Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Data Collection and Outcome Parameters
2.3. Immunohistochemical Staining
2.4. Immune Cell Quantification
2.5. Laser Capture Microdissection
2.6. Protein Quantification in Stromal and Tumor Epithelial Compartment
2.7. Measurement of White Blood Cells (WBC) and c-Reactive Protein (CRP)
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Patients by Sex
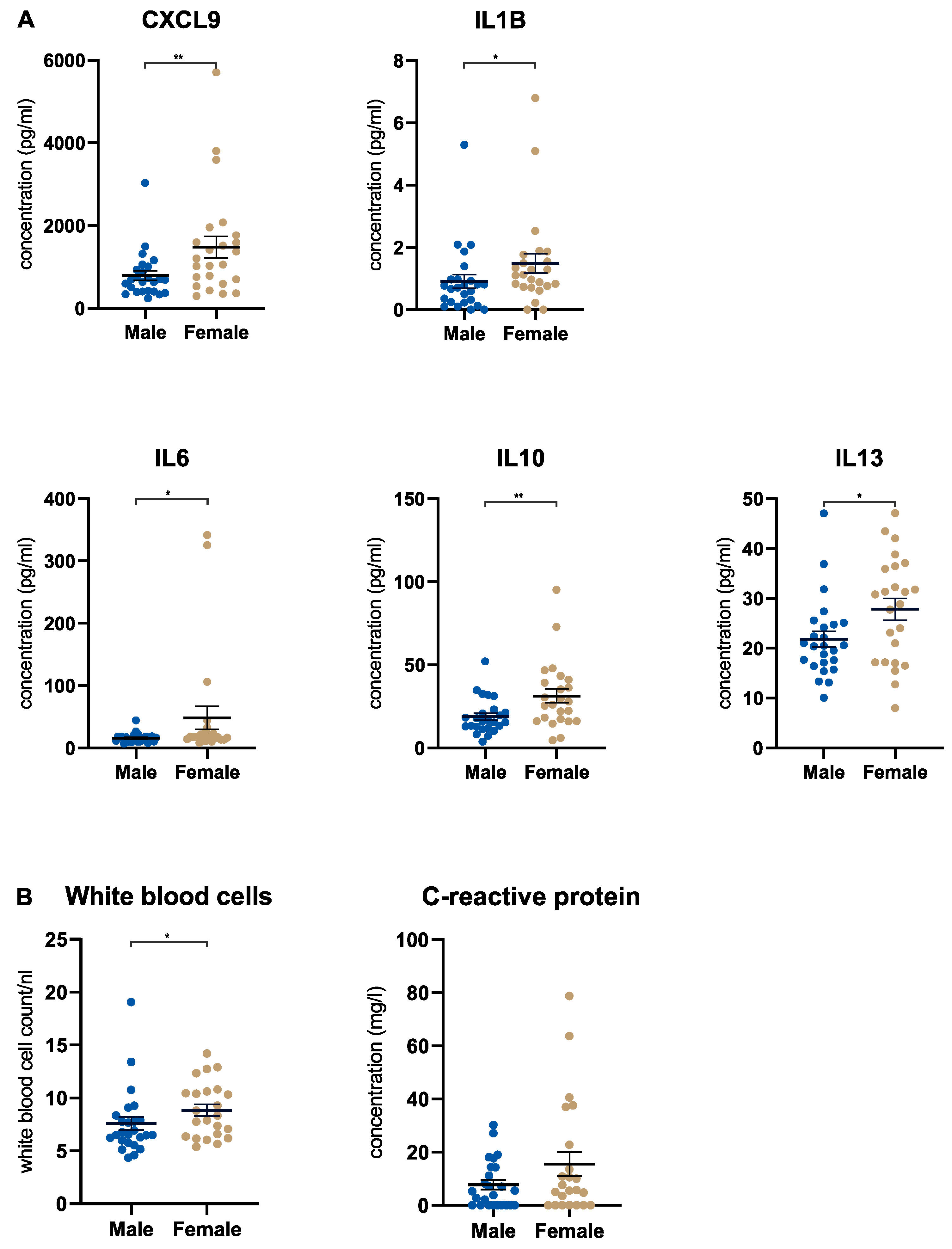
3.2. Female Pancreatic Cancer Patients Show a Stronger Systemic Immune Response
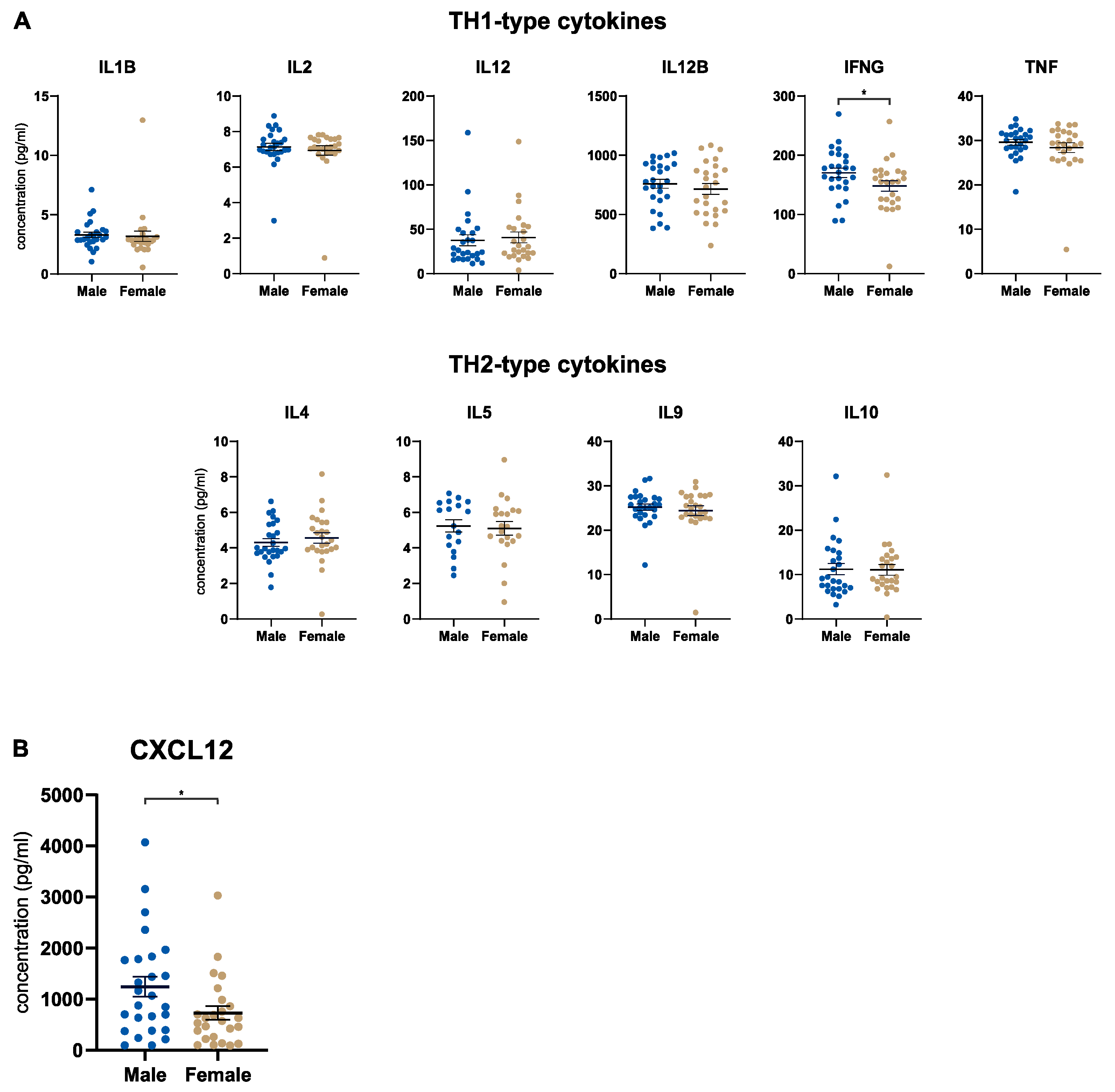
3.3. Male Patients Express Higher Tumoral Levels of CXCL12
3.4. Local Cytokine Changes upon Blockade of CXCL12 (Anti-CXCL12, NOX-A12) in Patients with PDA of the OPERA Trial
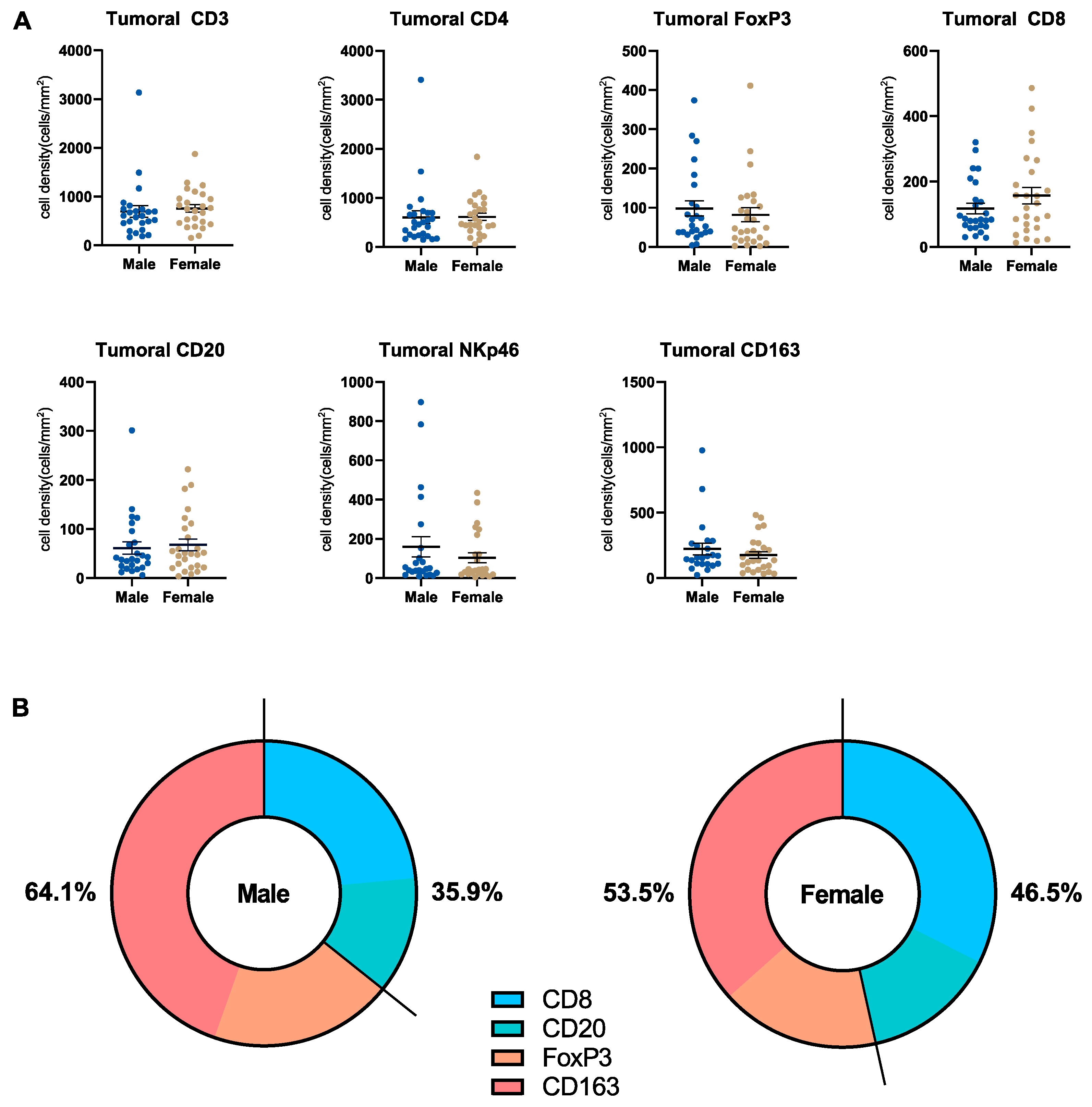
3.5. Microenvironmental Immune Cell Infiltrate in Pancreatic Cancer Patients Stratified by Sex
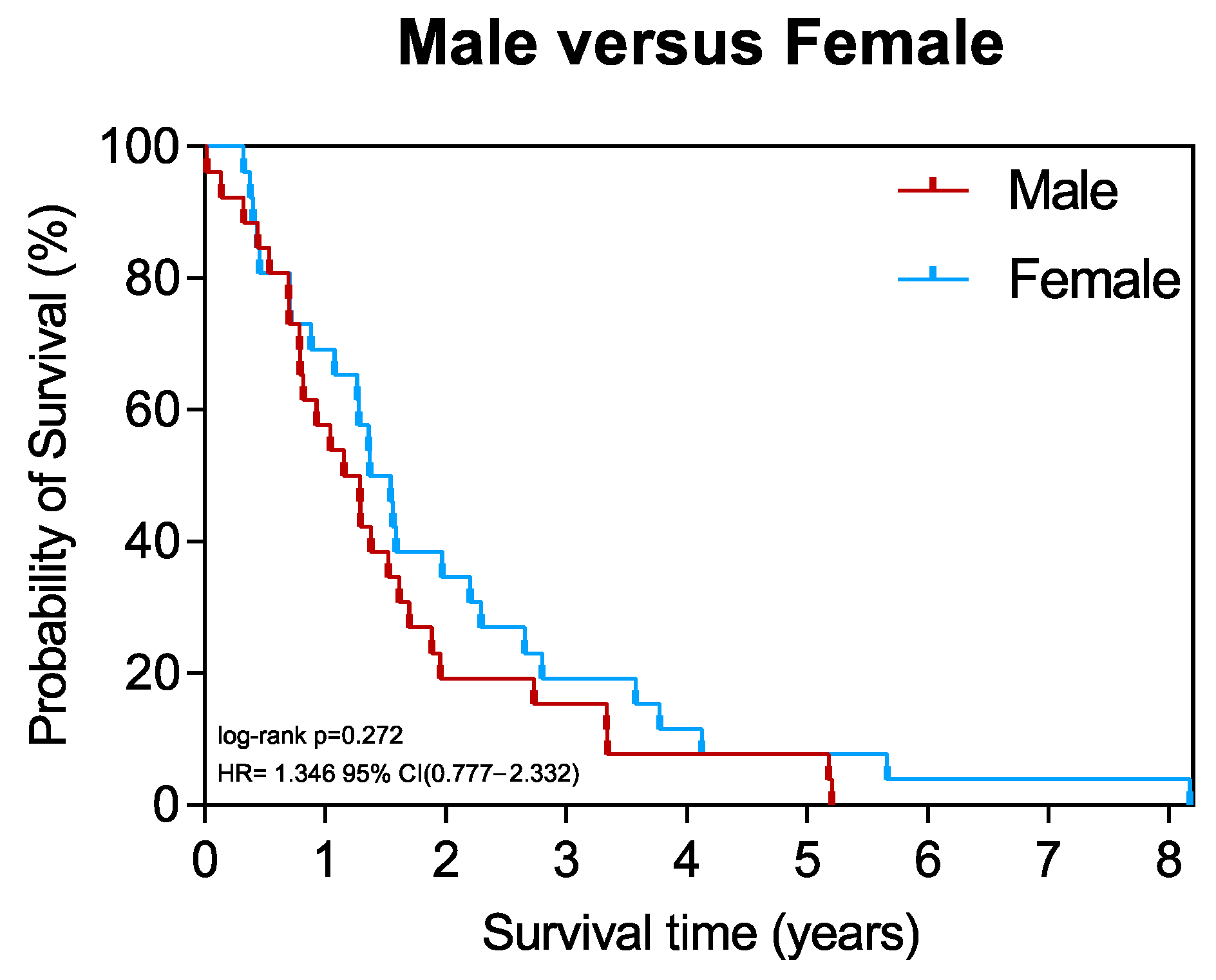
3.6. Survival of Patients Stratified by Sex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer AnalysisSex Differences in Cancer Incidence and Survival. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Ramos, C.M.; Quackenbush, J.; DeMeo, D.L. Genome-wide sex and gender differences in cancer. Front. Oncol. 2020, 10, 597788. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.B. The spectrum of sex differences in cancer. Trends Cancer 2022, 8, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.B.; Lagas, J.S.; Broestl, L.; Sponagel, J.; Rockwell, N.; Rhee, G.; Rosen, S.F.; Chen, S.; Klein, R.S.; Imoukhuede, P. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020, 11, 17. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cowley, L.A.; Liu, X.-S. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Pierdominici, M.; Rider, V. Sex hormones and gender differences in immune responses. Front. Immunol. 2019, 10, 1076. [Google Scholar]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Karp, N.A.; Reavey, N. Sex bias in preclinical research and an exploration of how to change the status quo. Br. J. Pharmacol. 2019, 176, 4107–4118. [Google Scholar] [CrossRef]
- Fields, R.D. NIH policy: Mandate goes too far. Nature 2014, 510, 340. [Google Scholar] [CrossRef] [PubMed]
- Pijnappel, E.N.; Schuurman, M.; Wagner, A.D.; de Vos-Geelen, J.; van der Geest, L.G.; de Groot, J.-W.B.; Koerkamp, B.G.; de Hingh, I.H.; Homs, M.Y.; Creemers, G.-J. Sex, Gender and Age Differences in Treatment Allocation and Survival of Patients With Metastatic Pancreatic Cancer: A Nationwide Study. Front. Oncol. 2022, 12, 839779. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, M.M.; Asch, D.; Yu, S.; O’Dwyer, P.J.; Domchek, S.M.; Nathanson, K.L.; Rosen, M.A.; Beatty, G.L.; Siegelman, E.S.; Reiss, K.A. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br. J. Cancer 2020, 122, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Heise, L.; Greene, M.E.; Opper, N.; Stavropoulou, M.; Harper, C.; Nascimento, M.; Zewdie, D.; Darmstadt, G.L.; Greene, M.E.; Hawkes, S. Gender inequality and restrictive gender norms: Framing the challenges to health. Lancet 2019, 393, 2440–2454. [Google Scholar] [CrossRef]
- Sathyamurthy, A.; Chela, H.K.; Romana, B.; Yousef, M.H.; Winn, J.; Madsen, R.; Bechtold, M.; Asombang, A.W. Pancreatic Cancer Survival Outcomes Based on Gender and Geographical Location: Retrospective Study at a Tertiary Medical Center: 2258. Off. J. Am. Coll. Gastroenterol. ACG 2015, 110, S937. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R.; Potluri, T.; Fink, A.L.; Klein, S.L. The confluence of sex hormones and aging on immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Suarez-Carmona, M.; Williams, A.; Schreiber, J.; Hohmann, N.; Pruefer, U.; Krauss, J.; Jäger, D.; Frömming, A.; Beyer, D.; Eulberg, D. Combined inhibition of CXCL12 and PD-1 in MSS colorectal and pancreatic cancer: Modulation of the microenvironment and clinical effects. J. Immunother. Cancer 2021, 9, e002505. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Halama, N.; Zoernig, I.; Berthel, A.; Kahlert, C.; Klupp, F.; Suarez-Carmona, M.; Suetterlin, T.; Brand, K.; Krauss, J.; Lasitschka, F. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 2016, 29, 587–601. [Google Scholar] [CrossRef]
