Neuropsychiatric Adverse Drug Reactions with Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors: An Analysis from the European Spontaneous Adverse Event Reporting System
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
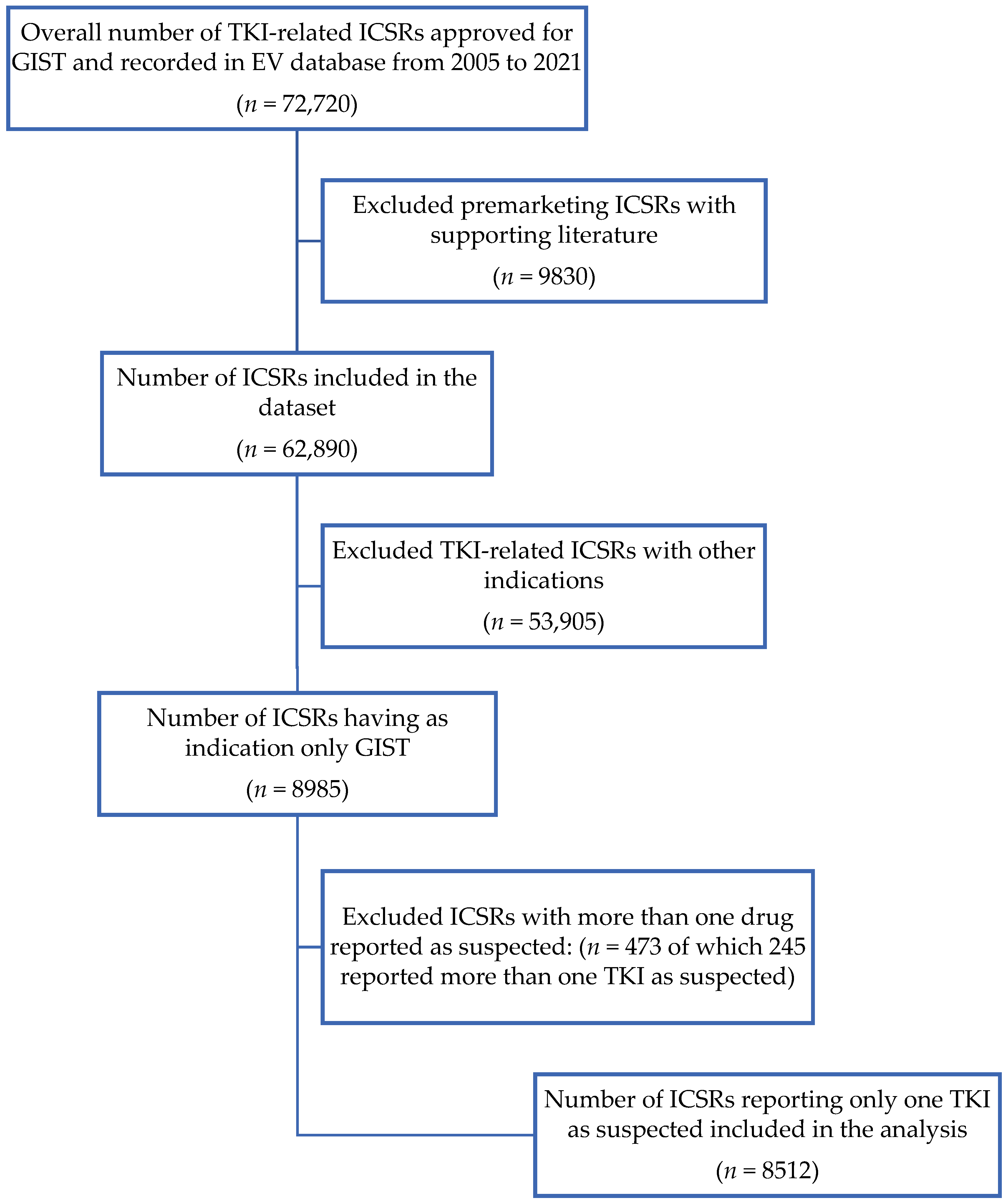
2.1. Data Sources and Selection Process
2.2. Data Analyses
3. Results
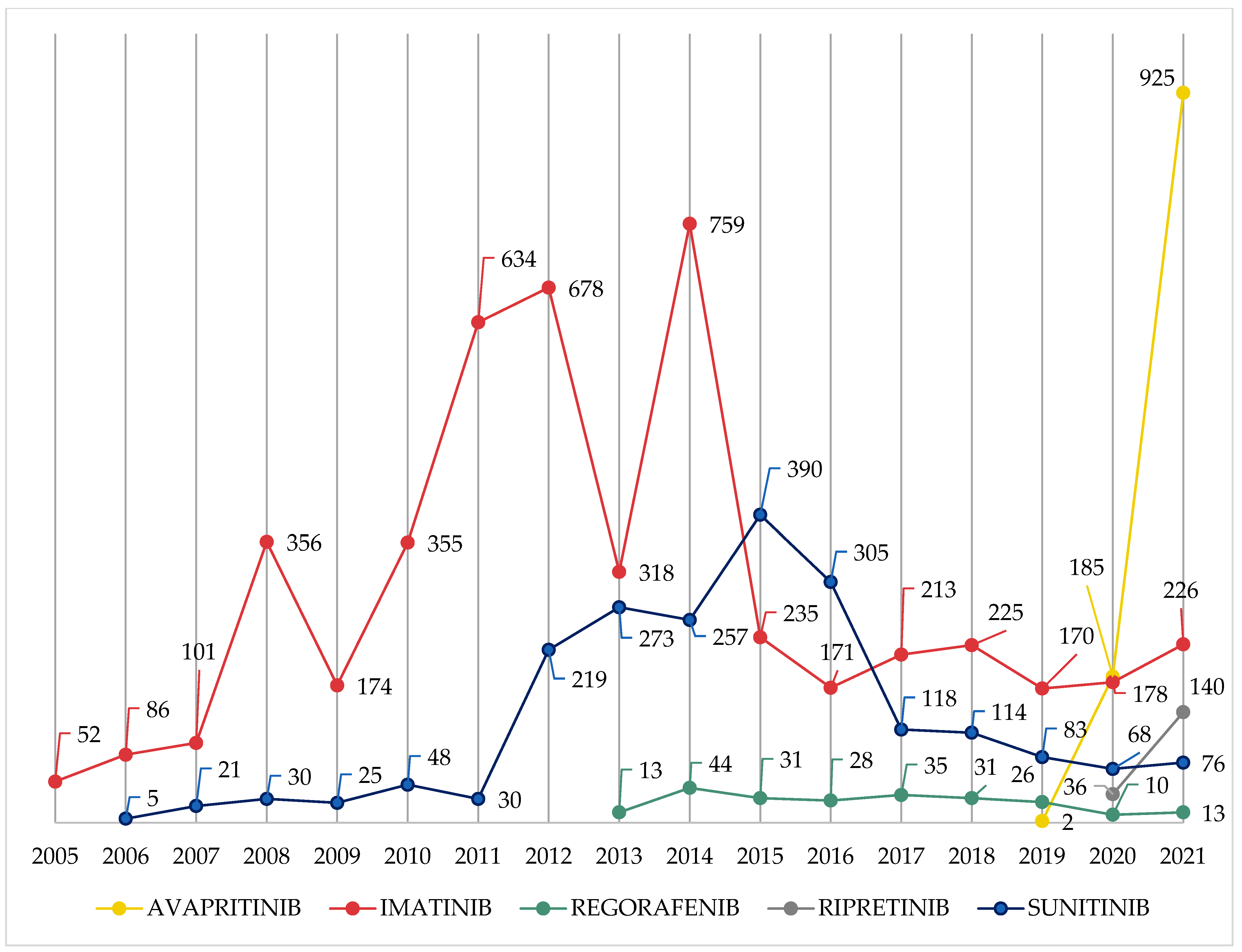
3.1. Characteristics of ICSRs
3.2. Characteristics of ADRs
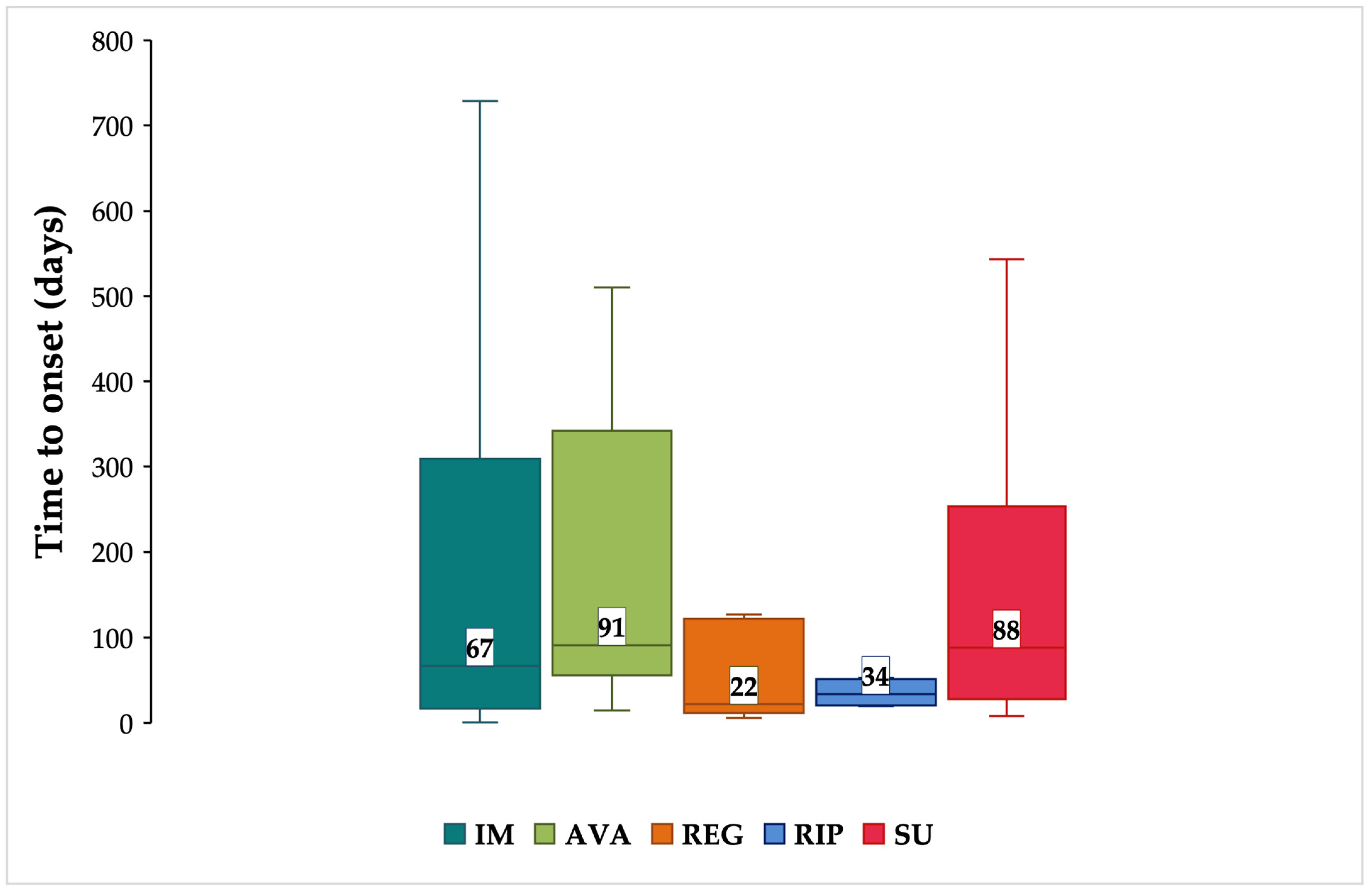
3.3. Neuropsychiatric ADRs
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaefer, I.-M.; DeMatteo, R.P.; Serrano, C. The GIST of Advances in Treatment of Advanced Gastrointestinal Stromal Tumor. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 885–899. [Google Scholar] [CrossRef]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; et al. Gastrointestinal stromal tumours: ESMO–EURACAN–GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Corless, C.L.; Barnett, C.M.; Heinrich, M.C. Gastrointestinal stromal tumours: Origin and molecular oncology. Nat. Rev. Cancer 2011, 11, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Mazzocca, A.; Minelli, A.; Paternostro, F.; Silletta, M.; Napolitano, A.; Vincenzi, B. New molecularly targeted drugs for gist after imatinib, sunitinib and regorafenib: A narrative review. Gastrointest. Stromal Tumor 2022, 5, 4. [Google Scholar] [CrossRef]
- European Medicines Agency. Glivec®, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/glivec-epar-product-information_en.pdf (accessed on 20 December 2022).
