Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
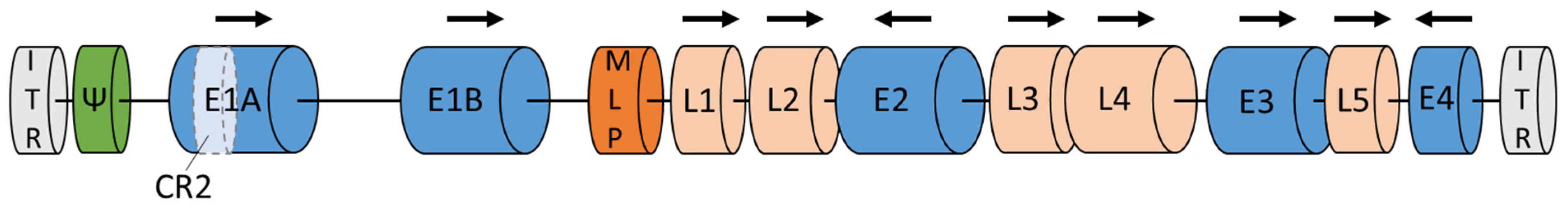
2. Characterization of the Adenovirus Genome
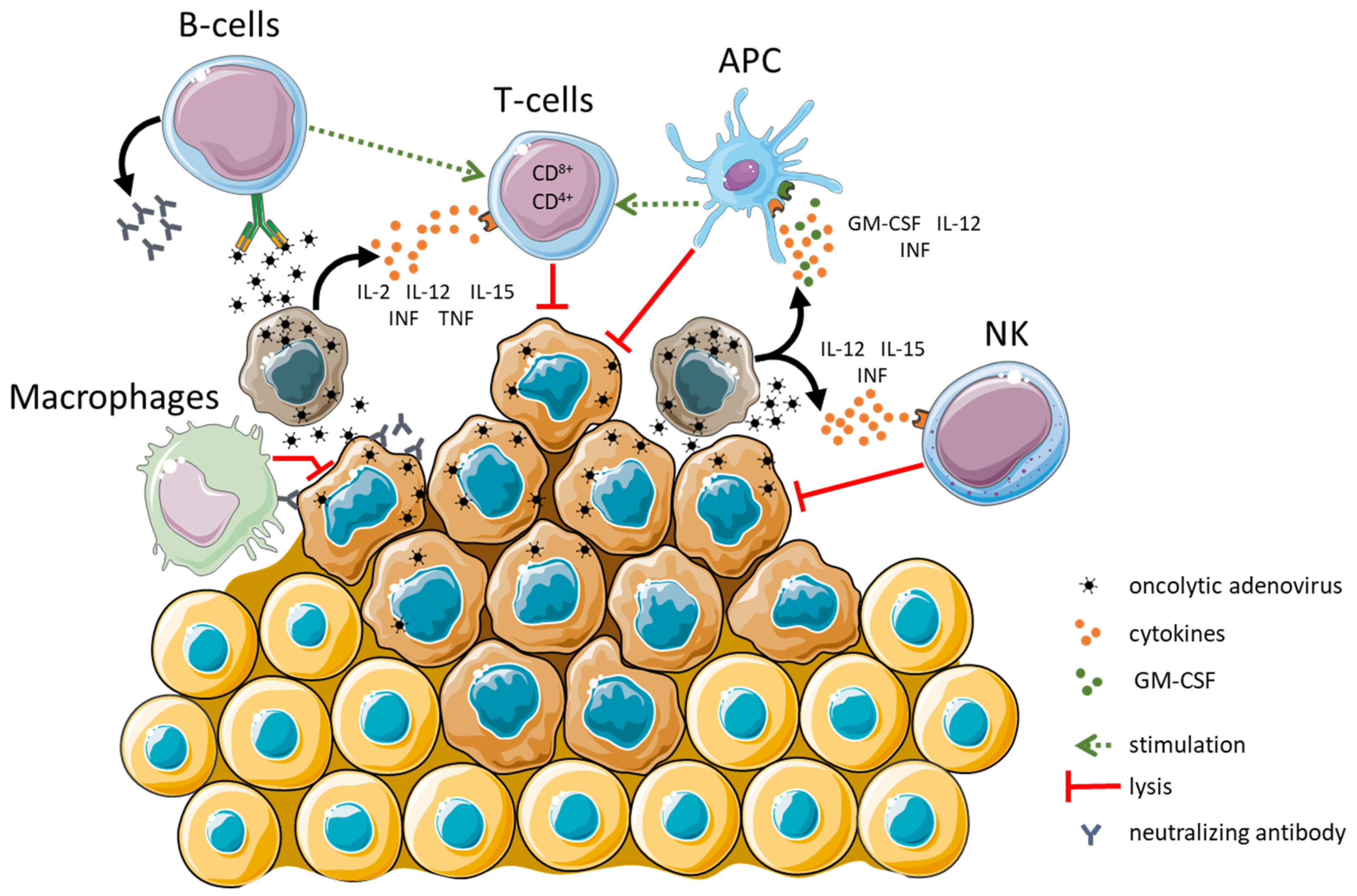
3. Adenovirus Cell Entry, Replication, and Immunogenicity
4. Oncolytic Adenoviruses—Genetic Modifications to Enhance Oncolytic Activity
4.1. Oncolytic Species B and C and Their Chimer-Versatile Platforms for Cancer Therapy
4.2. Species D and Its Chimer with Species C Adenoviruses in Oncolytic Therapies
4.3. Species E, F, and G Adenoviruses—Limited in Oncolytic Therapy
4.4. Species A Adenoviruses Excluded from Anticancer Therapy
5. Transgenes Enhancing Efficiency
5.1. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
5.2. Interleukin 2 (IL-2)
5.3. Interleukin 12 (IL-12)
5.4. Interleukin 15 (IL-15)
5.5. Interferons (INF)
5.6. Tumor Necrosis Factor (TNFα)
6. Oncolytic Adenoviruses: Clinical Progress
7. Limitations of Adenoviruses in Clinical Trials
8. Commercial Companies Developing Virology-Based Technologies
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2019: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Jayalie, V.F.; Sekarutami, S.M. Combining Oncolytic Virus and Radiation Therapy for Cancer Management. J. Cancer Metastasis Treat 2022, 8, 17. [Google Scholar] [CrossRef]
- Diaz Arguello, O.A.; Haisma, H.J. Apoptosis-Inducing TNF Superfamily Ligands for Cancer Therapy. Cancers 2021, 13, 1543. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.T.F.; Kemp, V.; Cramer, S.J.; van den Wollenberg, D.J.M.; Hornsveld, M.; Lamfers, M.L.M.; van der Pluijm, G.; Hoeben, R.C. Nonhuman Primate Adenoviruses of the Human Adenovirus B Species Are Potent and Broadly Acting Oncolytic Vector Candidates. Hum. Gene Ther. 2022, 33, 275–289. [Google Scholar] [CrossRef]
- Hoare, J.; Campbell, N.; Carapuça, E. Oncolytic Virus Immunotherapies in Ovarian Cancer: Moving beyond Adenoviruses. Porto Biomed. J. 2018, 3, e7. [Google Scholar] [CrossRef]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing Oncolytic Virotherapy in Cancer Treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-O.; Hong, J.; Yoon, A.-R. Current Clinical Landscape of Oncolytic Viruses as Novel Cancer Immunotherapeutic and Recent Preclinical Advancements. Front. Immunol. 2022, 13, 953410. [Google Scholar] [CrossRef] [PubMed]
- Boozari, B.; Mundt, B.; Woller, N.; Struver, N.; Gurlevik, E.; Schache, P.; Kloos, A.; Knocke, S.; Manns, M.P.; Wirth, T.C.; et al. Antitumoural Immunity by Virus-Mediated Immunogenic Apoptosis Inhibits Metastatic Growth of Hepatocellular Carcinoma. Gut 2010, 59, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.M.; Stoff-Khalili, M.A.; Curiel, D.T. Oncolytic Adenoviruses—Selective Retargeting to Tumor Cells. Oncogene 2005, 24, 7775–7791. [Google Scholar] [CrossRef]
- Abudoureyimu, M.; Lai, Y.; Tian, C.; Wang, T.; Wang, R.; Chu, X. Oncolytic Adenovirus—A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC. Front. Oncol. 2019, 9, 1182. [Google Scholar] [CrossRef]
- Daussy, C.F.; Pied, N.; Wodrich, H. Understanding Post Entry Sorting of Adenovirus Capsids; A Chance to Change Vaccine Vector Properties. Viruses 2021, 13, 1221. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, W.; Ehrhardt, A. Expanding the Spectrum of Adenoviral Vectors for Cancer Therapy. Cancers 2020, 12, 1139. [Google Scholar] [CrossRef] [PubMed]
- Short, J.J.; Vasu, C.; Holterman, M.J.; Curiel, D.T.; Pereboev, A. Members of Adenovirus Species B Utilize CD80 and CD86 as Cellular Attachment Receptors. Virus Res. 2006, 122, 144–153. [Google Scholar] [CrossRef]
- Segerman, A.; Atkinson, J.P.; Marttila, M.; Dennerquist, V.; Wadell, G.; Arnberg, N. Adenovirus Type 11 Uses CD46 as a Cellular Receptor. J. Virol. 2003, 77, 9183–9191. [Google Scholar] [CrossRef]
- Hall, K.; Blair Zajdel, M.E.; Blair, G.E. Defining the Role of CD46, CD80 and CD86 in Mediating Adenovirus Type 3 Fiber Interactions with Host Cells. Virology 2009, 392, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, D.J.; Segerman, A.; Lindman, K.; Mei, Y.-F.; Wadell, G. The Arg279Glu Substitution in the Adenovirus Type 11p (Ad11p) Fiber Knob Abolishes EDTA-Resistant Binding to A549 and CHO-CD46 Cells, Converting the Phenotype to That of Ad7p. J. Virol. 2006, 80, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Pache, L.; Venkataraman, S.; Reddy, V.S.; Nemerow, G.R. Structural Variations in Species B Adenovirus Fibers Impact CD46 Association. J. Virol. 2008, 82, 7923–7931. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liaw, Y.-C.; Stone, D.; Kalyuzhniy, O.; Amiraslanov, I.; Tuve, S.; Verlinde, C.L.M.J.; Shayakhmetov, D.; Stehle, T.; Roffler, S.; et al. Identification of CD46 Binding Sites within the Adenovirus Serotype 35 Fiber Knob. J. Virol. 2007, 81, 12785–12792. [Google Scholar] [CrossRef]
