R.; El Dika, I.; et al. Prospective Genotyping of Hepatocellular Carcinoma: Clinical Implications of Next-Generation Sequencing for Matching Patients to Targeted and Immune Therapies. Clin. Cancer Res. 2019, 25, 2116–2126. [Google Scholar] [CrossRef]
- Morita, M.; Nishida, N.; Sakai, K.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; et al. Immunological Microenvironment Predicts the Survival of the Patients with Hepatocellular Carcinoma Treated with Anti-Pd-1 Antibody. Liver Cancer 2021, 10, 380–393. [Google Scholar] [CrossRef]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-Intrinsic Β-Catenin Signalling Prevents Anti-Tumour Immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Galarreta, M.; Bresnahan, E.; Molina-Sánchez, P.; Lindblad, K.E.; Maier, B.; Sia, D.; Puigvehi, M.; Miguela, V.; Casanova-Acebes, M.; Dhainaut, M.; et al. Β-Catenin Activation Promotes Immune Escape and Resistance to Anti-Pd-1 Therapy in Hepatocellular Carcinoma. Cancer Discov. 2019, 9, 1124–1141. [Google Scholar] [CrossRef]
- Pylayeva-Gupta, Y.; Lee, K.E.; Hajdu, C.H.; Miller, G.; Bar-Sagi, D. Oncogenic Kras-Induced Gm-Csf Production Promotes the Development of Pancreatic Neoplasia. Cancer Cell 2021, 21, 836–847. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of Pten Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Borcoman, E.; De La Rochere, P.; Richer, W.; Vacher, S.; Chemlali, W.; Krucker, C.; Sirab, N.; Radvanyi, F.; Allory, Y.; Pignot, G.; et al. Inhibition of Pi3k Pathway Increases Immune Infiltrate in Muscle-Invasive Bladder Cancer. Oncoimmunology 2019, 8, e1581556. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. Pi3kγ Is a Molecular Switch That Controls Immune Suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef]
- Wang, G.; Lu, X.; Dey, P.; Deng, P.; Wu, C.C.; Jiang, S.; Fang, Z.; Zhao, K.; Konaparthi, R.; Hua, S.; et al. Targeting Yap-Dependent Mdsc Infiltration Impairs Tumor Progression. Cancer Discov. 2016, 6, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Lachenmayer, A.; Finn, R.S. Advances in Targeted Therapies for Hepatocellular Carcinoma in the Genomic Era. Nat. Rev. Clin. Oncol. 2015, 12, 408–424. [Google Scholar] [CrossRef]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4. [Google Scholar] [CrossRef]
- Nishida, N.; Sakai, K.; Morita, M.; Aoki, T.; Takita, M.; Hagiwara, S.; Komeda, Y.; Takenaka, M.; Minami, Y.; Ida, H.; et al. Association between Genetic and Immunological Background of Hepatocellular Carcinoma and Expression of Programmed Cell Death-1. Liver Cancer 2020, 9, 426–439. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kushihara, Y.; Saito, N.; Yamaguchi, S.; Kakimi, K. A Novel Scoring Method Based on Rna-Seq Immunograms Describing Individual Cancer-Immunity Interactions. Cancer Sci. 2020, 111, 4031–4040. [Google Scholar] [CrossRef] [PubMed]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. Pd-L1 Expression Is Mainly Regulated by Interferon Gamma Associated with Jak-Stat Pathway in Gastric Cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating Pd-L1 and Pd-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; de Moura, M.C.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-Specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826. [Google Scholar] [CrossRef]
