The Matrix Reloaded—The Role of the Extracellular Matrix in Cancer
Abstract
Simple Summary
Abstract
1. Introduction
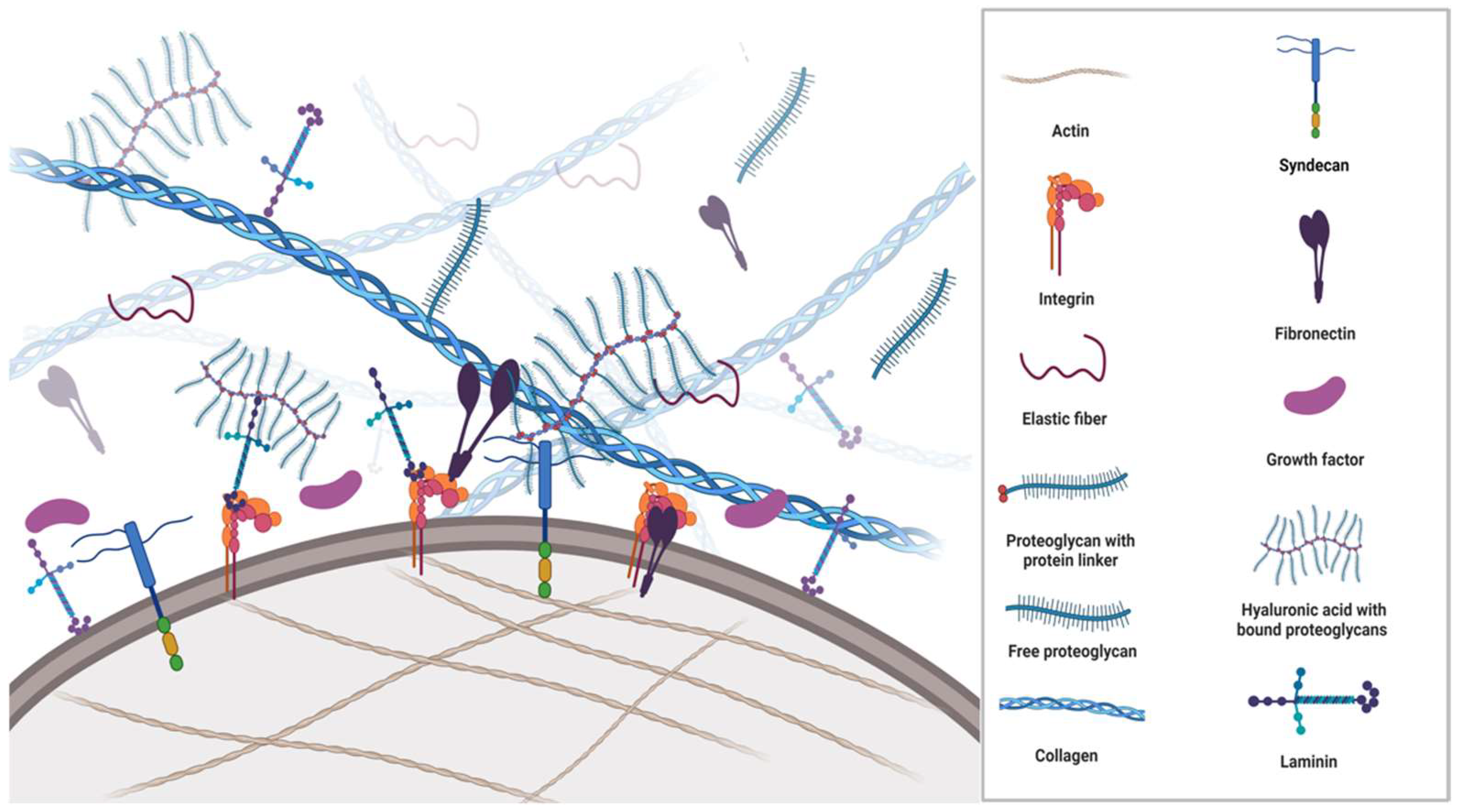
2. Components of ECM
3. Fibrous ECM Proteins
3.1. Collagen
3.2. Elastin
4. The Glycoproteins
4.1. Fibronectin
4.2. Laminin
5. Proteoglycans
Hyaluronic Acid
6. Sensing and Communication in ECM
7. ECM in Solid Cancers
8. Desmoplasia and CAF
9. ECM Stiffness
10. Therapeutical Targets
11. Discussion
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Mishra, Y.G.; Manavathi, B. Focal adhesion dynamics in cellular function and disease. Cell. Signal. 2021, 85, 110046. [Google Scholar] [CrossRef]
- Li, W.; Chi, N.; Rathnayake, R.A.C.; Wang, R. Distinctive roles of fibrillar collagen I and collagen III in mediating fibroblast-matrix interaction: A nanoscopic study. Biochem. Biophys. Res. Commun. 2021, 560, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Shivers, J.L.; MacKintosh, F.C.; Patteson, A.E.; Janmey, P.A. Cell-induced confinement effects in soft tissue mechanics. J. Appl. Phys. 2021, 129, 140901. [Google Scholar] [CrossRef]
- Müller, P.; Rogers, K.W.; Yu, S.R.; Brand, M.; Schier, A.F. Morphogen transport. Development 2013, 140, 1621–1638. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021, 599, 673–678. [Google Scholar] [CrossRef]
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063. [Google Scholar] [CrossRef]
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of Collagen. Methods Mol. Biol. 2021, 2347, 17–25. [Google Scholar] [PubMed]
- Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J.P. Collagen Structure-Function Mapping Informs Applications for Re-generative Medicine. Bioengineering 2020, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-S.; Dunleavey, J.M.; Szot, C.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Feng, Y.; Lutz, E.M.; Koogle, R.; et al. Cancer cell survival depends on collagen uptake into tumor-associated stroma. Nat. Commun. 2022, 13, 7078. [Google Scholar] [CrossRef]
- Ma, W.; Ma, H.; Fogerty, F.J.; Mosher, D.F. Bivalent ligation of the collagen-binding modules of fibronectin by SFS, a non-anchored bacterial protein of streptococcus equi. J. Biol. Chem. 2015, 290, 4866–4876. [Google Scholar] [CrossRef] [PubMed]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef]
- Burke, L.; Guterman, I.; Gallego, R.P.; Britton, R.G.; Burschowsky, D.; Tufarelli, C.; Rufini, A. The Janus-like role of proline metabolism in cancer. Cell Death Discov. 2020, 6, 104. [Google Scholar] [CrossRef]
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Tromp, G.; Prockop, D. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum. Mutat. 1999, 9, 300–331. Available online: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1098-1004(1997)9:4%3C300::AID-HUMU2%3E3.0.CO;2-9 (accessed on 13 February 2023). [CrossRef]
- Hwang, S.J.; Ha, G.H.; Seo, W.Y.; Kim, C.K.; Kim, K.J.; Lee, S.B. Human collagen alpha-2 type I stimulates collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs). BMB Rep. 2020, 53, 539–544. [Google Scholar] [CrossRef]
- Wanjare, M.; Agarwal, N.; Gerecht, S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Physiol. 2015, 309, C271–C281. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Heinz, A.; Troilo, H.; Lockhart-Cairns, M.P.; Jowitt, T.A.; Marchand, M.F.; Bidault, L.; Bignon, M.; Hedtke, T.; Barret, A.; et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. FASEB J. 2019, 33, 5468–5481. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.; Pierce, J.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef]
- Tembely, D.; Henry, A.; Vanalderwiert, L.; Toussaint, K.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Jaisson, S.; Galés, C.; Martiny, L.; et al. The Elastin Receptor Complex: An Emerging Therapeutic Target Against Age-Related Vascular Diseases. Front. Endocrinol. 2022, 13, 136. [Google Scholar] [CrossRef]
- Thorlacius-Ussing, J.; Kehlet, S.N.; Rønnow, S.R.; Karsdal, M.A.; Willumsen, N. Non-invasive profiling of protease-specific elastin turnover in lung cancer: Biomarker potential. J. Cancer Res. Clin. Oncol. 2018, 145, 383–392. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Jiang, Y.; Hansbro, N.G.; Hansbro, P.M.; Xu, J.; Liu, G. Elastin is a key factor of tumour development in colorectal cancer. BMC Cancer 2020, 20, 217. [Google Scholar]