- Sirena, D.; Lilienfeld, B.; Eisenhut, M.; Kälin, S.; Boucke, K.; Beerli, R.R.; Vogt, L.; Ruedl, C.; Bachmann, M.F.; Greber, U.F.; et al. The Human Membrane Cofactor CD46 Is a Receptor for Species B Adenovirus Serotype 3. J. Virol. 2004, 78, 4454–4462. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, R.; Agrawal, B. Adenoviral Vector-Based Vaccines and Gene Therapies: Current Status and Future Prospects. In Adenoviruses; Desheva, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-990-5. [Google Scholar]
- Wu, C.; Wei, F.; Xu, Z.; Wen, R.; Chen, J.; Wang, J.; Mao, L. Tropism and Transduction of Oncolytic Adenovirus Vectors in Prostate Cancer Therapy. Front. Biosci. (Landmark Ed.) 2021, 26, 866–872. [Google Scholar] [CrossRef]
- Kuryk, L.; Møller, A.-S.; Vuolanto, A.; Pesonen, S.; Garofalo, M.; Cerullo, V.; Jaderberg, M. Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks. Int. J. Mol. Sci. 2019, 20, 621. [Google Scholar] [CrossRef]
- Tseha, S.T. Role of Adenoviruses in Cancer Therapy. Front. Oncol. 2022, 12, 772659. [Google Scholar] [CrossRef] [PubMed]
- Payne, S. Viruses, 1st ed.; Elsevier: Boston, MA, USA, 2017; ISBN 978-0-12-803109-4. [Google Scholar]
- Barry, M.A.; Rubin, J.D.; Lu, S. Retargeting Adenoviruses for Therapeutic Applications and Vaccines. FEBS Lett. 2020, 594, 1918–1946. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Møller, A.W. Chimeric Oncolytic Ad5/3 Virus Replicates and Lyses Ovarian Cancer Cells through Desmoglein-2 Cell Entry Receptor. J. Med. Virol. 2020, 92, 1309–1315. [Google Scholar] [CrossRef]
- Jiang, H.; Rivera-Molina, Y.; Gomez-Manzano, C.; Clise-Dwyer, K.; Bover, L.; Vence, L.M.; Yuan, Y.; Lang, F.F.; Toniatti, C.; Hossain, M.B.; et al. Oncolytic Adenovirus and Tumor-Targeting Immune Modulatory Therapy Improve Autologous Cancer Vaccination. Cancer Res. 2017, 77, 3894–3907. [Google Scholar] [CrossRef]
- Lynch, D.H. The Promise of 4-1BB (CD137)-Mediated Immunomodulation and the Immunotherapy of Cancer. Immunol. Rev. 2008, 222, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.R.; Suzuki, M. Immunology of Adenoviral Vectors in Cancer Therapy. Mol. Ther.—Methods Clin. Dev. 2019, 15, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Kühnel, F. Oncolytic Adenovirus in Cancer Immunotherapy. Cancers 2020, 12, 3354. [Google Scholar] [CrossRef]
- Kuryk, L.; Møller, A.-S.W.; Garofalo, M.; Cerullo, V.; Pesonen, S.; Alemany, R.; Jaderberg, M. Antitumor-Specific T-Cell Responses Induced by Oncolytic Adenovirus ONCOS-102 (AdV5/3-D24-GM-CSF) in Peritoneal Mesothelioma Mouse Model. J. Med. Virol. 2018, 90, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Leoni, V.; Campadelli-Fiume, G. Targeted Delivery of IL-12 Adjuvants Immunotherapy by Oncolytic Viruses. In Tumor Microenvironment; Birbrair, A., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1290, pp. 67–80. ISBN 978-3-030-55616-7. [Google Scholar]
- Weitzman, M.D. Functions of the Adenovirus E4 Proteins and Their Impact on Viral Vectors. Front. Biosci. 2005, 10, 1106. [Google Scholar] [CrossRef]
- Kulanayake, S.; Tikoo, S. Adenovirus Core Proteins: Structure and Function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Leppard, K.N. E4 Gene Function in Adenovirus, Adenovirus Vector and Adeno-Associated Virus Infections. J. Gen. Virol. 1997, 78, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Mese, K.; Bunz, O.; Ehrhardt, A. State-of-the-art Human Adenovirus Vectorology for Therapeutic Approaches. FEBS Lett. 2019, 593, 3609–3622. [Google Scholar] [CrossRef]
- Baker, A.; Aguirre-Hernández, C.; Halldén, G.; Parker, A. Designer Oncolytic Adenovirus: Coming of Age. Cancers 2018, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Gao, J.; Schaffarczyk, L.; Janz, S.; Ehrke-Schulz, E.; Dittmar, T.; Ehrhardt, A.; Zhang, W. Spectrum-Wide Exploration of Human Adenoviruses for Breast Cancer Therapy. Cancers 2020, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, O.; Diaconu, I.; Cerullo, V.; Pesonen, S.K.; Kanerva, A.; Joensuu, T.; Kairemo, K.; Laasonen, L.; Partanen, K.; Kangasniemi, L.; et al. Ad3-HTERT-E1A, a Fully Serotype 3 Oncolytic Adenovirus, in Patients With Chemotherapy Refractory Cancer. Mol. Ther. 2012, 20, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Ono, R.; Takayama, K.; Sakurai, F.; Mizuguchi, H. Efficient Antitumor Effects of a Novel Oncolytic Adenovirus Fully Composed of Species B Adenovirus Serotype 35. Mol. Ther.-Oncolytics 2021, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T.; Davies, J.A.; Bates, E.A.; Moses, E.; Mundy, R.M.; Marlow, G.; Cole, D.K.; Bliss, C.M.; Rizkallah, P.J.; Parker, A.L. The Fiber Knob Protein of Human Adenovirus Type 49 Mediates Highly Efficient and Promiscuous Infection of Cancer Cell Lines Using a Novel Cell Entry Mechanism. J. Virol. 2021, 95, e01849-20. [Google Scholar] [CrossRef]
- Huang, Y.; Lv, S.; Liu, P.; Ye, Z.; Yang, H.; Li, L.; Zhu, H.; Wang, Y.; Cui, L.; Jiang, D.; et al. A SIRPα-Fc Fusion Protein Enhances the Antitumor Effect of Oncolytic Adenovirus against Ovarian Cancer. Mol. Oncol. 2020, 14, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.Y.L.; Ennis, D.P.; Kennedy, P.R.; Hansell, C.; Dowson, S.; Farquharson, M.; Spiliopoulou, P.; Nautiyal, J.; McNamara, S.; Carlin, L.M.; et al. NK Cells Augment Oncolytic Adenovirus Cytotoxicity in Ovarian Cancer. Mol. Ther.-Oncolytics 2020, 16, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Weaver, E.A.; Khare, R.; May, S.M.; Barry, M.A. Mining the Adenovirus Virome for Oncolytics against Multiple Solid Tumor Types. Cancer Gene 2011, 18, 744–750. [Google Scholar] [CrossRef]