- Fu, W.C.; Jamison, T.F. Modular Continuous Flow Synthesis of Imatinib and Analogues. Org. Lett. 2019, 21, 6112–6116. [Google Scholar] [CrossRef]
- Raut, C.P.; Espat, N.J.; Maki, R.G.; Araujo, D.M.; Trent, J.; Williams, T.F.; Purkayastha, D.D.; DeMatteo, R.P. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients with Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor. JAMA Oncol. 2018, 4, e184060. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, L.; Xu, P.; Hu, W. An overview of agents and treatments for PDGFRA-mutated gastrointestinal stromal tumors. Front. Oncol. 2022, 12, 927587. [Google Scholar] [CrossRef]
- European Medicines Agency. Sutent®, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/sutent-epar-product-information_en.pdf (accessed on 20 December 2022).
- European Medicines Agency. Stivarga®, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/stivarga-epar-product-information_en.pdf (accessed on 19 January 2023).
- Chamberlain, F.; Farag, S.; Williams-Sharkey, C.; Collingwood, C.; Chen, L.; Mansukhani, S.; Engelmann, B.; Al-Muderis, O.; Chauhan, D.; Thway, K.; et al. Toxicity management of regorafenib in patients with gastro-intestinal stromal tumour (GIST) in a tertiary cancer centre. Clin. Sarcoma Res. 2020, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Shiri, P.; Ramezanpour, S.; Amani, A.M.; Dehaen, W. A patent review on efficient strategies for the total synthesis of pazopanib, regorafenib and lenvatinib as novel anti-angiogenesis receptor tyrosine kinase inhibitors for cancer therapy. Mol. Divers. 2022, 26, 2981–3002. [Google Scholar] [CrossRef]
- European Medicines Agency. Qinlock®, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/qinlock-epar-product-information_en.pdf (accessed on 19 January 2023).
- Liang, X.; Yang, Q.; Wu, P.; He, C.; Yin, L.; Xu, F.; Yin, Z.; Yue, G.; Zou, Y.; Li, L.; et al. The synthesis review of the approved tyrosine kinase inhibitors for anticancer therapy in 2015–2020. Bioorg. Chem. 2021, 113, 105011. [Google Scholar] [CrossRef] [PubMed]
- Zalcberg, J.R. Ripretinib for the treatment of advanced gastrointestinal stromal tumor. Therap. Adv. Gastroenterol. 2021, 14, 175628482110081. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.H.; Ryu, M.-H.; Ryoo, B.-Y.; Beck, M.; Choi, D.R.; Cho, Y.; Lee, J.-L.; Chang, H.-M.; Kim, T.W.; Kang, Y.-K. Sunitinib as a second-line therapy for advanced GISTs after failure of imatinib: Relationship between efficacy and tumor genotype in Korean patients. Investig. New Drugs 2012, 30, 819–827. [Google Scholar] [CrossRef]
- Bauer, S.; George, S.; von Mehren, M.; Heinrich, M.C. Early and Next-Generation KIT/PDGFRA Kinase Inhibitors and the Future of Treatment for Advanced Gastrointestinal Stromal Tumor. Front. Oncol. 2021, 11, 672500. [Google Scholar] [CrossRef]
- European Medicines Agency. Ayvakit®, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ayvakyt-epar-product-information_en.pdf (accessed on 20 December 2022).
- Trullas-Jimeno, A.; Delgado, J.; Garcia-Ochoa, B.; Wang, I.; Sancho-Lopez, A.; Payares-Herrera, C.; Dalhus, M.L.; Strøm, B.O.; Egeland, E.J.; Enzmann, H.; et al. The EMA assessment of avapritinib in the treatment of gastrointestinal stromal tumours harbouring the PDGFRA D842V mutation. ESMO Open 2021, 6, 100159. [Google Scholar] [CrossRef]
- Patel, S.R.; Reichardt, P. An updated review of the treatment landscape for advanced gastrointestinal stromal tumors. Cancer 2021, 127, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, J.; Qian, Y.; Chen, L.; Li, Q.; Xu, K.; Chen, M.; Sun, L.; He, Z.; Yang, L.; et al. Association of Imatinib Plasma Concentration and Single-nucleotide Polymorphisms with Adverse Drug Reactions in Patients with Gastrointestinal Stromal Tumors. Mol. Cancer Ther. 2018, 17, 2780–2787. [Google Scholar] [CrossRef]
- Chang, Y.-R.; Huang, W.-K.; Wang, S.-Y.; Wu, C.-E.; Chen, J.-S.; Yeh, C.-N. A Nomogram Predicting Progression Free Survival in Patients with Gastrointestinal Stromal Tumor Receiving Sunitinib: Incorporating Pre-Treatment and Post-Treatment Parameters. Cancers 2021, 13, 2587. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Jones, R.L.; Bauer, S.; Kang, Y.-K.; Schöffski, P.; Eskens, F.; Mir, O.; Cassier, P.A.; Serrano, C.; Tap, W.D.; et al. Avapritinib in Patients With Advanced Gastrointestinal Stromal Tumors Following at Least Three Prior Lines of Therapy. Oncologist 2021, 26, e639–e649. [Google Scholar] [CrossRef]
- Nannini, M.; Rizzo, A.; Nigro, M.C.; Vincenzi, B.; Mazzocca, A.; Grignani, G.; Merlini, A.; D’Ambrosio, L.; Tolomeo, F.; Badalamenti, G.; et al. Standard versus personalized schedule of regorafenib in metastatic gastrointestinal stromal tumors: A retrospective, multicenter, real-world study. ESMO Open 2021, 6, 100222. [Google Scholar] [CrossRef]
- Lostes-Bardaji, M.J.; García-Illescas, D.; Valverde, C.; Serrano, C. Ripretinib in gastrointestinal stromal tumor: The long-awaited step forward. Ther. Adv. Med. Oncol. 2021, 13, 175883592098649. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Radia, D.H.; George, T.I.; Robinson, W.A.; Quiery, A.T.; Drummond, M.W.; Bose, P.; Hexner, E.O.; Winton, E.F.; Horny, H.P.; et al. Safety and efficacy of avapritinib in advanced systemic mastocytosis: The phase 1 EXPLORER trial. Nat. Med. 2021, 27, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Summary of Risk Management Plan for AYVAKYT (Avapritinib). Available online: https://www.ema.europa.eu/en/documents/rmp-summary/saphnelo-epar-risk-management-plan-summary_en.pdf (accessed on 6 December 2022).