- Halsey, L.G. The reign of the p-value is over: What alternative analyses could we employ to fill the power vacuum? Biol. Lett. 2019, 15, 20190174. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA statement on p-values: Context, process, and purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Chai, P.-S.; Chong, M.-Y.; Tohit, E.R.M.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef]
- D’agostino, P.; Milano, S.; Barbera, C.; Di Bella, G.; La Rosa, M.; Ferlazzo, V.; Farruggio, R.; Miceli, D.; Miele, M.; Castagnetta, L. Sex hormones modulate inflammatory mediators produced by macrophages a. Ann. N. Y. Acad. Sci. 1999, 876, 426–429. [Google Scholar] [CrossRef]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet-Hudson, Y.M.; Marriott, I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol. Reprod. 2008, 78, 432–437. [Google Scholar] [CrossRef]
- Hou, J.; Wu, F.Z. Effect of sex hormones on NK and ADDC activity of mice. Int. J. Immunopharmacol. 1988, 10, 15–22. [Google Scholar] [CrossRef]
- Paharkova-Vatchkova, V.; Maldonado, R.; Kovats, S. Estrogen preferentially promotes the differentiation of CD11c+ CD11bintermediate dendritic cells from bone marrow precursors. J. Immunol. 2004, 172, 1426–1436. [Google Scholar] [CrossRef]