- Efthymiou, G.; Saint, A.; Ruff, M.; Rekad, Z.; Ciais, D.; van Obberghen-Schilling, E. Shaping Up the Tumour Microenvironment with Cellular Fibronectin. Front. Oncol. 2020, 10, 641. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yan, L.; Xu, Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 2014, 34, 3315–3324. [Google Scholar] [CrossRef]
- Patankar, M.; Eskelinen, S.; Tuomisto, A.; Karttunen, T.J. KRAS and BRAF mutations induce anoikis resistance and characteristic 3D phenotypes in Caco-2 cells. Mol. Med. Rep. 2019, 20, 4634–4644. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, B.; Li, X.; Lu, D.; Yang, L.; Chen, L.; Li, Y.; Cheng, L.; Lv, F.; Zhang, P.; et al. ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells. Cell Death Dis. 2022, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and NF-κB. Oncogene 2006, 25, 4341–4349. [Google Scholar] [CrossRef] [PubMed]
- Gee, E.P.S.; Yüksel, D.; Stultz, C.M.; Ingber, D.E. SLLISWD sequence in the 10FNIII domain initiates fibronectin fibrillogenesis. J. Biol. Chem. 2013, 288, 21329–21340. [Google Scholar] [CrossRef]
- Ohashi, T.; Kiehart, D.P.; Erickson, H.P. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc. Natl. Acad. Sci. USA 1999, 96, 2153–2158. [Google Scholar] [CrossRef]
- Paten, J.A.; Martin, C.L.; Wanis, J.T.; Siadat, S.M.; Figueroa-Navedo, A.M.; Ruberti, J.W.; Deravi, L.F. Molecular Interactions between Collagen and Fibronectin: A Reciprocal Relationship that Regulates De Novo Fibrillogenesis. Chem 2019, 5, 2126–2145. [Google Scholar] [CrossRef]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Griggs, L.A.; Hassan, N.T.; Malik, R.S.; Griffin, B.P.; Martinez, B.A.; Elmore, L.W.; Lemmon, C.A. Fibronectin fibrils regulate TGF-β1-induced Epithelial-Mesenchymal Transition. Matrix Biol. 2017, 60–61, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumour aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum. Pathol. 2013, 44, 2028–2037. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Li, M.; Wang, C. Fibronectin and colorectal cancer: Signaling pathways and clinical implications. J. Recept. Signal Transduct. 2020, 41, 313–320. [Google Scholar] [CrossRef]
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191. [Google Scholar] [CrossRef]
- Joseph, J.V.; Conroy, S.; Pavlov, K.; Sontakke, P.; Tomar, T.; Eggens-Meijer, E.; Balasubramaniyan, V.; Wagemakers, M.; Dunnen, W.F.D.; Kruyt, F.A. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α–ZEB1 axis. Cancer Lett. 2015, 359, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.H.; Park, H.M.; Chung, J.; Lee, C.H.; Park, H.R. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma in-vasion via upregulation of alpha5 integrin and fibronectin. Biochem. Biophys. Res. Commun. 2010, 393, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care 2015, 4, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2018, 75–76, 12–26. [Google Scholar] [CrossRef]
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Thummarati, P.; Kaewkong, P.; Sripa, B.; Suthiphongchai, T. Role of laminin and cognate receptors in cholangiocarcinoma cell migration. Cell Adhes. Migr. 2021, 15, 152–165. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Gopal, S.; Amran, A.; Elton, A.; Ng, L.; Pocock, R. A somatic proteoglycan controls Notch-directed germ cell fate. Nat. Commun. 2021, 12, 6708. [Google Scholar] [CrossRef]
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat. Res. Commun. 2021, 27, 100312. [Google Scholar] [CrossRef]
- Scott, J. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645. [Google Scholar] [CrossRef]
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C. Molecular Cell Biology, 5th ed.; W.H Freeman: New York, NY, USA, 2003. [Google Scholar]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef]
- Enemark, M.B.; Hybel, T.E.; Madsen, C.; Lauridsen, K.L.; Honoré, B.; Plesner, T.L.; Hamilton-Dutoit, S.; d’Amore, F.; Ludvigsen, M. Tumour-Tissue Expression of the Hyaluronic Acid Receptor RHAMM Predicts Histological Transformation in Follicular Lymphoma Patients. Cancers 2022, 14, 1316. [Google Scholar] [CrossRef]
- Auvinen, P.; Tammi, R.; Kosma, V.-M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int. J. Cancer 2012, 132, 531–539. [Google Scholar] [CrossRef]
- Michalczyk, M.; Humeniuk, E.; Adamczuk, G.; Korga-Plewko, A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int. J. Mol. Sci. 2022, 24, 103. [Google Scholar] [CrossRef]