- Drom, C.; Schenheit, K.; Matzke, M.; Obeidat, A.Z.; Molinaro, J.; Charlson, J.; Knight, J.M. Abrupt onset of severe parkinsonism in a patient with metastatic gastrointestinal stromal tumor receiving treatment with avapritinib: A case report. Brain Behav. Immun.—Health 2023, 27, 100570. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Kalaiselvan, V.; Bin Jardan, Y.A.; Zameer, S.; Sarwat, M. Comparative Study of Adverse Drug Reactions Associated with Filgrastim and Pegfilgrastim Using the EudraVigilance Database. Biology 2022, 11, 340. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Gaio, M.; Zinzi, A.; Scavone, C.; Gargano, F.; Coscioni, E.; Rossi, F.; Capuano, A. Cardiac events potentially associated to remdesivir: An analysis from the european spontaneous adverse event reporting system. Pharmaceuticals 2021, 14, 611. [Google Scholar] [CrossRef] [PubMed]
- di Mauro, G.; Zinzi, A.; Scavone, C.; Mascolo, A.; Gaio, M.; Sportiello, L.; Ferrajolo, C.; Rafaniello, C.; Rossi, F.; Capuano, A. PCSK9 Inhibitors and Neurocognitive Adverse Drug Reactions: Analysis of Individual Case Safety Reports from the Eudravigilance Database. Drug Saf. 2021, 44, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Scavone, C.; Ferrajolo, C.; Rafaniello, C.; Danesi, R.; Del Re, M.; Russo, A.; Coscioni, E.; Rossi, F.; Alfano, R.; et al. Immune Checkpoint Inhibitors and Cardiotoxicity: An Analysis of Spontaneous Reports in Eudravigilance. Drug Saf. 2021, 44, 957–971. [Google Scholar] [CrossRef]
- Rutkowski, P.; Bylina, E.; Lugowska, I.; Teterycz, P.; Klimczak, A.; Streb, J.; Czarnecka, A.M.; Osuch, C. Treatment outcomes in older patients with advanced gastrointestinal stromal tumor (GIST). J. Geriatr. Oncol. 2018, 9, 520–525. [Google Scholar] [CrossRef]
- Demetri, G.D.; van Oosterom, A.T.; Garrett, C.R.; Blackstein, M.E.; Shah, M.H.; Verweij, J.; McArthur, G.; Judson, I.R.; Heinrich, M.C.; Morgan, J.A.; et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006, 368, 1329–1338. [Google Scholar] [CrossRef]
- Demetri, G.D.; Reichardt, P.; Kang, Y.; Blay, J.; Rutkowski, P.; Gelderblom, H.; Hohenberger, P.; Leahy, M.; von Mehren, M.; Joensuu, H.; et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 295–302. [Google Scholar] [CrossRef]
- Heinrich, M.C.; Jones, R.L.; von Mehren, M.; Schöffski, P.; Serrano, C.; Kang, Y.-K.; Cassier, P.A.; Mir, O.; Eskens, F.; Tap, W.D.; et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): A multicentre, open-label, phase 1 trial. Lancet Oncol. 2020, 21, 935–946. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, H.; Zhao, J. Risk of fatal adverse events in cancer patients treated with sunitinib. Crit. Rev. Oncol. Hematol. 2019, 137, 115–122. [Google Scholar] [CrossRef]
- Hamnvik, O.-P.R.; Choueiri, T.K.; Turchin, A.; McKay, R.R.; Goyal, L.; Davis, M.; Kaymakcalan, M.D.; Williams, J.S. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015, 121, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Vaziri, S.A.J.; Rini, B.I.; Elson, P.; Garcia, J.A.; Wirka, R.; Dreicer, R.; Ganapathi, M.K.; Ganapathi, R. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer 2012, 118, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhao, H. Incidence and risk of regorafenib-induced hepatotoxicity. Oncotarget 2017, 8, 84102–84111. [Google Scholar] [CrossRef] [PubMed]
- Seidel, C.; Fenner, M.; Länger, F.; Bantel, H.; Ganser, A.; Grünwald, V. Imatinib-induced liver cirrhosis in a patient with advanced gastrointestinal stroma tumor (GIST). BMC Cancer 2012, 12, 186. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.; Chu, B.; Aggarwal, P. Photosensitivity From Avapritinib: Pharamacovigilance Analysis. JMIR Dermatology 2022, 5, e39229. [Google Scholar] [CrossRef]
- Rautiola, J.; Donskov, F.; Peltola, K.; Joensuu, H.; Bono, P. Sunitinib-induced hypertension, neutropaenia and thrombocytopaenia as predictors of good prognosis in patients with metastatic renal cell carcinoma. BJU Int. 2016, 117, 110–117. [Google Scholar] [CrossRef]
- Banka, R.; Udwadia, Z. Imatinib-induced pleural effusion: A case report. J. Postgrad. Med. 2017, 63, 55. [Google Scholar] [CrossRef]
- BC Cancer Agency. BC Cancer Drug Manual Ripretinib. Available online: http://www.bccancer.bc.ca/drug-database-site/DrugIndex/Ripretinib_handout.pdf (accessed on 19 January 2023).
- Chi, P.; Janku, F.; Heinrich, M.; Ganjoo, K.; Gelderblom, H.; Gordon, M.; Jones, R.; Razak, A.; Trent, J.; von Mehren, M.; et al. Abstract C077: Updated results of phase 1 study of ripretinib (DCC-2618), a broad-spectrum KIT and PDGFRA inhibitor, in patients with gastrointestinal stromal tumor (GIST) by line of therapy (NCT02571036). Mol. Cancer Ther. 2019, 18, C077. [Google Scholar] [CrossRef]
- Joseph, C.P.; Abaricia, S.N.; Angelis, M.A.; Polson, K.; Jones, R.L.; Kang, Y.-K.; Riedel, R.F.; Schöffski, P.; Serrano, C.; Trent, J.; et al. Optimal Avapritinib Treatment Strategies for Patients with Metastatic or Unresectable Gastrointestinal Stromal Tumors. Oncologist 2021, 26, e622–e631. [Google Scholar] [CrossRef]
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal Cognitive Aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Serrano, C.; von Mehren, M.; George, S.; Heinrich, M.C.; Kang, Y.-K.; Schöffski, P.; Cassier, P.A.; Mir, O.; Chawla, S.P.; et al. Avapritinib in unresectable or metastatic PDGFRA D842V-mutant gastrointestinal stromal tumours: Long-term efficacy and safety data from the NAVIGATOR phase I trial. Eur. J. Cancer 2021, 145, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.; Bast, T.; Gaily, E.; Golla, G.; Gorman, K.M.; Griffiths, L.R.; Hahn, A.; Hukin, J.; King, M.; Korff, C.; et al. Clinical and genetic spectrum of SCN2A-associated episodic ataxia. Eur. J. Paediatr. Neurol. 2019, 23, 438–447. [Google Scholar] [CrossRef]
- Serrano, C.; George, S. Gastrointestinal Stromal Tumor: Challenges and Opportunities for a New Decade. Clin. Cancer Res. 2020, 26, 5078–5085. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Deng, Y.; Wu, X.; Zheng, Z.; Zhou, Y.; Cai, S.; Zhang, Y.; Zhang, J.; Tao, K.; et al. Efficacy and Safety of Avapritinib in Treating Unresectable or Metastatic Gastrointestinal Stromal Tumors: A Phase I/II, Open-Label, Multicenter Study. Oncologist 2023, 28, 187-e114. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Ragueneau-Majlessi, I. Pharmacokinetic Drug-Drug Interactions with Drugs Approved by the US Food and Drug Administration in 2020: Mechanistic Understanding and Clinical Recommendations. Drug Metab. Dispos. 2022, 50, 1–7. [Google Scholar] [CrossRef]
- Ozdemir, N.; Toptas, S.; Sendur, M.A.N.; Yazici, O.; Öksüzoğlu, B.; Silay, K.; Zengin, N. Tyrosine kinase inhibitors (TKI): Awareness of drug-drug interaction. Ann. Oncol. 2017, 28, v399. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) Public Dashboard. Available online: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis (accessed on 1 February 2023).