- Zafar, S.; Basnet, S.; Launonen, I.-M.; Quixabeira, D.C.A.; Santos, J.; Hemminki, O.; Malmstedt, M.; Cervera-Carrascon, V.; Aronen, P.; Kalliokoski, R.; et al. Oncolytic Adenovirus Type 3 Coding for CD40L Facilitates Dendritic Cell Therapy of Prostate Cancer in Humanized Mice and Patient Samples. Hum. Gene Ther. 2021, 32, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Duffy, M.R.; Lei-Rossmann, J.; Muntzer, A.; Scott, E.M.; Hagel, J.; Campo, L.; Bryant, R.J.; Verrill, C.; Lambert, A.; et al. An Oncolytic Virus Expressing a T-Cell Engager Simultaneously Targets Cancer and Immunosuppressive Stromal Cells. Cancer Res. 2018, 78, 6852–6865. [Google Scholar] [CrossRef]
- Bahlmann, N.A.; Tsoukas, R.L.; Erkens, S.; Wang, H.; Jönsson, F.; Aydin, M.; Naumova, E.A.; Lieber, A.; Ehrhardt, A.; Zhang, W. Properties of Adenovirus Vectors with Increased Affinity to DSG2 and the Potential Benefits of Oncolytic Approaches and Gene Therapy. Viruses 2022, 14, 1835. [Google Scholar] [CrossRef]
- Kalyuzhniy, O.; Di Paolo, N.C.; Silvestry, M.; Hofherr, S.E.; Barry, M.A.; Stewart, P.L.; Shayakhmetov, D.M. Adenovirus Serotype 5 Hexon Is Critical for Virus Infection of Hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 5483–5488. [Google Scholar] [CrossRef]
- Hensen, L.C.M.; Hoeben, R.C.; Bots, S.T.F. Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2020, 21, 6828. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Lemckert, A.A.C.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Smits, S.; Holterman, L.; Damen, I.; Vogels, R.; Thorner, A.R.; et al. Comparative Seroprevalence and Immunogenicity of Six Rare Serotype Recombinant Adenovirus Vaccine Vectors from Subgroups B and D. J. Virol. 2007, 81, 4654–4663. [Google Scholar] [CrossRef]
- Dyer, A.; Di, Y.; Calderon, H.; Illingworth, S.; Kueberuwa, G.; Tedcastle, A.; Jakeman, P.; Chia, S.L.; Brown, A.; Silva, M.A.; et al. Oncolytic Group B Adenovirus Enadenotucirev Mediates Non-Apoptotic Cell Death with Membrane Disruption and Release of Inflammatory Mediators. Mol. Ther.-Oncolytics 2017, 4, 18–30. [Google Scholar] [CrossRef]
- Di, Y.; Seymour, L.; Fisher, K. Activity of a Group B Oncolytic Adenovirus (ColoAd1) in Whole Human Blood. Gene 2014, 21, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Salazar, R.; Duran, I.; Osman-Garcia, I.; Paz-Ares, L.; Bozada, J.M.; Boni, V.; Blanc, C.; Seymour, L.; Beadle, J.; et al. Phase 1 Study of Intravenous Administration of the Chimeric Adenovirus Enadenotucirev in Patients Undergoing Primary Tumor Resection. J. Immunother. Cancer 2017, 5, 71. [Google Scholar] [CrossRef]
- Illingworth, S.; Di, Y.; Bauzon, M.; Lei, J.; Duffy, M.R.; Alvis, S.; Champion, B.; Lieber, A.; Hermiston, T.; Seymour, L.W.; et al. Preclinical Safety Studies of Enadenotucirev, a Chimeric Group B Human-Specific Oncolytic Adenovirus. Mol. Ther.-Oncolytics 2017, 5, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.D.; John, L.; Rafie, K.; Strebl, M.; Frängsmyr, L.; Ballmann, M.Z.; Mindler, K.; Havenga, M.; Lemckert, A.; Stehle, T.; et al. Human Species D Adenovirus Hexon Capsid Protein Mediates Cell Entry through a Direct Interaction with CD46. Proc. Natl. Acad. Sci. USA 2021, 118, e2020732118. [Google Scholar] [CrossRef] [PubMed]
- Hemsath, J.R.; Liaci, A.M.; Rubin, J.D.; Parrett, B.J.; Lu, S.-C.; Nguyen, T.V.; Turner, M.A.; Chen, C.Y.; Cupelli, K.; Reddy, V.S.; et al. Ex Vivo and In Vivo CD46 Receptor Utilization by Species D Human Adenovirus Serotype 26 (HAdV26). J. Virol. 2022, 96, 14. [Google Scholar] [CrossRef]
- Zhang, W.; Mese, K.; Schellhorn, S.; Bahlmann, N.; Mach, N.; Bunz, O.; Dhingra, A.; Hage, E.; Lafon, M.-E.; Wodrich, H.; et al. High-Throughput Cloning and Characterization of Emerging Adenovirus Types 70, 73, 74, and 75. Int. J. Mol. Sci. 2020, 21, 6370. [Google Scholar] [CrossRef]
- Chen, C.Y.; Senac, J.S.; Weaver, E.A.; May, S.M.; Jelinek, D.F.; Greipp, P.; Witzig, T.; Barry, M.A. Species D Adenoviruses as Oncolytics against B-Cell Cancers. Clin. Cancer Res. 2011, 17, 6712–6722. [Google Scholar] [CrossRef]
- Senac, J.S.; Doronin, K.; Russell, S.J.; Jelinek, D.F.; Greipp, P.R.; Barry, M.A. Infection and Killing of Multiple Myeloma by Adenoviruses. Hum. Gene Ther. 2010, 21, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Chen, C.Y.; May, S.M.; Barry, M.E.; Barry, M.A. Comparison of Adenoviruses as Oncolytics and Cancer Vaccines in an Immunocompetent B Cell Lymphoma Model. Hum. Gene Ther. 2011, 22, 1095–1100. [Google Scholar] [CrossRef]
- Clinicaltrial.Gov. Clinical Trials Data Base. 2022. Available online: https://clinicaltrials.gov/ct2/about-site/new (accessed on 4 March 2023).