- Kim, P.K.; Halbrook, C.J.; Kerk, S.A.; Radyk, M.; Wisner, S.; Kremer, D.M.; Sajjakulnukit, P.; Andren, A.; Hou, S.W.; Trivedi, A.; et al. Hyaluronic acid fuels pancreatic cancer cell growth. eLife 2021, 10, e62645. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, L.; Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Gao, F. A novel role of breast cancer-derived hyaluronan on inducement of M2-like tumour-associated macrophages formation. Oncoimmunology 2016, 5, e1172154. [Google Scholar] [CrossRef]
- Kuang, D.M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.M.; Zheng, L. Tumour-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007, 110, 587–595. [Google Scholar] [CrossRef]
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients: A protocol for systematic review and meta analysis. Medicine 2020, 99, e20438. [Google Scholar] [CrossRef]
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immuno-therapy. Nat. Biotechnol. 2020, 38, 947–953. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. Ebiomedicine 2022, 76, 103873. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Feng, X.Q.; Li, X.; Lu, Y.; Wang, Z.; Rezgui, Y. Predictive assembling model reveals the self-adaptive elastic properties of lamellipodial actin networks for cell migration. Commun. Biol. 2020, 3, 616. [Google Scholar] [CrossRef]
- Grandy, C.; Port, F.; Pfeil, J.; Gottschalk, K.-E. Influence of ROCK Pathway Manipulation on the Actin Cytoskeleton Height. Cells 2022, 11, 430. [Google Scholar] [CrossRef]
- Northey, J.J.; Przybyla, L.; Weaver, V.M. Tissue force programs cell fate and tumour aggression. Cancer Discov. 2017, 7, 1224–1237. [Google Scholar] [CrossRef]
- Han, S.J.; Azarova, E.V.; Whitewood, A.J.; Bachir, A.; Guttierrez, E.; Groisman, A.; Horwitz, A.R.; Goult, B.T.; Dean, K.M.; Danuser, G.; et al. Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation. eLife 2021, 10, e66151. [Google Scholar] [CrossRef]
- Seetharaman, S.; Vianay, B.; Roca, V.; Farrugia, A.J.; De Pascalis, C.; Boëda, B.; Dingli, F.; Loew, D.; Vassilopoulos, S.; Bershadsky, A.; et al. Microtubules tune mechanosensitive cell responses. Nat. Mater. 2021, 21, 366–377. [Google Scholar] [CrossRef]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905. [Google Scholar] [CrossRef]
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020, 133, jcs230425. [Google Scholar] [CrossRef]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2021, 21, 60–78. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Luo, W.; Yuan, J.; Cui, C.; Guo, L.; Wu, C. Kindlin-2 Acts as a Key Mediator of Lung Fibroblast Activation and Pulmonary Fibrosis Progression. Am. J. Respir. Cell Mol. Biol. 2021, 65, 54–69. [Google Scholar] [CrossRef]
- Guo, L.; Cui, C.; Zhang, K.; Wang, J.; Wang, Y.; Lu, Y.; Chen, K.; Yuan, J.; Xiao, G.; Tang, B.; et al. Kindlin-2 links mechano-environment to proline synthesis and tumour growth. Nat. Commun. 2019, 10, 845. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumour Microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef]
- Silsirivanit, A. Chapter Five: Glycosylation markers in cancer. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 189–213. [Google Scholar]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Tumour Vasculature: Improving Drug Delivery and Efficacy. Trends Cancer 2018, 4, 258–259. [Google Scholar] [CrossRef]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Miskolczi, Z.; Smith, M.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene 2018, 37, 3166–3182. [Google Scholar] [CrossRef]
- Ajeti, V.; Nadiarnykh, O.; Ponik, S.M.; Keely, P.J.; Eliceiri, K.W.; Campagnola, P.J. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: Implications for probing stromal alterations in human breast cancer. Biomed. Opt. Express 2011, 2, 2307–2316. [Google Scholar] [CrossRef]
- Kauppila, S.; Stenbäck, F.; Risteli, J.; Jukkola, A.; Risteli, L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1999, 186, 262–268. [Google Scholar] [CrossRef]
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456. [Google Scholar] [CrossRef]
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin Deposition Participates in Extracellular Matrix Assembly and Vascular Morphogenesis. PLoS ONE 2016, 11, e0147600. [Google Scholar] [CrossRef] [PubMed]
- Romero-López, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.S.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumour microenvironment: Colon tumour-derived extracellular matrix promotes angiogenesis and tumour cell growth. Biomaterials 2017, 116, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Ruehle, M.A.; Eastburn, E.A.; LaBelle, S.A.; Krishnan, L.; Weiss, J.A.; Boerckel, J.D.; Wood, L.B.; Guldberg, R.E.; Willett, N.J. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci. Adv. 2020, 6, eabb6351. Available online: https://www.science.org/doi/10.1126/sciadv.abb6351 (accessed on 21 November 2022). [CrossRef]