- Pinyol, R.; Sia, D.; Llovet, J.M. Immune Exclusion-Wnt/Ctnnb1 Class Predicts Resistance to Immunotherapies in Hcc. Clin. Cancer Res. 2019, 25, 2021–2023. [Google Scholar] [CrossRef]
- Kurebayashi, Y.; Ojima, H.; Tsujikawa, H.; Kubota, N.; Maehara, J.; Abe, Y.; Kitago, M.; Shinoda, M.; Kitagawa, Y.; Sakamoto, M. Landscape of Immune Microenvironment in Hepatocellular Carcinoma and Its Additional Impact on Histological and Molecular Classification. Hepatology 2018, 68, 1025–1041. [Google Scholar] [CrossRef]
- Fujita, M.; Yamaguchi, R.; Hasegawa, T.; Shimada, S.; Arihiro, K.; Hayashi, S.; Maejima, K.; Nakano, K.; Fujimoto, A.; Ono, A.; et al. Classification of Primary Liver Cancer with Immunosuppression Mechanisms and Correlation with Genomic Alterations. EBioMedicine 2020, 53, 102659. [Google Scholar] [CrossRef]
- Montironi, C.; Castet, F.; Haber, P.K.; Pinyol, R.; Torres-Martin, M.; Torrens, L.; Mesropian, A.; Wang, H.; Puigvehi, M.; Maeda, M.; et al. Inflamed and Non-Inflamed Classes of Hcc: A Revised Immunogenomic Classification. Gut 2023, 72, 129–140. [Google Scholar] [CrossRef]
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. Nash Limits Anti-Tumour Surveillance in Immunotherapy-Treated Hcc. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-Aggressive Cxcr6(+) Cd8 T Cells Cause Liver Immune Pathology in Nash. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Pinyol, R.; Torrecilla, S.; Wang, H.; Montironi, C.; Piqué-Gili, M.; Torres-Martin, M.; Wei-Qiang, L.; Willoughby, C.E.; Ramadori, P.; Andreu-Oller, C.; et al. Molecular Characterisation of Hepatocellular Carcinoma in Patients with Non-Alcoholic Steatohepatitis. J. Hepatol. 2021, 75, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Chiang, D.Y.; Villanueva, A.; Hoshida, Y.; Peix, J.; Newell, P.; Minguez, B.; LeBlanc, A.C.; Donovan, D.J.; Thung, S.N.; Solé, M.; et al. Focal Gains of Vegfa and Molecular Classification of Hepatocellular Carcinoma. Cancer Res. 2008, 68, 6779–6788. [Google Scholar] [CrossRef]
- Wong, A.M.; Ding, X.; Wong, A.M.; Xu, M.; Zhang, L.; Leung, H.H.; Chan, A.W.; Song, Q.X.; Kwong, J.; Chan, L.K.; et al. Unique Molecular Characteristics of Nafld-Associated Liver Cancer Accentuate Β-Catenin/Tnfrsf19-Mediated Immune Evasion. J. Hepatol. 2022, 77, 410–423. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/Β-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Angers, S.; Moon, R.T. Proximal Events in Wnt Signal Transduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Cadigan, K.M.; Waterman, M.L. Tcf/Lefs and Wnt Signaling in the Nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar]
- De Lau, W.; Peng, W.C.; Gros, P.; Clevers, H. The R-Spondin/Lgr5/Rnf43 Module: Regulator of Wnt Signal Strength. Genes. Dev. 2014, 28, 305–316. [Google Scholar] [CrossRef]