- Othman, M.; Baker, A.T.; Gupalo, E.; Elsebaie, A.; Bliss, C.M.; Rondina, M.T.; Lillicrap, D.; Parker, A.L. To Clot or Not to Clot? Ad Is the Question—Insights on Mechanisms Related to Vaccine-induced Thrombotic Thrombocytopenia. J. Thromb. Haemost. 2021, 19, 2845–2856. [Google Scholar] [CrossRef]
- Dehghan, S.; Seto, J.; Liu, E.B.; Walsh, M.P.; Dyer, D.W.; Chodosh, J.; Seto, D. Computational Analysis of Four Human Adenovirus Type 4 Genomes Reveals Molecular Evolution through Two Interspecies Recombination Events. Virology 2013, 443, 197–207. [Google Scholar] [CrossRef]
- Paris, R.; Kuschner, R.A.; Binn, L.; Thomas, S.J.; Colloca, S.; Nicosia, A.; Cortese, R.; Bailer, R.T.; Sullivan, N.; Koup, R.A. Adenovirus Type 4 and 7 Vaccination or Adenovirus Type 4 Respiratory Infection Elicits Minimal Cross-Reactive Antibody Responses to Nonhuman Adenovirus Vaccine Vectors. Clin. Vaccine Immunol. 2014, 21, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tian, X. Vaccine Development for Human Mastadenovirus. J. Thorac. Dis. 2018, 10, S2280–S2294. [Google Scholar] [CrossRef] [PubMed]
- Walters, N.J. Characterisation of Wild-Type Human Adenovirus Serotypes Ad4, Ad11, Ad12 and Ad17 and Modified Viruses Ad5HVR48 and Ad5f35 in Comparison to Ad5 for Potential Use as Oncolytic Agents. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2010. [Google Scholar]
- Yokoda, R.; Nagalo, B.; Borad, M. Oncolytic Adenoviruses in Gastrointestinal Cancers. Biomedicines 2018, 6, 33. [Google Scholar] [CrossRef]
- Haller, S.D.; Monaco, M.L.; Essani, K. The Present Status of Immuno-Oncolytic Viruses in the Treatment of Pancreatic Cancer. Viruses 2020, 12, 1318. [Google Scholar] [CrossRef]
- Farrera-Sal, M.; Moreno, R.; Mato-Berciano, A.; Maliandi, M.V.; Bazan-Peregrino, M.; Alemany, R. Hyaluronidase Expression within Tumors Increases Virotherapy Efficacy and T Cell Accumulation. Mol. Ther.-Oncolytics 2021, 22, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Årdahl, C.; Rajan, A.; Nilsson, E.; Bradford, W.; Kaeshammer, L.; Jones, M.S.; Frängsmyr, L.; et al. Human Adenovirus 52 Uses Sialic Acid-Containing Glycoproteins and the Coxsackie and Adenovirus Receptor for Binding to Target Cells. PLoS Pathog. 2015, 11, e1004657. [Google Scholar] [CrossRef] [PubMed]
- Liaci, A.M. Structural and Functional Studies on the Early Steps of Polyomavirus and Adenovirus Life Cycles. Ph.D. Thesis, Eberhard Karls Universität Tübingen, Tübingen, Germany, 2017. [Google Scholar]
- Li, Z.; Langhans, S.A. In Vivo and Ex Vivo Pediatric Brain Tumor Models: An Overview. Front. Oncol. 2021, 11, 620831. [Google Scholar] [CrossRef]
- Ogawa, K.; Hamaya, K.; Fujii, Y.; Matsuura, K.; Endo, T. Tumor Induction by Adenovirus Type 12 and Its Target Cells in the Central Nervous System. Gan 1969, 60, 383–392. [Google Scholar]
- Li, D.; Tian, G.; Wang, J.; Zhao, L.Y.; Co, O.; Underill, Z.C.; Mymryk, J.S.; Claessens, F.; Dehm, S.M.; Daaka, Y.; et al. Inhibition of Androgen Receptor Transactivation Function by Adenovirus Type 12 E1A Undermines Prostate Cancer Cell Survival. Prostate 2018, 78, 1140–1156. [Google Scholar] [CrossRef]
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I Study with ONCOS-102 for the Treatment of Solid Tumors—An Evaluation of Clinical Response and Exploratory Analyses of Immune Markers. J. Immunother. Cancer 2016, 4, 17. [Google Scholar] [CrossRef]
- Diaconu, I.; Cerullo, V.; Hirvinen, M.L.M.; Escutenaire, S.; Ugolini, M.; Pesonen, S.K.; Bramante, S.; Parviainen, S.; Kanerva, A.; Loskog, A.S.I.; et al. Immune Response Is an Important Aspect of the Antitumor Effect Produced by a CD40L-Encoding Oncolytic Adenovirus. Cancer Res. 2012, 72, 2327–2338. [Google Scholar] [CrossRef]
- Wenthe, J.; Naseri, S.; Labani-Motlagh, A.; Enblad, G.; Wikström, K.I.; Eriksson, E.; Loskog, A.; Lövgren, T. Boosting CAR T-Cell Responses in Lymphoma by Simultaneous Targeting of CD40/4-1BB Using Oncolytic Viral Gene Therapy. Cancer Immunol. Immunother. 2021, 70, 2851–2865. [Google Scholar] [CrossRef]
- Rojas, J.M.; Alejo, A.; Avia, J.M.; Rodríguez-Martín, D.; Sánchez, C.; Alcamí, A.; Sevilla, N.; Martín, V. Activation of OX40 and CD27 Costimulatory Signalling in Sheep through Recombinant Ovine Ligands. Vaccines 2020, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Ponce, R. Adverse Consequences of Immunostimulation. J. Immunotoxicol. 2008, 5, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Moon, D.; Kong, S.J.; Lee, Y.S.; Yoo, Y.; Kim, S.; Kim, C.; Chon, H.J.; Kim, J.-H.; Choi, K.-J. Antitumor Effects of IL-12 and GM-CSF Co-Expressed in an Engineered Oncolytic HSV-1. Gene 2021, 28, 186–198. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Liu, X.; Hoffman, R.D.; Shi, R.; Lv, G.; Gao, J. G-CSF/GM-CSF-Induced Hematopoietic Dysregulation in the Progression of Solid Tumors. FEBS Open Bio 2022, 12, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A. GM-CSF in Inflammation. J. Exp. Med. 2020, 217, e20190945. [Google Scholar] [CrossRef]
- van de Laar, L.; Coffer, P.J.; Woltman, A.M. Regulation of Dendritic Cell Development by GM-CSF: Molecular Control and Implications for Immune Homeostasis and Therapy. Blood 2012, 119, 3383–3393. [Google Scholar] [CrossRef]