- Daub, J.T.; Merks, R.M.H. A Cell-Based Model of Extracellular-Matrix-Guided Endothelial Cell Migration During Angiogenesis. Bull. Math. Biol. 2013, 75, 1377–1399. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Muranen, T.; Iwanicki, M.P.; Curry, N.L.; Hwang, J.; DuBois, C.D.; Coloff, J.L.; Hitchcock, D.S.; Clish, C.B.; Brugge, J.S.; Kalaany, N.Y. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 2017, 8, 13989. [Google Scholar] [CrossRef] [PubMed]
- Rainero, E.; Howe, J.D.; Caswell, P.T.; Jamieson, N.B.; Anderson, K.; Critchley, D.R.; Machesky, L.; Norman, J.C. Ligand-Occupied Integrin Internalization Links Nutrient Signaling to Invasive Migration. Cell Rep. 2015, 10, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Hoffman, G.R.; Poulogiannis, G.; Buel, G.R.; Jang, Y.J.; Lee, K.W.; Kim, B.Y.; Erikson, R.L.; Cantley, L.C.; Choo, A.Y.; et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell 2012, 49, 172–185. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.; Rainero, E. Cross-Talk Between the Tumour Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol. 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Muranen, T.; Selfors, L.M.; Worster, D.T.; Iwanicki, M.P.; Song, L.; Morales, F.C.; Gao, S.; Mills, G.B.; Brugge, J.S. Inhibition of PI3K/mTOR Leads to Adaptive Resistance in Matrix-Attached Cancer Cells. Cancer Cell 2012, 21, 227–239. [Google Scholar] [CrossRef]
- Koorman, T.; Jansen, K.A.; Khalil, A.; Haughton, P.D.; Visser, D.; Rätze, M.A.K.; Haakma, W.E.; Sakalauskaitè, G.; van Diest, P.J.; de Rooij, J.; et al. Spatial collagen stiffening promotes collective breast cancer cell invasion by reinforcing extracellular matrix alignment. Oncogene 2022, 41, 2458–2469. [Google Scholar] [CrossRef]
- Esfahani, P.; Levine, H.; Mukherjee, M.; Sun, B. Three-dimensional cancer cell migration directed by dual mechanochemical guidance. Phys. Rev. Res. 2022, 4, L022007. [Google Scholar] [CrossRef]
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Liu, R.; Qu, J. Oriented collagen fibers direct tumour cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213. [Google Scholar] [CrossRef]
- Piotrowski-Daspit, A.S.; Nerger, B.A.; Wolf, A.E.; Sundaresan, S.; Nelson, C.M. Dynamics of Tissue-Induced Alignment of Fibrous Extracellular Matrix. Biophys. J. 2017, 113, 702–713. [Google Scholar] [CrossRef]
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The use of mixed collagen-Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS ONE 2020, 15, e0220019. [Google Scholar] [CrossRef]
- Gaggar, A.; Weathington, N. Bioactive extracellular matrix fragments in lung health and disease. J. Clin. Investig. 2016, 126, 3176–3184. [Google Scholar] [CrossRef]
- He, X.; Lee, B.; Jiang, Y. Extracellular matrix in cancer progression and therapy. Med. Rev. 2022, 2, 125–139. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Olabi, S.; Ucar, A.; Brennan, K.; Streuli, C.H. Integrin-Rac signalling for mammary epithelial stem cell self-renewal 06 Biological Sciences 0601 Biochemistry and Cell Biology. Breast Cancer Res. 2018, 20, 128. [Google Scholar] [CrossRef]
- Wei, S.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.; et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar] [CrossRef]
- Barrera, L.N.; Ridley, P.M.; Bermejo-Rodriguez, C.; Costello, E.; Perez-Mancera, P.A. The role of microRNAs in the modulation of cancer-associated fibroblasts activity during pancreatic cancer pathogenesis. J. Physiol. Biochem. 2022, 79, 193–204. [Google Scholar] [CrossRef]
- Ishimoto, T.; Miyake, K.; Nandi, T.; Yashiro, M.; Onishi, N.; Huang, K.K.; Lin, S.J.; Kalpana, R.; Tay, S.T.; Suzuki, Y.; et al. Activation of Transforming Growth Factor Beta 1 Signaling in Gastric Cancer-associated Fibroblasts Increases Their Motility, via Expression of Rhomboid 5 Homolog 2, and Ability to Induce Invasiveness of Gastric Cancer Cells. Gastroenterology 2017, 153, 191–204.e16. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef]