- Fearon, E.R. Parsing the Phrase “All in for Axin”-Wnt Pathway Targets in Cancer. Cancer Cell 2009, 16, 366–368. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/Beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; Bienz, M. Inhibition of Gsk3 by Wnt Signalling—Two Contrasting Models. J. Cell Sci. 2011, 124, 3537–3544. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. The Complex World of Wnt Receptor Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.P.; Reddien, P.W. Wnt Signaling and the Polarity of the Primary Body Axis. Cell 2009, 139, 1056–1068. [Google Scholar] [CrossRef]
- Sokol, S.Y. Maintaining Embryonic Stem Cell Pluripotency with Wnt Signaling. Development 2011, 138, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- van Amerongen, R.; Nusse, R. Towards an Integrated View of Wnt Signaling in Development. Development 2009, 136, 3205–3214. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The Many Faces and Functions of Β-Catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Li, Q.; Sun, M.; Wang, M.; Feng, M.; Yang, F.; Li, L.; Zhao, J.; Chang, C.; Dong, H.; Xie, T.; et al. Dysregulation of Wnt/Β-Catenin Signaling by Protein Kinases in Hepatocellular Carcinoma and Its Therapeutic Application. Cancer Sci. 2021, 112, 1695–1706. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.C.; Chen, Y.; Zhao, J.L.; Gao, C.C.; Han, H.; Liu, W.C.; Qin, H.Y. Crosstalk between Hepatic Tumor Cells and Macrophages Via Wnt/Β-Catenin Signaling Promotes M2-Like Macrophage Polarization and Reinforces Tumor Malignant Behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef]
- Mikels, A.J.; Nusse, R. Purified Wnt5a Protein Activates or Inhibits Beta-Catenin-Tcf Signaling Depending on Receptor Context. PLoS Biol. 2006, 4, e115. [Google Scholar] [CrossRef]
- Grumolato, L.; Liu, G.; Mong, P.; Mudbhary, R.; Biswas, R.; Arroyave, R.; Vijayakumar, S.; Economides, A.N.; Aaronson, S.A. Canonical and Noncanonical Wnts Use a Common Mechanism to Activate Completely Unrelated Coreceptors. Genes. Dev. 2010, 24, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Shen, J.; Huang, Y.L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell Cycle Control of Wnt Receptor Activation. Dev. Cell 2009, 17, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rajasekaran, M.; Xia, H.; Kong, S.N.; Deivasigamani, A.; Sekar, K.; Gao, H.; Swa, H.L.; Gunaratne, J.; Ooi, L.L.; et al. Cdk1-Mediated Bcl9 Phosphorylation Inhibits Clathrin to Promote Mitotic Wnt Signalling. EMBO J. 2018, 37, e99395. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Su, Y.; Wesslowski, J.; Hagemann, A.I.; Ramialison, M.; Wittbrodt, J.; Scholpp, S.; Davidson, G. Tyrosine Phosphorylation of Lrp6 by Src and Fer Inhibits Wnt/Β-Catenin Signalling. EMBO Rep. 2014, 15, 1254–1267. [Google Scholar] [CrossRef]
- Krejci, P.; Aklian, A.; Kaucka, M.; Sevcikova, E.; Prochazkova, J.; Masek, J.K.; Mikolka, P.; Pospisilova, T.; Spoustova, T.; Weis, M.; et al. Receptor Tyrosine Kinases Activate Canonical Wnt/Β-Catenin Signaling Via Map Kinase/Lrp6 Pathway and Direct Β-Catenin Phosphorylation. PLoS ONE 2012, 7, e35826. [Google Scholar] [CrossRef]