- Aliper, A.M.; Frieden-Korovkina, V.P.; Buzdin, A.; Roumiantsev, S.A.; Zhavoronkov, A. A Role for G-CSF and GM-CSF in Nonmyeloid Cancers. Cancer Med. 2014, 3, 737–746. [Google Scholar] [CrossRef]
- Jenner, A.L.; Frascoli, F.; Yun, C.-O.; Kim, P.S. Optimising Hydrogel Release Profiles for Viro-Immunotherapy Using Oncolytic Adenovirus Expressing IL-12 and GM-CSF with Immature Dendritic Cells. Appl. Sci. 2020, 10, 2872. [Google Scholar] [CrossRef]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef]
- Tähtinen, S.; Kaikkonen, S.; Merisalo-Soikkeli, M.; Grönberg-Vähä-Koskela, S.; Kanerva, A.; Parviainen, S.; Vähä-Koskela, M.; Hemminki, A. Favorable Alteration of Tumor Microenvironment by Immunomodulatory Cytokines for Efficient T-Cell Therapy in Solid Tumors. PLoS ONE 2015, 10, e0131242. [Google Scholar] [CrossRef] [PubMed]
- Shoushtari, A.N.; Olszanski, A.J.; Nyakas, M.; Hornyak, T.J.; Wolchok, J.D.; Levitsky, V.; Kuryk, L.; Hansen, T.B.; Jäderberg, M. Pilot Study of ONCOS-102 and Pembrolizumab: Remodeling of the Tumor Microenvironment and Clinical Outcomes in Anti–PD-1–Resistant Advanced Melanoma. Clin. Cancer Res. 2023, 29, 100–109. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Interleukin-2 Therapy of Cancer-Clinical Perspectives. Int. Immunopharmacol. 2021, 98, 107836. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, Y.; Wang, H.; Ma, C.; Feist, M.; Ju, S.; Guo, Z.S.; Bartlett, D.L. Modifying the Cancer-Immune Set Point Using Vaccinia Virus Expressing Re-Designed Interleukin-2. Nat. Commun. 2018, 9, 4682. [Google Scholar] [CrossRef]
- Pearl, T.M.; Markert, J.M.; Cassady, K.A.; Ghonime, M.G. Oncolytic Virus-Based Cytokine Expression to Improve Immune Activity in Brain and Solid Tumors. Mol. Ther.-Oncolytics 2019, 13, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A. The Shared and Contrasting Roles of IL2 and IL15 in the Life and Death of Normal and Neoplastic Lymphocytes: Implications for Cancer Therapy. Cancer Immunol. Res. 2015, 3, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Khong, H.; Fa’ak, F.; Bentebibel, S.-E.; Janssen, L.M.E.; Chesson, B.C.; Creasy, C.A.; Forget, M.-A.; Kahn, L.M.S.; Pazdrak, B.; et al. Bempegaldesleukin Selectively Depletes Intratumoral Tregs and Potentiates T Cell-Mediated Cancer Therapy. Nat. Commun. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H. Anti-Cancer Immunotherapy: Breakthroughs and Future Strategies. Semin. Immunopathol. 2019, 41, 1–3. [Google Scholar] [CrossRef]
- Havunen, R.; Kalliokoski, R.; Siurala, M.; Sorsa, S.; Santos, J.M.; Cervera-Carrascon, V.; Anttila, M.; Hemminki, A. Cytokine-Coding Oncolytic Adenovirus TILT-123 Is Safe, Selective, and Effective as a Single Agent and in Combination with Immune Checkpoint Inhibitor Anti-PD-1. Cells 2021, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.O.; Zhang, Y.; Siddiqui, F. Preclinical Toxicology of Oncolytic Adenovirus-Mediated Cytotoxic and Interleukin-12 Gene Therapy for Prostate Cancer. Gene Ther. 2015, 7, 15006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-N.; Choi, I.-K.; Huang, J.-H.; Yoo, J.-Y.; Choi, K.-J.; Yun, C.-O. Optimizing DC Vaccination by Combination With Oncolytic Adenovirus Coexpressing IL-12 and GM-CSF. Mol. Ther. 2011, 19, 1558–1568. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Guz-Montgomery, K.; Saha, D. Oncolytic Virus Encoding a Master Pro-Inflammatory Cytokine Interleukin 12 in Cancer Immunotherapy. Cells 2020, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Oh, J.-E.; Hong, J.; Chung, Y.; Lee, Y.; Park, K.D.; Kim, S.; Yun, C.-O. Optimized Biodegradable Polymeric Reservoir-Mediated Local and Sustained Co-Delivery of Dendritic Cells and Oncolytic Adenovirus Co-Expressing IL-12 and GM-CSF for Cancer Immunotherapy. J. Control. Release 2017, 259, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, X.; Wang, J.; Gao, D.; Li, Y.; Li, H.; Chu, Y.; Zhang, Z.; Liu, H.; Jiang, G.; et al. Re-Designing Interleukin-12 to Enhance Its Safety and Potential as an Anti-Tumor Immunotherapeutic Agent. Nat. Commun. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Tian, Y.; Zhu, G.; Liu, S.; Liu, F. Efficacy of a Novel Double-Controlled Oncolytic Adenovirus Driven by the Ki67 Core Promoter and Armed with IL-15 against Glioblastoma Cells. Cell Biosci. 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Fu, S.; Zhao, Q. 2022 Update on the Scientific Premise and Clinical Trials for IL-15 Agonists as Cancer Immunotherapy. J. Leukoc. Biol. 2022, 112, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Kowalsky, S.J.; Liu, Z.; Feist, M.; Berkey, S.E.; Ma, C.; Ravindranathan, R.; Dai, E.; Roy, E.J.; Guo, Z.S.; Bartlett, D.L. Superagonist IL-15-Armed Oncolytic Virus Elicits Potent Antitumor Immunity and Therapy That Are Enhanced with PD-1 Blockade. Mol. Ther. 2018, 26, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Knudson, K.M.; Hodge, J.W.; Schlom, J.; Gameiro, S.R. Rationale for IL-15 Superagonists in Cancer Immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, K.; Bourgeois-Daigneault, M.-C. The Pros and Cons of Interferons for Oncolytic Virotherapy. Cytokine Growth Factor Rev. 2020, 56, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, A.; Zhu, R.; Zhou, C.; Su, H.; Xie, G.; Deng, Y.; Xu, X. The Different Effects of IFN-β and IFN-γ on the Tumor-Suppressive Activity of Human Amniotic Fluid-Derived Mesenchymal Stem Cells. Stem. Cells Int. 2019, 2019, 4592701. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Lu, J. Interferon Gamma in Cancer Immunotherapy. Cancer Med. 2018, 7, 4509–4516. [Google Scholar] [CrossRef]