- van Elst, J.M.; IJzerman, N.S.; Mathijssen, R.H.J.; Steeghs, N.; Reyners, A.K.L.; de Haan, J.J. Taste, smell and mouthfeel disturbances in patients with gastrointestinal stromal tumors treated with tyrosine-kinase inhibitors. Support. Care Cancer 2022, 30, 2307–2315. [Google Scholar] [CrossRef]
- Food and Drug Administration. Multi-Disciplinary Review and Evaluation NDA 212608 AYVAKIT (Avapritinib). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212608Orig1s000MultidisciplineR.pdf (accessed on 24 January 2023).
- Chen, B.; Zhang, Y.; Chen, S.; Li, X.; Dong, J.; Chen, W.; Tao, S.; Yang, W.; Zhang, Y. The role of vascular endothelial growth factor in ischemic stroke. Pharmazie 2021, 76, 127–131. [Google Scholar] [CrossRef]
- Zuo, P.-Y.; Chen, X.-L.; Liu, Y.-W.; Xiao, C.-L.; Liu, C.-Y. Increased Risk of Cerebrovascular Events in Patients with Cancer Treated with Bevacizumab: A Meta-Analysis. PLoS ONE 2014, 9, e102484. [Google Scholar] [CrossRef]
- Strom, B.L.; Kimmel, S.E.; Hennessy, S. Pharmacoepidemiology, 6th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; ISBN 9781119413417. [Google Scholar]
- Raschi, E.; Moretti, U.; Salvo, F.; Pariente, A.; Cosimo Antonazzo, I.; De Ponti, F.; Poluzzi, E. Evolving Roles of Spontaneous Reporting Systems to Assess and Monitor Drug Safety. In Pharmacovigilance; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Eichler, M.; Pink, D.; Menge, F.; Jakob, J.; Hentschel, L.; Richter, S.; Hohenberger, P.; Kasper, B.; Andreou, D.; Singer, S.; et al. Quality of life of GIST patients with and without current tyrosine kinase inhibitor treatment: Cross-sectional results of a German multicentre observational study (PROSa). Eur. J. Cancer Care 2021, 30, e13484. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Sorbara, E.E.; Cicala, G.; Santoro, V.; Cutroneo, P.M.; Franchina, T.; Santarpia, M.; Silvestris, N.; Spina, E. Safety profile of tyrosine kinase inhibitors used in non-small-cell lung cancer: An analysis from the Italian pharmacovigilance database. Front. Oncol. 2022, 12, 1005626. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.A.; Sorbara, E.E.; Cicala, G.; Santoro, V.; Cutroneo, P.M.; Franchina, T.; Spina, E. Adverse Drug Reactions with HER2-Positive Breast Cancer Treatment: An Analysis from the Italian Pharmacovigilance Database. Drugs—Real World Outcomes 2022, 9, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.N.; Duncombe, C.; Falzon, D.; Olsson, S. WHO strategy for collecting safety data in public health programmes: Complementing spontaneous reporting systems. Drug Saf. 2013, 36, 75–81. [Google Scholar] [CrossRef]
- Barbieri, M.A.; Cicala, G.; Cutroneo, P.M.; Mocciaro, E.; Sottosanti, L.; Freni, F.; Galletti, F.; Arcoraci, V.; Spina, E. Ototoxic Adverse Drug Reactions: A Disproportionality Analysis Using the Italian Spontaneous Reporting Database. Front. Pharmacol. 2019, 10, 1161. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Rong, C.; He, X.; Chen, L. Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitor-Associated Cardiotoxicity: A Recent Five-Year Pharmacovigilance Study. Front. Pharmacol. 2022, 13, 858279. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Meng, L.; Yang, B.; Sun, S.; Luo, Z.; Chen, H. Safety Profile of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: A Disproportionality Analysis of FDA Adverse Event Reporting System. Sci. Rep. 2020, 10, 4803. [Google Scholar] [CrossRef] [PubMed]
| Characteristic, n (%) | AVA (n = 1112) | IM (n = 4931) | REG (n = 231) | RIP (n = 176) | SU (n = 2062) | Total (n = 8512) |
|---|---|---|---|---|---|---|
| Age Group | ||||||
| Infant | 1 (0.1) | 2 (<0.1) | 3 (<0.1) | |||
| Child | 1 (0.1) | 2 (<0.1) | 3 (<0.1) | |||
| Adolescent | 7 (0.1) | 2 (0.1) | 9 (0.1) | |||
| Adult | 480 (43.2) | 2027 (41.1) | 111 (48.1) | 85 (48.3) | 1099 (53.3) | 3802 (44.7) |
| Elderly | 621 (55.8) | 1783 (36.2) | 82 (35.5) | 88 (50.0) | 785 (38.1) | 3359 (39.5) |
| Not Specified | 9 (0.8) | 1110 (22.5) | 38 (16.5) | 3 (1.7) | 176 (8.5) | 1336 (15.7) |
| Patient Sex | ||||||
| Female | 511 (46.0) | 1871 (37.9) | 88 (38.1) | 73 (41.5) | 780 (37.8) | 3323 (39.0) |
| Male | 593 (53.3) | 2373 (48.1) | 121 (52.4) | 102 (58.0) | 1212 (58.8) | 4401 (51.7) |
| Not Specified | 8 (0.7) | 687 (13.9) | 22 (9.5) | 1 (0.6) | 70 (3.4) | 788 (9.3) |