- Villarroel, A.; Del Valle-Pérez, B.; Fuertes, G.; Curto, J.; Ontiveros, N.; de Herreros, A.G.; Duñach, M. Src and Fyn Define a New Signaling Cascade Activated by Canonical and Non-Canonical Wnt Ligands and Required for Gene Transcription and Cell Invasion. Cell. Mol. Life Sci. 2020, 77, 919–935. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, A.M.; Li, Y.H.; Luo, R.C.; Zou, Y.J.; Liu, Y.Y.; Liu, C.; Xie, Y.Y.; Zuo, S.; Liu, Z.; et al. Silencing Myh9 Blocks Hbx-Induced Gsk3β Ubiquitination and Degradation to Inhibit Tumor Stemness in Hepatocellular Carcinoma. Signal. Transduct. Target. Ther. 2020, 5, 13. [Google Scholar] [CrossRef]
- Tey, S.K.; Wong, S.W.K.; Chan, J.Y.T.; Mao, X.; Ng, T.H.; Yeung, C.L.S.; Leung, Z.; Fung, H.L.; Tang, A.H.N.; Wong, D.K.H.; et al. Patient Pigr-Enriched Extracellular Vesicles Drive Cancer Stemness, Tumorigenesis and Metastasis in Hepatocellular Carcinoma. J. Hepatol. 2022, 76, 883–895. [Google Scholar] [CrossRef]
- Zhao, G.; Song, Y.; Dong, L.; Shi, H.; Li, H.; Yang, L.; Wang, J. Silencing of Lemur Tyrosine Kinase 2 Restricts the Proliferation and Invasion of Hepatocellular Carcinoma through Modulation of Gsk-3β/Wnt/Β-Catenin Signaling. Biochem. Biophys. Res. Commun. 2019, 517, 722–728. [Google Scholar] [CrossRef]
- Jho, E.; Lomvardas, S.; Costantini, F. A Gsk3beta Phosphorylation Site in Axin Modulates Interaction with Beta-Catenin and Tcf-Mediated Gene Expression. Biochem. Biophys. Res. Commun. 1999, 266, 28–35. [Google Scholar] [CrossRef]
- Deng, R.; Zuo, C.; Li, Y.; Xue, B.; Xun, Z.; Guo, Y.; Wang, X.; Xu, Y.; Tian, R.; Chen, S.; et al. The Innate Immune Effector Isg12a Promotes Cancer Immunity by Suppressing the Canonical Wnt/Β-Catenin Signaling Pathway. Cell. Mol. Immunol. 2020, 17, 1163–1179. [Google Scholar] [CrossRef]
- Klimowski, L.K.; Garcia, B.A.; Shabanowitz, J.; Hunt, D.F.; Virshup, D.M. Site-Specific Casein Kinase 1epsilon-Dependent Phosphorylation of Dishevelled Modulates Beta-Catenin Signaling. FEBS J. 2006, 273, 4594–4602. [Google Scholar] [CrossRef]
- de Groot, R.E.; Ganji, R.S.; Bernatik, O.; Lloyd-Lewis, B.; Seipel, K.; Šedová, K.; Zdráhal, Z.; Dhople, V.M.; Dale, T.C.; Korswagen, H.C.; et al. Huwe1-Mediated Ubiquitylation of Dishevelled Defines a Negative Feedback Loop in the Wnt Signaling Pathway. Sci. Signal. 2014, 7, ra26. [Google Scholar] [CrossRef]
- Cervenka, I.; Valnohova, J.; Bernatik, O.; Harnos, J.; Radsetoulal, M.; Sedova, K.; Hanakova, K.; Potesil, D.; Sedlackova, M.; Salasova, A.; et al. Dishevelled Is a Nek2 Kinase Substrate Controlling Dynamics of Centrosomal Linker Proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 9304–9309. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Sun, J.; Chen, X.; Liu, L.; Wu, D. Nek2 Augments Sorafenib Resistance by Regulating the Ubiquitination and Localization of Β-Catenin in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 316. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, N.; Malbon, C.C. Dishevelled-2 Docks and Activates Src in a Wnt-Dependent Manner. J. Cell Sci. 2009, 122, 4439–4451. [Google Scholar] [CrossRef] [PubMed]
- Dajani, R.; Fraser, E.; Roe, S.M.; Yeo, M.; Good, V.M.; Thompson, V.; Dale, T.C.; Pearl, L.H. Structural Basis for Recruitment of Glycogen Synthase Kinase 3beta to the Axin-Apc Scaffold Complex. EMBO J. 2003, 22, 494–501. [Google Scholar] [CrossRef]