- Hasselbalch, H.C. Perspectives on Interferon-Alpha in the Treatment of Polycythemia Vera and Related Myeloproliferative Neoplasms: Minimal Residual Disease and Cure? Semin. Immunopathol. 2019, 41, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Sano, E.; Hanashima, Y.; Yamamuro, S.; Sumi, K.; Ueda, T.; Nakayama, T.; Hara, H.; Yoshino, A.; Katayama, Y. IFN-β Sensitizes TRAIL-induced Apoptosis by Upregulation of Death Receptor 5 in Malignant Glioma Cells. Oncol. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Blaauboer, A.; Van Koetsveld, P.; Mustafa, D.; Dumas, J.; Dogan, F.; Van Zwienen, S.; Van Eijck, C.; Hofland, L. Immunomodulatory Antitumor Effect of Interferon-beta Combined with Gemcitabine in Pancreatic Cancer. Int. J. Oncol. 2022, 61, 97. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Raković, N. The Role of Cytokines in the Evolution of Cancer: IFN-γ Paradigm. Cytokine 2022, 151, 155442. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, Z.; Zhao, Q.; Li, M.; Wu, X.; Zhang, L.; Hu, W.; Cho, C.H. Anti-Cancer Therapy with TNFα and IFNγ: A Comprehensive Review. Cell Prolif. 2018, 51, e12441. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hu, Y.; Qiu, L. Vesicular IFN-γ as a Cooperative Attacker to Enhance Anti-Cancer Effect of 5-Fluorouracil via Thymidine Phosphorylase Upregulation and Tumor Microenvironment Normalization. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102501. [Google Scholar] [CrossRef]
- Bourgeois-Daigneault, M.-C.; Roy, D.G.; Falls, T.; Twumasi-Boateng, K.; St-Germain, L.E.; Marguerie, M.; Garcia, V.; Selman, M.; Jennings, V.A.; Pettigrew, J.; et al. Oncolytic Vesicular Stomatitis Virus Expressing Interferon-σ Has Enhanced Therapeutic Activity. Mol. Ther.-Oncolytics 2016, 3, 16001. [Google Scholar] [CrossRef] [PubMed]
- Salzwedel, A.O.; Han, J.; LaRocca, C.J.; Shanley, R.; Yamamoto, M.; Davydova, J. Combination of Interferon-Expressing Oncolytic Adenovirus with Chemotherapy and Radiation Is Highly Synergistic in Hamster Model of Pancreatic Cancer. Oncotarget 2018, 9, 18041–18052. [Google Scholar] [CrossRef]
- Zhang, L.; Steele, M.B.; Jenks, N.; Grell, J.; Suksanpaisan, L.; Naik, S.; Federspiel, M.J.; Lacy, M.Q.; Russell, S.J.; Peng, K.-W. Safety Studies in Tumor and Non-Tumor-Bearing Mice in Support of Clinical Trials Using Oncolytic VSV-IFNβ-NIS. Hum. Gene Ther. Clin. Dev. 2016, 27, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.-Q.; Lei, T.-R.; Yu, T.-B.; Zhou, P.-H. Nanocarrier-Based Activation of Necroptotic Cell Death Potentiates Cancer Immunotherapy. Nanoscale 2021, 13, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Josephs, S.F.; Ichim, T.E.; Prince, S.M.; Kesari, S.; Marincola, F.M.; Escobedo, A.R.; Jafri, A. Unleashing Endogenous TNF-Alpha as a Cancer Immunotherapeutic. J. Transl. Med. 2018, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Carrascon, V.; Siurala, M.; Santos, J.M.; Havunen, R.; Tähtinen, S.; Karell, P.; Sorsa, S.; Kanerva, A.; Hemminki, A. TNFa and IL-2 Armed Adenoviruses Enable Complete Responses by Anti-PD-1 Checkpoint Blockade. OncoImmunology 2018, 7, e1412902. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.-P.; Salazar, R.; Rottey, S.; Duran, I.; Dirix, L.; Geboes, K.; Wilkinson-Blanc, C.; Pover, G.; Alvis, S.; Champion, B.; et al. A Phase 1 Dose Escalation Study of the Oncolytic Adenovirus Enadenotucirev, Administered Intravenously to Patients with Epithelial Solid Tumors (EVOLVE). J. Immunother. Cancer 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Barretina-Ginesta, M.-P.; García-Donas, J.; Jayson, G.C.; Roxburgh, P.; Vázquez, R.M.; Michael, A.; Antón-Torres, A.; Brown, R.; Krige, D.; et al. Safety and Efficacy of the Tumor-Selective Adenovirus Enadenotucirev with or without Paclitaxel in Platinum-Resistant Ovarian Cancer: A Phase 1 Clinical Trial. J. Immunother. Cancer 2021, 9, e003645. [Google Scholar] [CrossRef]
- O’Cathail, S.M.; Davis, S.; Holmes, J.; Brown, R.; Fisher, K.; Seymour, L.; Adams, R.; Good, J.; Sebag-Montefiore, D.; Maughan, T.; et al. A Phase 1 Trial of the Safety, Tolerability and Biological Effects of Intravenous Enadenotucirev, a Novel Oncolytic Virus, in Combination with Chemoradiotherapy in Locally Advanced Rectal Cancer (CEDAR). Radiat. Oncol. 2020, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carbonero, R.; Bazan-Peregrino, M.; Gil-Martín, M.; Álvarez, R.; Macarulla, T.; Riesco-Martinez, M.C.; Verdaguer, H.; Guillén-Ponce, C.; Farrera-Sal, M.; Moreno, R.; et al. Phase I, Multicenter, Open-Label Study of Intravenous VCN-01 Oncolytic Adenovirus with or without Nab-Paclitaxel plus Gemcitabine in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2022, 10, e003255. [Google Scholar] [CrossRef] [PubMed]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with Oncolytic Viruses: Progress and Challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Shoaf, M.L.; Peters, K.B. Clinical Trials of Oncolytic Viruses in Glioblastoma. Adv. Oncol. 2022, 2, 139–158. [Google Scholar] [CrossRef]