- Nolan-Stevaux, O.; Lau, J.; Truitt, M.L.; Chu, G.C.; Hebrok, M.; Fernández-Zapico, M.E.; Hanahan, D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009, 23, 24–36. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Junttila, M.R.; Karrison, T.; Bahary, N.; Horiba, M.N.; Nattam, S.R.; Marsh, R.; Wallace, J.; Kozloff, M.; Rajdev, L.; et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients with Metastatic Pancreatic Cancer. J. Clin. Oncol. 2015, 33, 4284–4292. [Google Scholar] [CrossRef]
- Amakye, D.; Jagani, Z.; Dorsch, M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 2013, 19, 1410–1422. [Google Scholar] [CrossRef]
- Elyada, E.; Bolisetty, M.; Laise, P.; Flynn, W.F.; Courtois, E.T.; Burkhart, R.A.; Teinor, J.A.; Belleau, P.; Biffi, G.; Lucito, M.S.; et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019, 9, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Laklai, H.; Miroshnikova, Y.A.; Pickup, M.W.; Collisson, E.A.; Kim, G.E.; Barrett, A.S.; Hill, R.C.; Lakins, J.N.; Schlaepfer, D.D.; Mouw, J.K.; et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumour progression. Nat. Med. 2016, 22, 497–505. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Pink, D.; Chronopoulos, A.; Karim, S.A.; Morton, J.P.; Hernández, A.E.D.R. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci. Rep. 2017, 7, 2506. [Google Scholar] [CrossRef]
- Mpekris, F.; Papageorgis, P.; Polydorou, C.; Voutouri, C.; Kalli, M.; Pirentis, A.P.; Stylianopoulos, T. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumour microenvironment to improve chemo- and nanotherapy. J. Control. Release 2017, 261, 105–112. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Martin, J.D.; Chauhan, V.P.; Jain, S.R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumours. Proc. Natl. Acad. Sci. USA 2012, 109, 15101–15108. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Parag-Sharma, K.; Terajima, M.; Musicant, A.M.; Murphy, R.M.; Ramsey, M.R.; Hibi, H.; Yamauchi, M.; Amelio, A.L. Lysyl hydroxylase 2-induced collagen cross-link switching promotes metastasis in head and neck squamous cell carcinomas. Neoplasia 2021, 23, 594–606. [Google Scholar] [CrossRef]
- García-Gareta, E.; Pérez, M.; García-Aznar, J.M. Decellularization of tumours: A new frontier in tissue engineering. J. Tissue Eng. 2022, 13, 204173142210916. [Google Scholar] [CrossRef]
- Verginadis, I.I.; Avgousti, H.; Monslow, J.; Skoufos, G.; Chinga, F.; Kim, K.; Leli, N.M.; Karagounis, I.V.; Bell, B.I.; Velalopoulou, A.; et al. A stromal Integrated Stress Response activates perivascular cancer-associated fibroblasts to drive angiogenesis and tumour progression. Nat. Cell Biol. 2022, 24, 940–953. [Google Scholar] [CrossRef]
- Diop-Frimpong, B.; Chauhan, V.P.; Krane, S.; Boucher, Y.; Jain, R.K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumours. Proc. Natl. Acad. Sci. USA 2011, 108, 2909–2914. [Google Scholar] [CrossRef]
- Arrieta, O.; Guevara, P.; Escobar, E.; García-Navarrete, R.; Pineda, B.; Sotelo, J. Blockage of angiotensin II type I receptor decreases the synthesis of growth factors and induces apoptosis in C6 cultured cells and C6 rat glioma. Br. J. Cancer 2005, 92, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, Z.; Li, Z.; Zhang, A.; Jin, Y.; Chen, H.; Le, X. Angiotensin II Enhances Proliferation and Inflammation through AT1/PKC/NF-κB Signaling Pathway in Hepatocellular Carcinoma Cells. Cell. Physiol. Biochem. 2016, 39, 13–32. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total Neoadjuvant Therapy with FOLFIRINOX in Combination with Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1020–1027. [Google Scholar] [CrossRef]
- Wu, H.; Huang, D.; Zhou, H.; Sima, X.; Wu, Z.; Sun, Y.; Wang, L.; Ruan, Y.; Wu, Q.; Wu, F.; et al. Metformin: A promising drug for human cancers (Review). Oncol. Lett. 2022, 24, 204. [Google Scholar] [CrossRef] [PubMed]
- Yenmis, G.; Yaprak Sarac, E.; Besli, N.; Soydas, T.; Tastan, C.; Dilek Kancagi, D.; Yilanci, M.; Senol, K.; Karagulle, O.O.; Ekmekci, C.G.; et al. Anti-cancer effect of metformin on the metastasis and invasion of primary breast cancer cells through mediating NF-κB activity. Acta Histochem. 2021, 123, 151709. [Google Scholar] [CrossRef]
- Liang, F.; Wang, Y.-G.; Wang, C. Metformin Inhibited Growth, Invasion and Metastasis of Esophageal Squamous Cell Carcinoma In Vitro and In Vivo. Cell. Physiol. Biochem. 2018, 51, 1276–1286. [Google Scholar] [CrossRef]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; De Censi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Chen, B.E.; Gelmon, K.A.; Whelan, T.J.; Ennis, M.; Lemieux, J.; Ligibel, J.A.; Hershman, D.L.; Mayer, I.A.; Hobday, T.J.; et al. Effect of Metformin vs Placebo on Invasive Disease–Free Survival in Patients with Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA 2022, 327, 1963–1973. [Google Scholar] [CrossRef]