- Rubinfeld, B.; Albert, I.; Porfiri, E.; Fiol, C.; Munemitsu, S.; Polakis, P. Binding of Gsk3beta to the Apc-Beta-Catenin Complex and Regulation of Complex Assembly. Science 1996, 272, 1023–1026. [Google Scholar] [CrossRef]
- Su, Y.; Fu, C.; Ishikawa, S.; Stella, A.; Kojima, M.; Shitoh, K.; Schreiber, E.M.; Day, B.W.; Liu, B. Apc Is Essential for Targeting Phosphorylated Beta-Catenin to the Scfbeta-Trcp Ubiquitin Ligase. Mol. Cell 2008, 32, 652–661. [Google Scholar] [CrossRef]
- Davidson, G.; Wu, W.; Shen, J.; Bilic, J.; Fenger, U.; Stannek, P.; Glinka, A.; Niehrs, C. Casein Kinase 1 Gamma Couples Wnt Receptor Activation to Cytoplasmic Signal Transduction. Nature 2005, 438, 867–872. [Google Scholar] [CrossRef]
- Zeng, X.; Tamai, K.; Doble, B.; Li, S.; Huang, H.; Habas, R.; Okamura, H.; Woodgett, J.; He, X. A Dual-Kinase Mechanism for Wnt Co-Receptor Phosphorylation and Activation. Nature 2005, 438, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of Beta-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Ha, N.C.; Tonozuka, T.; Stamos, J.L.; Choi, H.J.; Weis, W.I. Mechanism of Phosphorylation-Dependent Binding of Apc to Beta-Catenin and Its Role in Beta-Catenin Degradation. Mol. Cell 2004, 15, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Salic, A.; Kirschner, M.W. Physiological Regulation of [Beta]-Catenin Stability by Tcf3 and Ck1epsilon. J. Cell Biol. 2023, 154, 983–993. [Google Scholar] [CrossRef]
- Song, D.H.; Dominguez, I.; Mizuno, J.; Kaut, M.; Mohr, S.C.; Seldin, D.C. Ck2 Phosphorylation of the Armadillo Repeat Region of Beta-Catenin Potentiates Wnt Signaling. J. Biol. Chem. 2003, 278, 24018–24025. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jones, K.A. Ck2 Controls the Recruitment of Wnt Regulators to Target Genes in Vivo. Curr. Biol. 2006, 16, 2239–2244. [Google Scholar] [CrossRef]
- Monga, S.P.; Mars, W.M.; Pediaditakis, P.; Bell, A.; Mulé, K.; Bowen, W.C.; Wang, X.; Zarnegar, R.; Michalopoulos, G.K. Hepatocyte Growth Factor Induces Wnt-Independent Nuclear Translocation of Beta-Catenin after Met-Beta-Catenin Dissociation in Hepatocytes. Cancer Res. 2002, 62, 2064–2071. [Google Scholar]
- Li, Y.; Shao, Y.; Tong, Y.; Shen, T.; Zhang, J.; Li, Y.; Gu, H.; Li, F. Nucleo-Cytoplasmic Shuttling of Pak4 Modulates Β-Catenin Intracellular Translocation and Signaling. Biochim. Biophys. Acta 2012, 1823, 465–475. [Google Scholar] [CrossRef]
- Ishitani, T.; Ninomiya-Tsuji, J.; Nagai, S.; Nishita, M.; Meneghini, M.; Barker, N.; Waterman, M.; Bowerman, B.; Clevers, H.; Shibuya, H.; et al. The Tak1-Nlk-Mapk-Related Pathway Antagonizes Signalling between Beta-Catenin and Transcription Factor Tcf. Nature 1999, 399, 798–802. [Google Scholar] [CrossRef]
- Qu, C.; He, D.; Lu, X.; Dong, L.; Zhu, Y.; Zhao, Q.; Jiang, X.; Chang, P.; Jiang, X.; Wang, L.; et al. Salt-Inducible Kinase (Sik1) Regulates Hcc Progression and Wnt/Β-Catenin Activation. J. Hepatol. 2016, 64, 1076–1089. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, J.; Wang, L.; Xiao, S.; Li, L.; Yan, X.; Yao, W.; Wu, L.; Zhang, S.; Zhang, Y.; et al. Ptk2 Promotes Cancer Stem Cell Traits in Hepatocellular Carcinoma by Activating Wnt/Β-Catenin Signaling. Cancer Lett. 2019, 450, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Wang, K.; Hu, J.; Yan, C.; Li, M.; Yu, X.; Liu, X.; Lei, J.; Guo, W.; Wu, L.; et al. Ubiquitin-Like Protein Fat10 Promotes the Invasion and Metastasis of Hepatocellular Carcinoma by Modifying Β-Catenin Degradation. Cancer Res. 2014, 74, 5287–5300. [Google Scholar] [CrossRef] [PubMed]