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Yung, W.K.A.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. JCO 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Chen, S.R.; Chen, M.M.; Ene, C.; Lang, F.F.; Kan, P. Perfusion-Guided Endovascular Super-Selective Intra-Arterial Infusion for Treatment of Malignant Brain Tumors. J. NeuroInterv. Surg. 2021, 14, 533–538. [Google Scholar] [CrossRef]
- Kuryk, L.; Møller, A.-S.W. Next Generation Oncolytic Viruses Expressing PADI1 and TIMP2 Exhibit Anti-Tumor Activity against Melanoma in Nude and Humanized Mouse Models. Mol. Ther.-Oncolytics 2023, 28, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Jaderberg, M.; Cedres, S.; Paz-Ares, L.; Serres, X.; Ricordel, C.; Isambert, N.; Aix, S.P.; Levitsky, V.; Kuryk, L.; Moller, A.-S.; et al. 361 A Randomised Open-Label Phase I/II Study Adding ONCOS-102 to Pemetrexed/Cisplatin in Patients with Unresectable Malignant Pleural Mesothelioma—12 Month Analysis of Biomarkers and Clinical Outcomes. In Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd.: London, UK, 2020; pp. A220–A221. [Google Scholar]
- Musher, B.L.; Smaglo, B.G.; Abidi, W.; Othman, M.; Patel, K.; Jawaid, S.; Jing, J.; Brisco, A.; Wenthe, J.; Eriksson, E.; et al. A Phase I/II Study of LOAd703, a TMZ-CD40L/4-1BBL-Armed Oncolytic Adenovirus, Combined with Nab-Paclitaxel and Gemcitabine in Advanced Pancreatic Cancer. JCO 2022, 40, 4138. [Google Scholar] [CrossRef]
- García, M.; Moreno, R.; Gil-Martin, M.; Cascallò, M.; de Olza, M.O.; Cuadra, C.; Piulats, J.M.; Navarro, V.; Domenech, M.; Alemany, R.; et al. A Phase 1 Trial of Oncolytic Adenovirus ICOVIR-5 Administered Intravenously to Cutaneous and Uveal Melanoma Patients. Hum. Gene Ther. 2019, 30, 352–364. [Google Scholar] [CrossRef] [PubMed]
- López González, M.; van de Ven, R.; de Haan, H.; Eck van der Sluijs, J.; Dong, W.; van Beusechem, V.W.; de Gruijl, T.D. Oncolytic Adenovirus ORCA-010 Increases the Type 1 T Cell Stimulatory Capacity of Melanoma-Conditioned Dendritic Cells. Clin. Exp. Immunol. 2020, 201, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Brachtlova, T.; Abramovitch, A.; Giddens, J.; Incze, P.; Jansz, K.; Casey, R.; van Beusechem, V.; Dong, W. 954 Clinical Results from a Phase I Dose Escalation Study in Treatment-Naïve Early Stage Prostate Cancer Patients with ORCA-010, a Potency Enhanced Oncolytic Replication Competent Adenovirus. J. Immunother. Cancer 2021, 9, A1004. [Google Scholar] [CrossRef]
- Morse, M.A.; Chaudhry, A.; Gabitzsch, E.S.; Hobeika, A.C.; Osada, T.; Clay, T.M.; Amalfitano, A.; Burnett, B.K.; Devi, G.R.; Hsu, D.S.; et al. Novel Adenoviral Vector Induces T-Cell Responses despite Anti-Adenoviral Neutralizing Antibodies in Colorectal Cancer Patients. Cancer Immunol. Immunother. 2013, 62, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, J.; Tong, A.; Yang, H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther.-Oncolytics 2019, 15, 234–247. [Google Scholar] [CrossRef]
- Hong, J.; Yun, C.-O. Overcoming the Limitations of Locally Administered Oncolytic Virotherapy. BMC Biomed. Eng. 2019, 1, 17. [Google Scholar] [CrossRef]
- Garofalo, M.; Pancer, K.W.; Wieczorek, M.; Staniszewska, M.; Salmaso, S.; Caliceti, P.; Kuryk, L. From Immunosuppression to Immunomodulation—Turning Cold Tumours into Hot. J. Cancer 2022, 13, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Kuryk, L.; Bertinato, L.; Staniszewska, M.; Pancer, K.; Wieczorek, M.; Salmaso, S.; Caliceti, P.; Garofalo, M. From Conventional Therapies to Immunotherapy: Melanoma Treatment in Review. Cancers 2020, 12, 3057. [Google Scholar] [CrossRef] [PubMed]
| Species | Virus Name | Virus Type | Phase | Posted | Clinical Endpoint | NCT Number |
|---|---|---|---|---|---|---|
| B | AdV-B3-hTERT-E1A | HAdV-B3 | 1 | 2012 | MTD | - |
| ColoAd1; EnAdV; Enadenotucirev | HAdV-B11-B3 | 1/2 | 2012 | MTD | NCT02028442 | |
| 1 | 2013 | Distribution | NCT02053220 | |||
| 1 | 2014 | MTD | NCT02028117 | |||
| 1 | 2016 | MTD | NCT02636036 | |||
| 1 | 2019 | MTD | NCT03916510 | |||
| NG-350A | HAdV-B11-B3 | 1 | 2019 | MTD | NCT03852511 | |
| NG-641 | HAdV-B11-B3 | 1 | 2020 | MTD | NCT04053283 | |
| 1 | 2021 | AE | NCT05043714 | |||
| VBIR-1; PrCa VBIR | HAdV-B68 | 1 | 2015 | DLT | NCT02616185 | |
| C | CG0070 | HAdV-C5-E2F | 1 | 2005 | MTD | NCT00109655 |
| 2/3 | 2011 | CR, DCR | NCT01438112 | |||
| 2 | 2014 | DCR | NCT02143804 | |||
| 2 | 2015 | DCR | NCT02365818 | |||
| 2 | 2020 | DOR | NCT04387461 | |||
| 3 | 2020 | DOR | NCT04452591 | |||
| 1 | 2020 | EAE | NCT04610671 | |||
| DNX2401 | HAdV-C5-Δ24-RGB | 1 | 2008 | MTD | NCT00805376 | |
| 1 | 2013 | DLT | NCT01956734 | |||
| 1 | 2014 | ORR | NCT02197169 | |||
| 2 | 2016 | ORR, OS | NCT02798406 | |||
| 1 | 2017 | OS, MTD | NCT03178032 | |||
| 1 | 2019 | MTD, AE, TR, TP, VS, IAA | NCT03896568 | |||
| DNX2440 | HAdV-C5-Δ24-RGB | 1 | 2018 | DLT | NCT03714334 | |
| 1 | 2021 | MTD | NCT04714983 | |||
| LOAd703 | HAdV-C5-Δ24 | 1/2 | 2016 | DLT | NCT02705196 | |