- Cortes, E.; Lachowski, D.; Robinson, B.; Sarper, M.; Teppo, J.S.; Thorpe, S.D.; Lieberthal, T.J.; Iwamoto, K.; Lee, D.A.; Okada-Hatakeyama, M.; et al. Tamoxifen mechanically reprograms the tumour microenvironment via HIF-1A and reduces cancer cell survival. EMBO Rep. 2019, 20, e46557. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, E.P.; Fink, D.; Stucki, M.; Imesch, P. Doxycycline reduces MMP-2 activity and inhibits invasion of 12Z epithelial endometriotic cells as well as MMP-2 and -9 activity in primary endometriotic stromal cells in vitro. Reprod. Biol. Endocrinol. 2019, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Mouratidis, P.X.; Colston, K.W.; Dalgleish, A.G. Doxycycline induces caspase-dependent apoptosis in human pancreatic cancer cells. Int. J. Cancer 2006, 120, 743–752. [Google Scholar] [CrossRef]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Nagasue, N. Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells. Int. J. Cancer 2006, 118, 1309–1315. [Google Scholar] [CrossRef]
- Rok, J.; Rzepka, Z.; Kowalska, J.; Banach, K.; Beberok, A.; Wrześniok, D. The Anticancer Potential of Doxycycline and Minocycline—A Comparative Study on Amelanotic Melanoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 831. [Google Scholar] [PubMed]
- Hadjimichael, A.; Foukas, A.F.; Savvidou, O.D.; Mavrogenis, A.F.; Psyrri, A.K.; Papagelopoulos, P.J. The anti-neoplastic effect of doxycycline in osteosarcoma as a metalloproteinase (MMP) inhibitor: A systematic review. Clin. Sarcoma Res. 2020, 10, 7. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumour Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Dobrotkova, V.; Chlapek, P.; Mazanek, P.; Sterba, J.; Veselska, R. Traffic lights for retinoids in oncology: Molecular markers of retinoid resistance and sensitivity and their use in the management of cancer differentiation therapy. BMC Cancer 2018, 18, 1059. [Google Scholar] [CrossRef]
- Froeling, F.E.M.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wntβ-catenin signaling to slow tumour progression. Gastroenterology 2011, 141, 1486–1497.e14. [Google Scholar] [CrossRef] [PubMed]
- Kocher, H.M.; Basu, B.; Froeling, F.E.M.; Sarker, D.; Slater, S.; Carlin, D.; Desouza, N.M.; De Paepe, K.N.; Goulart, M.R.; Hughes, C.; et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11, 4841. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Front. Immunol. 2019, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- van Cutsem, E.; Tempero, M.A.; Sigal, D.; Oh, D.-Y.; Fazio, N.; Macarulla, T.; Hitre, E.; Hammel, P.; Hendifar, A.E.; Bates, S.E.; et al. Randomized Phase III Trial of Pegvorhyaluronidase Alfa with Nab-Paclitaxel Plus Gemcitabine for Patients with Hyaluronan-High Metastatic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2020, 38, 3185–3194. [Google Scholar] [CrossRef]

| GF | ECM Protein | Growth Factor Functions |
|---|---|---|
| TGFβ | Type IV collagen Heparin/HS Fibronectin Fibrin/fibrinogen Betaglycan Decorin | Modulates cell growth and differentiation. Stimulates the synthesis of collagen, fibronectin and other ECM components, including HA, TSP, and tenascin. Increases production of protease inhibitors. Reduces the synthesis and secretion of proteases. |
| HGF | Type I, III, IV, V, and VI collagen Heparin/HS Fibronectin Fibrin/fibrinogen | Stimulates matrix remodelling and epithelial regeneration. Inhibits fibrosis. |
| IGF | IGF-binding protein | Stimulates cell mitogenesis, differentiation, and survival. Amplifies activity through the engagement of integrins or ECM glycosaminoglycans, heparin-binding domains, affecting cell adhesion and migration. |
| PDGF | SPARC Heparin/HS Fibronectin Fibrin/fibrinogen | Regulates angiogenesis. Attracts fibroblasts and monocytes and accelerates granulation tissue formation and ECM deposition. |
| VEGF | Collagen Heparin/HS Fibronectin Fibrin/fibrinogen | Controls blood vessel formation and growth. Binds to fibronectin to synergistically promote endothelial cell proliferation. |
| EGF | Collagen Fibronectin | Stimulates epithelial cell proliferation. Regulates a subset of G1 cell-cycle events. Elevates levels of EGFR. |
| FGF | Heparin/HS Fibronectin Fibrin/fibrinogen | Induces fibroblast proliferation and angiogenesis. Oligomerisation prolongs activity protection from proteolysis and endocytosis. |