| Primary Source Qualification | ||||||
| Healthcare Professional | 91 (8.2) | 4280 (86.8) | 214 (92.6) | 173 (98.3) | 960 (46.6) | 5718 (67.2) |
| Non-Healthcare Professional | 1021 (91.8) | 637 (12.9) | 17 (7.4) | 3 (1.7) | 1102 (53.4) | 2780 (32.7) |
| Not Specified | 14 (0.3) | 14 (0.2) | ||||
| Primary Source Country for Regulatory Purposes | ||||||
| European Economic Area | 5 (0.4) | 1082 (21.9) | 83 (35.9) | 220 (10.7) | 1390 (16.3) | |
| Non-European Economic Area | 1107 (99.6) | 3849 (78.1) | 148 (64.1) | 176 (100.0) | 1842 (89.3) | 7122 (83.7) |
| Serious | 347 (31.2) | 4719 (95.7) | 196 (84.8) | 176 (100.0) | 2012 (97.6) | 7450 (87.5) |
| Type of seriousness | ||||||
| Caused/Prolonged Hospitalization | 129 (37.2) | 829 (17.6) | 57 (29.1) | 90 (51.1) | 386 (19.2) | 1491 (20.0) |
| Congenital Anomaly | 1 (<0.1) | 1 (<0.1) | ||||
| Disabling | 2 (0.6) | 84 (1.8) | 4 (2.0) | 12 (0.6) | 102 (1.4) | |
| Life Threatening | 2 (0.6) | 88 (1.9) | 7 (3.6) | 32 (1.6) | 129 (1.7) | |
| Other Medically Important Condition | 174 (50.1) | 2694 (57.1) | 101 (51.5) | 54 (30.7) | 534 (26.5) | 3557 (47.7) |
| Results in Death | 40 (11.5) | 1023 (21.7) | 27 (13.8) | 32 (18.2) | 1048 (52.1) | 2170 (29.1) |
| Outcome | ||||||
| Recovered/resolved | 105 (9.4) | 670 (13.6) | 49 (21.2) | 15 (8.5) | 204 (9.9) | 1043 (12.3) |
| Recovering/resolving | 29 (2.6) | 582 (11.8) | 30 (13.0) | 16 (9.1) | 153 (7.4) | 810 (9.5) |
| Recovered/resolved with sequelae | 36 (0.7) | 1 (0.4) | 12 (0.6) | 49 (0.6) | ||
| Not recovered/not resolved | 674 (60.6) | 697 (14.1) | 48 (20.8) | 14 (8.0) | 271 (13.1) | 1704 (20.0) |
| Fatal | 40 (3.6) | 1023 (20.7) | 27 (11.7) | 32 (18.2) | 1048 (50.8) | 2170 (25.5) |
| Unknown | 264 (23.7) | 1923 (39.0) | 76 (32.9) | 99 (56.3) | 374 (18.1) | 2736 (32.1) |
| System Organ Class, n (%) | AVA (n = 1112) | IM (n = 4931) | REG (n = 231) | RIP (n = 176) | SU (n = 2062) | Total (n = 8512) |
|---|---|---|---|---|---|---|
| General disorders and administration site conditions | 559 (50.3) | 1847 (37.5) | 78 (33.8) | 102 (58.0) | 1343 (65.1) | 3929 (46.2) |
| General signs and symptoms NEC | 157 (14.1) | 292 (5.9) | 7 (3.0) | 31 (17.6) | 786 (38.1) | 1273 (15.0) |
| Asthenic conditions | 282 (25.4) | 350 (7.1) | 37 (16.0) | 33 (18.8) | 237 (11.5) | 939 (11.0) |
| Death and sudden death | 32 (2.9) | 481 (9.8) | 7 (3.0) | 24 (13.6) | 321 (15.6) | 865 (10.2) |
| Neoplasm benign, malignant and unspecified (incl cysts and polyps) | 71 (6.4) | 2042 (41.4) | 57 (24.7) | 33 (18.8) | 848 (41.1) | 3051 (35.8) |
| Gastrointestinal neoplasms malignant NEC | 1077 (21.8) | 46 (19.9) | 682 (33.1) | 1805 (21.2) | ||
| Neoplasms malignant site unspecified NEC | 12 (1.1) | 1482 (30.1) | 6 (3.4) | 14 (0.7) | 1514 (17.8) | |
| Neoplasms unspecified malignancy and site unspecified NEC | 44 (4.0) | 506 (10.3) | 11 (6.3) | 110 (5.3) | 671 (7.9) | |
| Gastrointestinal disorders | 404 (36.3) | 1139 (23.1) | 42 (18.2) | 46 (26.1) | 564 (27.4) | 2195 (25.8) |
| Nausea and vomiting symptoms | 168 (15.1) | 439 (8.9) | 4 (1.7) | 18 (10.2) | 143 (6.9) | 772 (9.1) |
| Diarrhoea (excl infective) | 152 (13.7) | 249 (5.0) | 18 (7.8) | 13 (7.4) | 189 (9.2) | 621 (7.3) |
| Gastrointestinal and abdominal pains (excl oral and throat) | 60 (5.4) | 234 (4.7) | 6 (2.6) | 14 (8.0) | 79 (3.8) | 393 (4.6) |
| Skin and subcutaneous tissue disorders | 170 (15.3) | 659 (13.4) | 80 (34.6) | 36 (20.5) | 310 (15.0) | 1255 (14.7) |
| Rashes, eruptions and exanthems NEC | 55 (4.9) | 342 (6.9) | 22 (9.5) | 9 (5.1) | 46 (2.2) | 474 (5.6) |
| Dermal and epidermal conditions NEC | 41 (3.7) | 127 (2.6) | 7 (3.0) | 5 (2.8) | 117 (5.7) | 297 (3.5) |
| Dermatitis ascribed to specific agent | 4 (0.4) | 44 (0.9) | 51 (22.1) | 7 (4.0) | 116 (5.6) | 222 (2.6) |
| Nervous system disorders | 375 (33.7) | 391 (7.9) | 26 (11.3) | 30 (17.0) | 286 (13.9) | 1108 (13.0) |
| Sensory abnormalities NEC | 55 (4.9) | 46 (0.9) | 1 (0.4) | 102 (4.5) | 204 (2.4) | |
| Neurological signs and symptoms NEC | 75 (6.7) | 72 (1.5) | 8 (4.5) | 44 (2.1) | 199 (2.3) | |
| Headaches NEC | 51 (4.6) | 46 (0.9) | 1 (0.4) | 6 (3.4) | 45 (2.2) | 149 (1.8) |
| Memory loss (excl dementia) | 126 (11.3) | 13 (0.3) | 10 (0.5) | 149 (1.8) | ||
| Blood and lymphatic system disorders | 73 (6.6) | 611 (12.4) | 12 (5.2) | 4 (2.3) | 335 (16.2) | 1035 (12.2) |