| 1/2 | 2017 | ST | NCT03225989 | |||
| 1/2 | 2018 | ORR, OS | NCT03555149 | |||
| 1/2 | 2019 | ORR, OS | NCT04123470 | |||
| ONCOS-102; CGTG-102 | HAdV-C5-Δ24-GMCSF | 1 | 2011 | ST | NCT01437280 | |
| 1 | 2012 | AE | NCT01598129 | |||
| 1/2 | 2016 | TEAE, DLT | NCT02963831 | |||
| 1 | 2016 | TEAE | NCT03003676 | |||
| 1/2 | 2016 | TEAE | NCT02879669 | |||
| 1/2 | 2018 | OS, ST | NCT03514836 | |||
| 2 | 2022 | TEAE, ORR | NCT05561491 | |||
| VCN-01 | HAdV-C5 | 1 | 2014 | AE | NCT02045602 | |
| 1 | 2014 | AE | NCT02045589 | |||
| - | 2017 | TEAE | NCT03284268 | |||
| 1 | 2019 | AE, ORR | NCT03799744 | |||
| 1 | 2021 | ORR, DOR, OS, PFS | NCT05057715 | |||
| - | 2023 | TEAE | NCT03284268 | |||
| ICOVIR | HAdV-C5 | 1 | 2013 | MTD | NCT01864759 | |
| 1/2 | 2013 | AE | NCT01844661 | |||
| 1/2 | 2021 | DLT | NCT04758533 | |||
| 1/2 | 2021 | OS, ORR | NCT05047276 | |||
| ORCA-010 | HAdV-C5 | 1/2 | 2019 | ST | NCT04097002 | |
| H101; Oncorine | HAdV-C5 | 3 | 2018 | OS, PFS, AE | NCT03780049 | |
| 2 | 2021 | AE, TTRP | NCT04771676 | |||
| 4 | 2021 | ORR, DCR | NCT05113290 | |||
| 4 | 2021 | PFS, ORR, DCR | NCT05124002 | |||
| 2 | 2022 | CR | NCT05564897 | |||
| 2 | 2022 | ORR, OS, DCR, PFS | NCT05234905 | |||
| 1 | 2023 | MTD, DLT, AE | NCT05675462 | |||
| C | Ad5yCD/mutTKSR39rep-ADP | HAdV-C5 | 1 | 2006 | TC | NCT00415454 |
| 2 | 2007 | MTD, OS, TC, FFF, CTL, AE | NCT00583492 | |||
| 1 | 2016 | AE | NCT02894944 | |||
| 1 | 2017 | MTD, OS, TC | NCT03029871 | |||
| 2 | 2021 | FFF, ORR | NCT04739046 | |||
| 1 | 2023 | MTD | NCT05686798 | |||
| Ad5-yCD/mutTKSR39rep-hIL12 | HAdV-C5 | 1 | 2015 | MTD, FFF, OS | NCT02555397 | |
| 1 | 2017 | TC, AE | NCT03281382 | |||
| Ad5.SSTR/TK.RGD | HAdV-C5 | 1 | 2009 | TC | NCT00964756 | |
| AdHER2.1 | HAdV-C5 | 1 | 2005 | TC | NCT00197522 | |
| 1 | 2006 | TC, MTD | NCT00307229 | |||
| ADV-hIL-12 | HAdV-C5 | 1 | 2005 | MCL | NCT00110526 | |
| 1 | 2006 | TC | NCT00301106 | |||
| 1 | 2009 | MTD, TC | NCT00849459 | |||
| Ad-RTS-hIL-12; INXN-2001 | HAdV-C5 | 1/2 | 2011 | ST | NCT01397708 | |
| 2 | 2012 | ST, ORR, CR | NCT01703754 | |||
| 1 | 2014 | ST, MTD | NCT02026271 | |||
| 1/2 | 2015 | ST, ORR, CR | NCT02423902 | |||
| 1/2 | 2017 | ST | NCT03330197 | |||
| 1 | 2018 | ST, ORR, PFS, OS | NCT03636477 | |||
| 1 | 2018 | ST, ORR, PFS, OS | NCT03679754 | |||
| 2 | 2019 | ST, PFS, OS | NCT04006119 | |||
| C | OBP-301; Telomelysin; Ad5-SGE-REIC/Dkk3 | HAdV-C5 | 1 | 2014 | ST, MTD, DLT | NCT02293850 |
| 1 | 2017 | DLT, RR, PFS, AE | NCT03172819 | |||
| 2 | 2017 | ORR, PFS, OS, CR | NCT03190824 | |||
| 1 | 2017 | DLT, AE | NCT03213054 | |||
| 2 | 2019 | ORR, DOR, OS, PFS | NCT03921021 | |||
| 1 | 2020 | DLT, AE, CR | NCT04391049 | |||
| 2 | 2020 | ORR, TC, OS, PFS, DOR | NCT04685499 | |||
| 1 | 2020 | CR, PFS, OS | NCT04391049 | |||
| TILT-123 | HAdV-C5 | 1 | 2020 | ST, AE | NCT04217473 | |
| 1 | 2021 | AE | NCT04695327 | |||
| 1 | 2022 | AE | NCT05222932 | |||
| 1 | 2022 | AE | NCT05271318 | |||
| MG1MA3 | HAdV-C5 | 1/2 | 2014 | AE, ORR, | NCT02285816 | |
| AdcuCD40L | HAdV-C5 | 1 | 2006 | TC, AE | NCT00328887 | |
| 1/2 | 2007 | PE | NCT00504322 | |||
| AdCD40L; Ad-ISF35 | HAdV-C5 | 1 | 2008 | TC, ST | NCT00772486 | |
| 1 | 2008 | TC, ST | NCT00783588 | |||
| 1 | 2008 | TC, ST | NCT00779883 | |||
| 1 | 2009 | MTD, ST | NCT00850057 | |||
| 1 | 2009 | ST | NCT00849524 | |||
| 2 | 2009 | ORR | NCT00942409 | |||
| 1/2 | 2011 | AE | NCT01455259 | |||
| 1/2 | 2016 | MTD, ORR | NCT02719015 | |||
| Ad5-hGCC-PADRE | HAdV-C5 | 1 | 2013 | AE | NCT01972737 | |
| Ad-E6E7 | HAdV-C5 | 1 | 2018 | ST, MTD | NCT03618953 | |
| ETBX-071—PSA ETBX-061—mucin1 ETBX-051—brachyury ETBX-011—CEA | HAdV-C5 | 1 | 2018 | DLT, CR, ORR | NCT03481816 | |
| ETBX-061—mucin1 ETBX-051—brachyury ETBX-011—CEA | HAdV-C5 | 1 | 2017 | AE, CR, | NCT03384316 | |
| VB-111 | HAdV-C5 | 1 | 2019 | ST, PFS, OS | NCT04166383 | |
| AdAPT-001 | HAdV-C5 | 1/2 | 2020 | MTD, DLT | NCT04673942 | |
| RSV-TK | HAdV-C5 | 1 | 2004 | - | NCT00005057 | |
| Ad-hCMV-TK Ad-hCMV-Flt3L | HAdV-C5 | 1 | 2013 | MTD, OS | NCT01811992 | |
| Ad-TD-nsIL12 | HAdV-C5 | 1 | 2023 | ST, OS, | NCT05717712 | |
| 1 | 2023 | ST, OS, | NCT05717699 | |||
| Ad5CMV-p53 | HAdV-C5 | 1 | 2004 | DLT | NCT00003147 | |
| Ad5 [E1-, E2B-]-CEA(6D) ETBX-011 | HAdV-C5 | 1/2 | 2010 | SF, TC, OS, CMI | NCT01147965 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryciuk, A.; Rogalska, M.; Baran, J.; Kuryk, L.; Staniszewska, M. Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment. Cancers 2023, 15, 1947. https://doi.org/10.3390/cancers15071947
Gryciuk A, Rogalska M, Baran J, Kuryk L, Staniszewska M. Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment. Cancers. 2023; 15(7):1947. https://doi.org/10.3390/cancers15071947
Chicago/Turabian StyleGryciuk, Aleksander, Marta Rogalska, Joanna Baran, Lukasz Kuryk, and Monika Staniszewska. 2023. "Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment" Cancers 15, no. 7: 1947. https://doi.org/10.3390/cancers15071947
APA StyleGryciuk, A., Rogalska, M., Baran, J., Kuryk, L., & Staniszewska, M. (2023). Oncolytic Adenoviruses Armed with Co-Stimulatory Molecules for Cancer Treatment. Cancers, 15(7), 1947. https://doi.org/10.3390/cancers15071947