- ten Berge, D.; Kurek, D.; Blauwkamp, T.; Koole, W.; Maas, A.; Eroglu, E.; Siu, R.K.; Nusse, R. Embryonic Stem Cells Require Wnt Proteins to Prevent Differentiation to Epiblast Stem Cells. Nat. Cell Biol. 2011, 13, 1070–1075. [Google Scholar] [CrossRef]
- Lu, J.; Ma, Z.; Hsieh, J.C.; Fan, C.W.; Chen, B.; Longgood, J.C.; Williams, N.S.; Amatruda, J.F.; Lum, L.; Chen, C. Structure-Activity Relationship Studies of Small-Molecule Inhibitors of Wnt Response. Bioorg Med. Chem. Lett. 2009, 19, 3825–3827. [Google Scholar] [CrossRef]
- Huang, S.M.; Mishina, Y.M.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase Inhibition Stabilizes Axin and Antagonizes Wnt Signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef]
- Thorne, C.A.; Hanson, A.J.; Schneider, J.; Tahinci, E.; Orton, D.; Cselenyi, C.S.; Jernigan, K.K.; Meyers, K.C.; Hang, B.I.; Waterson, A.G.; et al. Small-Molecule Inhibition of Wnt Signaling through Activation of Casein Kinase 1α. Nat. Chem. Biol. 2010, 6, 829–836. [Google Scholar] [CrossRef]
- Emami, K.H.; Nguyen, C.; Ma, H.; Kim, D.H.; Jeong, K.W.; Eguchi, M.; Moon, R.T.; Teo, J.L.; Kim, H.Y.; Moon, S.H.; et al. A Small Molecule Inhibitor of Beta-Catenin/Creb-Binding Protein Transcription [Corrected]. Proc. Natl. Acad. Sci. USA 2004, 101, 12682–12687. [Google Scholar] [CrossRef] [PubMed]
- Lepourcelet, M.; Chen, Y.N.; France, D.S.; Wang, H.; Crews, P.; Petersen, F.; Bruseo, C.; Wood, A.W.; Shivdasani, R.A. Small-Molecule Antagonists of the Oncogenic Tcf/Beta-Catenin Protein Complex. Cancer Cell 2004, 5, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hori, Y.; Inoue, S.; Yamamoto, Y.; Iso, K.; Kamiyama, H.; Yamaguchi, A.; Kimura, T.; Uesugi, M.; Ito, J.; et al. E7386, a Selective Inhibitor of the Interaction between Β-Catenin and Cbp, Exerts Antitumor Activity in Tumor Models with Activated Canonical Wnt Signaling. Cancer Res. 2021, 81, 1052–1062. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Kudo, M. Scientific Rationale for Combined Immunotherapy with Pd-1/Pd-L1 Antibodies and Vegf Inhibitors in Advanced Hepatocellular Carcinoma. Cancers 2020, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Ono, A.; Ishikawa, A.; Kodama, K.; Uchikawa, S.; Hatooka, H.; Zhang, P.; Teraoka, Y.; Morio, K.; Fujino, H.; et al. Tumor Fibroblast Growth Factor Receptor 4 Level Predicts the Efficacy of Lenvatinib in Patients with Advanced Hepatocellular Carcinoma. Clin. Transl. Gastroenterol. 2020, 11, e00179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Linley, A.; Hammond, D.E.; Hood, F.E.; Coulson, J.M.; MacEwan, D.J.; Ross, S.J.; Slupsky, J.R.; Smith, P.D.; Eyers, P.A.; et al. New Perspectives, Opportunities, and Challenges in Exploring the Human Protein Kinome. Cancer Res. 2018, 78, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT05091346 (accessed on 8 April 2023).