| Signalling Pathways Affected by ECM Dysregulation | Breast Cancer | NSCLC | CRC | PDAC | Prostate Cancer | Ovarian Cancer | HCC | Glioblastoma | Malignant Melanoma |
|---|---|---|---|---|---|---|---|---|---|
| EMT | + | + | + | + | + | + | |||
| EGFR | + | + | + | ||||||
| FAK | + | + | + | ||||||
| FAK/Src | + | + | + | + | + | ||||
| HA | + | ||||||||
| Hedgehog | + | + | + | + | + | ||||
| Hippo | + | + | + | ||||||
| Hippo/YAP | + | ||||||||
| Integrin | + | + | + | + | + | + | + | + | |
| MAPK/ERK | + | + | + | + | |||||
| Notch | + | + | + | ||||||
| PI3K/AKT | + | + | + | + | + | + | + | + | + |
| RAS/RAF/MAPK/ERK | + | + | |||||||
| RhoA/ROCK | + | ||||||||
| Rho GTPase | + | ||||||||
| TGF-β | + | + | + | + | + | ||||
| TGF-β/Smad | + | + | + | ||||||
| Wnt/β-catenin | + | + | + | + | + | + | + | + |
| Trial No and Phase | Cancer Type | Drug | Target | Mechanism of Action | Preliminary Results and Adverse Events |
|---|---|---|---|---|---|
| NCT00431561; Phase 2b | Recurrent and refractory glioma | Trabedersen (AP 12009) | TGFβ2 | Antisense oligodeoxynucleotide specifically inhibits TGF-beta2 and suppresses key mechanisms of tumour development, specifically immunosuppression, metastasis, angiogenesis, and proliferation. | 19/89 pts had CR or PR following robust lesion size reduction (med. time for 90% reduction of baseline tumour volume = 11.7 mo, 4.9–57.7 mo). 7 pts had an SD for ≥6 mo. For the group of 26 AA/GBM patients with favourable responses, the median PFS: 1109 days and OS: 1280 days (significantly better than seen in the group including non-responders (n = 89; p < 0.00001) (https://doi.org/10.3390/cancers11121892) |
| NCT00761280; Phase 3 | Recurrent or refractory AA and secondary glioblastoma | Trabedersen + Temozolomide + Carmustine + Lomustine | Terminated due to inability to recruit the projected patient number. | ||
| NCT01401062; Phase 2 | m-Breast cancer | Fresolimumab + Focal irradiation | TGFβ | Human IgG4-κ monoclonal antibody neutralises all TGFβ isoforms (i.e., β1, β2, and β3) with half-life ranging from 21–30 days. | 7 grade 3/4 AE in 5/11 pts (1 mg/kg arm) and in 2/12 pts (10 mg/kg arm), respectively. SD = 3. At 12 months follow-up, 20/23 pts deceased. Patients receiving the 10 mg/kg had a significantly higher OS than those receiving 1 mg/kg fresolimumab (HR: 2.73 with 95% CI: 1.02, 7.30; p = 0.039). (https://doi.org/10.1158/1078-0432.CCR-17-3322) |
| NCT01112293; Phase 2 | Relapsed malignant pleural mesothelioma | Fresolimumab (GC1008) | SD: 3/13 pts; serum from 5 patients showed increased levels of antibodies against MPM tumour lysates. Had increased OS (15 vs. 7.5 mo, p < 0.03) (https://doi.org/10.4161/onci.26218) | ||
| NCT01246986; Phase 2 | HCC | Galunisertib + Sofarenib + Ramucirumab | TGFβ-R1 | Galunisertib (LY2157299) acts as a small-molecule selective inhibitor of the TGF-β receptor type I, which is a serine/threonine kinase. | Median time-to-tumour progression was 4.1 mo for 150 mg Galunisertib cohort; OS: 18.8 mo; PR: 2 pts; SD: 21; and progressive disease: 13. TGF-β1 responders showed better OS compared to non-responders (22.8 vs. 12.0 months, p = 0.038). (https://doi.org/10.14309/ctg.0000000000000056) |
| NCT01373164; Phase 1, 2 | m-Neoplasms, pancreatic cancer | Galunisertib + Gemcitabine/ Placebo + Gemcitabine | OS: 10.9 vs. 7.2 mos (GG vs. GP) in the subgroup with baseline TGFβ1 levels ≤ 4224 pg/mL (n = 117). PFS: 3.65 vs. 2.79 mos (p = 0.215). ORR: 8.7 vs. 1.9 (p = 0.116). Grade 3/4 TR-AE (GG vs GP) were anaemia (7.8% vs. 13.5%), neutropenia (32.0% vs. 26.9%) and thrombocytopenia (7.8% vs. 9.6%). | ||
| NCT02149108; Phase 3 | Refractory m-CRC | Nintedanib (BIBF1120)/ Placebo | RTK | Oral small-molecule inhibitors of RTK, including FGFR-1 to 3, PDGFR-α and β, and VEGFR-1 to 3. It inhibits the release of proinflammatory and profibrotic mediators, migration and differentiation of fibrocytes and fibroblasts, and deposition of ECM. | OS 6.4 mo vs. 6.0 mo with placebo; HR 1.01; 95% CI 0.86–1.19; p = 0.8659. PFS 1.5 mo vs. 1.4 mo; HR 0.58; 95% CI 0.49–0.69; p < 0.0001). No CR or PR. AEs occurred in 97% of the treatment group (n = 384) and 93% of the placebo group (381). The most frequent grade ≥ 3 AEs were liver-related AEs (nintedanib 16%; placebo 8%) and fatigue (nintedanib 9%; placebo 6%). (https://doi.org/10.1093/annonc/mdy241) |