| Anaemias NEC | 28 (2.5) | 207 (4.2) | 2 (0.9) | 3 (1.7) | 60 (2.9) | 300 (3.5) |
| Thrombocytopenias | 11 (1.0) | 88 (1.8) | 5 (2.2) | 1 (0.6) | 171 (8.3) | 276 (3.2) |
| Leukopenias NEC | 31 (2.8) | 127 (2.6) | 2 (0.9) | 81 (3.9) | 241 (2.8) | |
| Metabolism and nutrition disorders | 193 (17.4) | 488 (9.9) | 26 (11.3) | 14 (8.0) | 274 (13.3) | 995 (11.7) |
| Appetite disorders | 81 (4.3) | 137 (2.8) | 19 (8.2) | 6 (3.4) | 127 (6.2) | 370 (4.3) |
| General nutritional disorders NEC | 54 (3.3) | 123 (2.5) | 4 (1.7) | 7 (4.0) | 96 (4.7) | 284 (3.3) |
| Metabolic disorders NEC | 5 (1.3) | 93 (1.9) | 9 (0.4) | 107 (1.3) |
| Characteristic, n (%) | AVA (n = 557) | IM (n = 528) | REG (n = 30) | RIP (n = 39) | SU (n = 357) | Total (n = 1511) |
|---|---|---|---|---|---|---|
| Age Group | ||||||
| Adolescent | 1 (1.2) | 2 (0.6) | 3 (0.2) | |||
| Adult | 187 (33.6) | 242 (45.8) | 10 (33.3) | 17 (43.6) | 161 (45.1) | 617 (40.8) |
| Elderly | 365 (65.5) | 220 (41.7) | 14 (46.7) | 21 (53.8) | 177 (49.6) | 797 (52.7) |
| Not Specified | 5 (0.9) | 65 (12.3) | 6 (20.0) | 1 (2.6) | 17 (4.8) | 94 (6.2) |
| Patient Sex | ||||||
| Female | 265 (47.6) | 245 (46.4) | 6 (20.0) | 14 (35.9) | 173 (48.5) | 703 (46.5) |
| Male | 287 (51.5) | 247 (46.8) | 23 (76.7) | 25 (64.1) | 178 (49.9) | 760 (50.3) |
| Not Specified | 5 (0.9) | 36 (6.8) | 1 (3.3) | 6 (1.7) | 48 (3.2) | |
| Primary Source Qualification | ||||||
| Healthcare Professional | 33 (5.9) | 398 (75.4) | 28 (93.3) | 39 (100) | 217 (60.8) | 715 (47.3) |
| Non-Healthcare Professional | 524 (94.1) | 127 (24.1) | 2 (6.7) | 140 (39.2) | 793 (52.5) | |
| Not Specified | 3 (0.6) | 3 (0.2) | ||||
| Primary Source Country for Regulatory Purposes | ||||||
| European Economic Area | 5 (0.9) | 159 (30.1) | 14 (46.7) | 40 (11.2) | 218 (14.4) | |
| Non-European Economic Area | 552 (99.1) | 369 (69.9) | 16 (53.3) | 39 (100) | 317 (88.8) | 1293 (85.6) |
| Serious | 191 (34.3) | 489 (92.6) | 29 (96.7) | 39 (100) | 349 (97.8) | 1097 (72.6) |
| Type of seriousness | ||||||
| Caused/Prolonged Hospitalization | 68 (35.6) | 146 (29.9) | 7 (24.1) | 20 (51.3) | 140 (40.1) | 381 (34.7) |
| Disabling | 2 (1.0) | 25 (5.1) | 1 (3.4) | 3 (0.9) | 31 (2.8) | |
| Life Threatening | 1 (0.5) | 16 (3.3) | 2 (6.9) | 8 (2.3) | 27 (2.5) | |
| Other Medically Important Condition | 117 (61.3) | 238 (48.7) | 13 (44.8) | 17 (43.6) | 135 (38.7) | 520 (47.4) |
| Results in Death | 3 (1.6) | 64 (13.1) | 6 (20.7) | 2 (5.1) | 63 (18.1) | 138 (12.6) |
| Outcome | ||||||
| Recovered/resolved | 8 (1.5) | 1 (3.3) | 4 (1.1) | 13 (0.9) | ||
| Recovering/resolving | 14 (2.5) | 80 (15.2) | 1 (3.3) | 6 (15.4) | 45 (12.6) | 146 (9.7) |
| Recovered/resolved with sequelae | 24 (4.3) | 69 (13.1) | 10 (33.3) | 3 (7.7) | 38 (10.6) | 144 (9.5) |
| Not recovered/not resolved | 428 (76.8) | 168 (31.8) | 4 (13.3) | 7 (17.9) | 137 (38.4) | 744 (49.2) |
| Fatal | 3 (0.5) | 64 (12.1) | 6 (20.0) | 2 (5.1) | 63 (17.6) | 138 (9.1) |
| Unknown | 88 (15.8) | 138 (26.1) | 8 (26.7) | 21 (53.8) | 69 (19.3) | 324 (21.4) |
| AVA (n = 1112) | IM (n = 4931) | REG (n = 231) | RIP (n = 176) | SU (n = 2062) | Total (n = 1511) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | ROR (95% CI) | SmPC | n | ROR (95% CI) | n | ROR (95% CI) | SmPC | n | ROR (95% CI) | SmPC | n | ROR (95% CI) | SmPC | ||
| Nervous system disorders | |||||||||||||||
| Central nervous system hemorrhages and cerebrovascular accidents | 2 | NC | 51 | 0.59 (0.41–0.86) | 9 | 3.19 (1.59–6.38) | NR | 4 | NC | 47 | 2.26 (1.55–3.29) | UN | 113 | ||
| Coordination and balance disturbances | 33 | 12.54 (7.04–22.35) | C | 6 | 0.10 (0.04–0.22) | 0 | - | 0 | - | 12 | 0.96 (0.50–1.84) | 51 | |||
| Cortical dysfuntion NEC | 33 | 16.14 (8.61–30.25) | C | 6 | 0.11 (0.04–0.25) | 0 | - | 0 | - | 8 | 0.64 (0.30–1.37) | 47 | |||
| Dementia (excl Alzheimer’s type) | 13 | 10.93 (4.52–26.43) | C | 6 | 0.29 (0.11–0.75) | 0 | - | 0 | - | 2 | NC | 21 | |||
| Disturbances in consciousness NEC | 31 | 2.07 (1.38–3.11) | C | 65 | 0.70 (0.50–0.99) | 2 | NC | 8 | 3.15 (1.52–6.55) | NR | 26 | 0.76 (0.50–1.18) | 132 | ||
| Encephalopathies NEC | 2 | NC | 2 | NC | 4 | NC | 0 | - | 10 | 3.92 (1.55–9.96) | R | 18 | |||