| Small Molecule | Molecular Target | Mechanism |
|---|---|---|
| IWP | Porcupine | IWP blocks Porcupine, the enzyme promoting acylation of Wnt proteins. |
| XAV939 | Tankyrase/Axin | XAV939 stimulates β-catenin degradation by stabilizing Axin. |
| IWR | Axin | IWR (IWR-1/2) antagonize Wnt signaling by stabilizing the Axin destruction complex. |
| Pyrvinium | CK1 | Pyrvinium appears to activate CK1, resulting in inhibition of the Wnt pathway. |
| ICG-001 | CBP | ICG-001 binds specifically to CBP and antagonizes β-catenin/TCF-mediated transcription. |
| E7386 | CBP/β-catenin | E7386 blocks the interaction between the exogenous N-terminal region of CBP and endogenous β-catenin |
| PKF115-584 | TCF/β-catenin | Antagonist of the TCF/β-catenin protein complex. |
| Target | Rationale | Inhibitors |
|---|---|---|
| MET | MET phosphorylates tyrosine residues of β-catenin, causing dissociation of β-catenin from MET and nuclear translocation of β-catenin. | Cabozantinib Tepotinib Golvatinib Capmatinib |
| EGFR | EGFR phosphorylates β-catenin at tyrosine residue 142, resulting in β-catenin release from membrane junctions and an increase in cytosolic β-catenin. | Erlotinib Gefitinib Lapatinib Cetuximab |
| FAK (PTK2) | FAK, also known as PTK2, reduces β-catenin degradation and increases the nuclear accumulation of β-catenin. | Defactinib |
| Src | Src phosphorylates β-catenin, resulting in the accumulation of β-catenin in the nucleus. This promotes TCF/LEF transcription. | Saracatinib Dasatinib |
| VEGF | VEGF was not proven to be involved in β-catenin activation. Nonetheless, VEGF is involved in tumor angiogenesis and affects the tumor microenvironment by inducing Tregs, TAMs, and MDSCs and inducing immunosuppressive cytokine release. VEGF inhibitors may improve the tumor microenvironment. | Bevacizumab Ramucirumab Aflibercept Beta Lenvatinib Sorafenib Regorafenib |
| FGFR | FGFR2 and FGFR3 phosphorylate β-catenin at tyrosine residue 142, which leads to the release of β-catenin from membrane junctions and an increase in cytoplasmic β-catenin. | Pemigatinib Futibatinib Infigratinib Lenvatinib |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, M.; Nishida, N.; Aoki, T.; Chishina, H.; Takita, M.; Ida, H.; Hagiwara, S.; Minami, Y.; Ueshima, K.; Kudo, M. Role of β-Catenin Activation in the Tumor Immune Microenvironment and Immunotherapy of Hepatocellular Carcinoma. Cancers 2023, 15, 2311. https://doi.org/10.3390/cancers15082311
Morita M, Nishida N, Aoki T, Chishina H, Takita M, Ida H, Hagiwara S, Minami Y, Ueshima K, Kudo M. Role of β-Catenin Activation in the Tumor Immune Microenvironment and Immunotherapy of Hepatocellular Carcinoma. Cancers. 2023; 15(8):2311. https://doi.org/10.3390/cancers15082311
Chicago/Turabian StyleMorita, Masahiro, Naoshi Nishida, Tomoko Aoki, Hirokazu Chishina, Masahiro Takita, Hiroshi Ida, Satoru Hagiwara, Yasunori Minami, Kazuomi Ueshima, and Masatoshi Kudo. 2023. "Role of β-Catenin Activation in the Tumor Immune Microenvironment and Immunotherapy of Hepatocellular Carcinoma" Cancers 15, no. 8: 2311. https://doi.org/10.3390/cancers15082311
APA StyleMorita, M., Nishida, N., Aoki, T., Chishina, H., Takita, M., Ida, H., Hagiwara, S., Minami, Y., Ueshima, K., & Kudo, M. (2023). Role of β-Catenin Activation in the Tumor Immune Microenvironment and Immunotherapy of Hepatocellular Carcinoma. Cancers, 15(8), 2311. https://doi.org/10.3390/cancers15082311