| NCT01015118; Phase 3 | Ovarian and peritoneal neoplasms | Nintedanib/Placebo + Carboplatin + Paclitaxel | 53% of nintedanib grp (486/911) had disease progression or death compared with 58% of placebo grp (266). PFS: 17.2 mo vs. 16.6 mo, HR 0.84; 95% CI 0.72–0.98; p = 0.024. The most common AE were diarrhoea, neutropenia, anaemia, and thrombocytopenia. SAE in 376/902 pts in nintedanib grp and 155/450 pts in placebo grp. 29 pts in the nintedanib group had SAE compared with 16 in the placebo group. TR-AE-related death in 3 vs. 1 pts in treatment vs the placebo group. | ||
| NCT01195415; Phase 2 | m-Pancreatic cancer | Vismodegib/Placebo + Gemcitabine | SMO | Vismodegib selectively binds to and inhibits the transmembrane G protein-coupled receptor protein, SMO, to inhibit the Hedgehog signalling pathway and reduce desmoplasia. | 75% pts had elevated SHH expression pretreatment. Post-treatment, GLI1 and PTCH1 decreased in 95.6% and 82.6% of 23 pts, fibrosis decreased in 45.4% of 22 and Ki-67 in 52.9% of 17 evaluable pts. PFS and OS for all pts was 2.8 and 5.3 mos. DCR: 65.2%. Grade > 3 AE seen in 56% pts. (n = 23) (https://doi.org/10.1158/1078-0432.CCR-14-1269) |
| NCT02667574; Phase 2 | laBCC | Vismodegib + Surgery | 44/55 patients had a procedure after vismodegib treatment (80.0%, 95% CI [67 to 90]). CR: 27/44. The main AEs were dysgeusia, muscle spasms, alopecia, fatigue, and weight loss (20% pts with grade ≥ 3). Vismodegib helps with downstaging before surgery for laBCC. (https://doi.org/10.1200/JCO.2018.36.15_suppl.9509) | ||
| NCT01130142, Phase 1, 2 | m-Pancreatic cancer | Patidegib (IPI-926) + Gemcitabine | SMO | Small-molecule, semi-synthetic cyclopamine analogue that inhibits SMO | Preliminary results: 3/9 radiographic PR with post-baseline scans. No Grade 4/5 AE and no TR-AEs. The most common TR-AEs: fatigue (40% total, 0% Grade 3), nausea (40%, 0%), ALT increased (13%, 7%), AST increased (13%, 7%), anaemia (13%, 0%), and vomiting (13%, 0%). The study was terminated early due to a lack of benefits. |
| NCT01479465; Phase 2a | m-CRC | A. Simtuzumab 700 mg + FOLFIRIB. Simtuzumab 200 mg + FOLFIRIC. Placebo + FOLFIRI | LOXL2 | A humanised IgG4 monoclonal antibody against LOXL2 inhibits its enzymatic activity and ECM remodelling required for tumour progression. | PFS: A. 5.5 mo, B. 5.4 mo, and C. 5.8 mo. OS: 11.4 mo (1.23 [0.80, 1.91]; p = 0.25), 10.5 mo (1.50 [0.98, 2.30]; p = 0.06), and 16.3 mo. ORR was 11.9%, 5.9%, and 10%. Simtuzumab was well tolerated; however, clinical outcomes did not improve. (https://doi.org/10.1634/theoncologist.2016-0479) |
| NCT00195091, Phase 2 | Breast Cancer, TNBC | Tetrathiomolybdate | LOX | TM is a copper chelator that targets the catalytic activity of LOX by binding to copper and depleting it. | Disease progression was seen in 14/74, and 17 died. EFS: 71.4% and OS: 64.7% for all patients. Cancer-specific OS: 79.9%. TNBC: EFS: 71.7%, and OS: 74.2%. non-TNBC: EFS: 71.2% and OS: 64.6% |
| NCT01839487, Phase 2 | m-PDAC | PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) or nab-paclitaxel/gemcitabine (AG) | Hyaluronic acid | PEGPH20 degrades tumour-associated HA and increases the efficacy of chemo- and immuno-therapeutic agents. | PFS significantly improved with PAG (HR, 0.73; 95% CI, 0.53–1.00; p = 0.049) and for patients with HA-high tumours. ORR: 45% vs. 31% (PAG vs. AG). OS: 11.5 vs. 8.5 mos (HR, 0.96; 95% CI, 0.57–1.61). The most common grade 3/4 TR-AE with significant differences between arms (PAG vs. AG): muscle spasms (13% vs. 1%), neutropenia (29% vs. 18%), and myalgia (5% vs. 0%) (https://doi.org/10.1200/JCO.2017.74.9564) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raskov, H.; Gaggar, S.; Tajik, A.; Orhan, A.; Gögenur, I. The Matrix Reloaded—The Role of the Extracellular Matrix in Cancer. Cancers 2023, 15, 2057. https://doi.org/10.3390/cancers15072057
Raskov H, Gaggar S, Tajik A, Orhan A, Gögenur I. The Matrix Reloaded—The Role of the Extracellular Matrix in Cancer. Cancers. 2023; 15(7):2057. https://doi.org/10.3390/cancers15072057
Chicago/Turabian StyleRaskov, Hans, Shruti Gaggar, Asma Tajik, Adile Orhan, and Ismail Gögenur. 2023. "The Matrix Reloaded—The Role of the Extracellular Matrix in Cancer" Cancers 15, no. 7: 2057. https://doi.org/10.3390/cancers15072057
APA StyleRaskov, H., Gaggar, S., Tajik, A., Orhan, A., & Gögenur, I. (2023). The Matrix Reloaded—The Role of the Extracellular Matrix in Cancer. Cancers, 15(7), 2057. https://doi.org/10.3390/cancers15072057