| Headaches NEC | 51 | 3.58 (2.54–5.05) | C | 46 | 0.32 (0.22–0.45) | 1 | NC | 6 | 2.02 (0.88–4.64) | 45 | 1.36 (0.96–1.94) | 149 | |||
| Lumbar spinal cord and nerve root disorders | 6 | 4.46 (1.58–12.54) | NR | 7 | 0.63 (0.23–1.75 | 0 | - | 0 | - | 2 | NC | 15 | |||
| Memory loss (excl dementia) | 126 | 40.99 (26.15–64.24) | C | 13 | 0.07 (0.04–0.12) | 0 | - | 0 | - | 10 | 0.22 (0.12–0.42) | 149 | |||
| Mental impairment (excl dementia and memory loss) | 72 | 26.89 (16.16–44.77) | C | 12 | 0.11 (0.06–0.20) | 2 | NC | 0 | - | 5 | 0.18 (0.07–0.44) | 91 | |||
| Narcolepsy and hypersomnia | 6 | 20.07 (4.05–99.55) | C | 0 | - | 0 | - | 0 | - | 2 | NC | 8 | |||
| Nervous system disorders NEC | 7 | 11.71 (3.42–40.08) | C | 2 | NC | 1 | NC | 0 | - | 1 | NC | 11 | |||
| Neurological signs and symptoms NEC | 75 | 4.24 (3.16–5.69) | C | 72 | 0.40 (0.30–0.54) | 0 | - | 8 | 2.03 (0.98–4.19) | 44 | 0.89 (0.63–1.24) | 199 | |||
| Olfactory nerve disorders | 6 | 8.02 (2.44–26.33) | NR | 5 | 0.60 (0.18–1.98) | 0 | - | 0 | - | 0 | 0.00 | 11 | |||
| Paraesthesias and dysaesthesias | 24 | 1.92 (1.22–3.04) | C | 43 | 0.48 (0.32–0.70) | 2 | NC | 3 | NC | 36 | 1.57 (1.05–2.36) | C | 108 | ||
| Sensory abnormalities NEC | 55 | 2.53 (1.85–3.47) | C | 46 | 0.20 (0.15–0.28) | 1 | NC | 0 | - | 102 | 3.24 (2.45–4.28) | VC | 204 | ||
| Speech and language abnormalities | 26 | 10.40 (5.62–19.22) | C | 9 | 0.19 (0.09–0.40) | 3 | NC | 1 | NC | 4 | NC | 43 | |||
| Transient cerebrovascular events | 1 | NC | 5 | 0.28 (0.10–0.78) | 2 | NC | 0 | - | 10 | 3.92 (1.55–9.96) | UN | 18 | |||
| Tremor (excl congenital) | 7 | 4.68 (1.78–12.32) | C | 7 | 0.51 (0.19–1.33) | 0 | NC | 1 | NC | 2 | NC | 17 | |||
| Psychiatric disorders | |||||||||||||||
| Anxiety symptoms | 40 | 10.19 (6.23–16.67) | C | 16 | 0.23 (0.13–0.40) | 2 | NC | 1 | NC | 8 | 0.42 (0.20–0.88) | 67 | |||
| Behavior and socialization disturbances | 3 | NC | 3 | NC | 1 | NC | 0 | - | 0 | - | 7 | ||||
| Confusion and disorientation | 39 | 8.37 (5.22–13.42) | C | 17 | 0.23 (0.13–0.39) | 1 | NC | 1 | NC | 13 | 0.70 (0.38–1.28) | 71 | |||
| Disturbances in initiating and maintaining sleep | 65 | 12.70 (8.41–19.18) | C | 23 | 0.21 (0.13–0.34) | 1 | NC | 3 | NC | 9 | 0.30 (0.15–0.60) | 101 | |||
| Eating disorders NEC | 3 | NC | 9 | 0.33 (0.15–0.72) | 0 | - | 1 | NC | 16 | 3.87 (1.86–8.06) | VC | 29 | |||
| Emotional and mood disturbances NEC | 7 | 3.90 (1.53–9.93) | C | 9 | 0.65 (0.27–1.61) | 0 | - | 0 | - | 3 | NC | 19 | |||
| Hallucinations (excl sleep-related) | 17 | 22.96 (8.45–62.36) | NR | 4 | NC | 0 | - | 0 | - | 1 | NC | 22 | |||
| Mood alterations with depressive symptoms | 12 | 6.72 (3.01–14.99) | C | 6 | 0.24 (0.10–0.61) | 0 | - | 1 | NC | 5 | 0.82 (0.31–2.21) | 24 | |||
| Thinking disturbances | 5 | NC | 0 | - | 0 | . | 0 | - | 2 | NC | 7 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, M.A.; Sorbara, E.E.; Russo, G.; Cicala, G.; Franchina, T.; Santarpia, M.; Silvestris, N.; Spina, E. Neuropsychiatric Adverse Drug Reactions with Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors: An Analysis from the European Spontaneous Adverse Event Reporting System. Cancers 2023, 15, 1851. https://doi.org/10.3390/cancers15061851
Barbieri MA, Sorbara EE, Russo G, Cicala G, Franchina T, Santarpia M, Silvestris N, Spina E. Neuropsychiatric Adverse Drug Reactions with Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors: An Analysis from the European Spontaneous Adverse Event Reporting System. Cancers. 2023; 15(6):1851. https://doi.org/10.3390/cancers15061851
Chicago/Turabian StyleBarbieri, Maria Antonietta, Emanuela Elisa Sorbara, Giulia Russo, Giuseppe Cicala, Tindara Franchina, Mariacarmela Santarpia, Nicola Silvestris, and Edoardo Spina. 2023. "Neuropsychiatric Adverse Drug Reactions with Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors: An Analysis from the European Spontaneous Adverse Event Reporting System" Cancers 15, no. 6: 1851. https://doi.org/10.3390/cancers15061851
APA StyleBarbieri, M. A., Sorbara, E. E., Russo, G., Cicala, G., Franchina, T., Santarpia, M., Silvestris, N., & Spina, E. (2023). Neuropsychiatric Adverse Drug Reactions with Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors: An Analysis from the European Spontaneous Adverse Event Reporting System. Cancers, 15(6), 1851. https://doi.org/10.3390/cancers15061851