- Escribese, M.M.; Kraus, T.; Rhee, E.; Fernandez-Sesma, A.; López, C.B.; Moran, T.M. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood J. Am. Soc. Hematol. 2008, 112, 4574–4584. [Google Scholar] [CrossRef]
- He, F.; Furones, A.R.; Landegren, N.; Fuxe, J.; Sarhan, D. Sex dimorphism in the tumor microenvironment–from bench to bedside and back. In Proceedings of the Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Reschke, R.; Gajewski, T.F. CXCL9 and CXCL10 bring the heat to tumors. Sci. Immunol. 2022, 7, eabq6509. [Google Scholar] [CrossRef]
- Liang, Y.-K.; Deng, Z.-K.; Chen, M.-T.; Qiu, S.-Q.; Xiao, Y.-S.; Qi, Y.-Z.; Xie, Q.; Wang, Z.-H.; Jia, S.-C.; Zeng, D. CXCL9 is a potential biomarker of immune infiltration associated with favorable prognosis in ER-negative breast cancer. Front. Oncol. 2021, 11, 710286. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 2019, 50, 1498–1512.e5. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wang, F.; Zhang, K.; Chen, Z. Comprehensive analysis of prognostic value and immune infiltration of CXC chemokines in pancreatic cancer. BMC Med. Genom. 2022, 15, 96. [Google Scholar] [CrossRef] [PubMed]
- Herremans, K.M.; Szymkiewicz, D.D.; Riner, A.N.; Bohan, R.P.; Tushoski, G.W.; Davidson, A.M.; Lou, X.; Leong, M.C.; Dean, B.D.; Gerber, M. The interleukin-1 axis and the tumor immune microenvironment in pancreatic ductal adenocarcinoma. Neoplasia 2022, 28, 100789. [Google Scholar] [CrossRef]
- Zhang, D.; Li, L.; Jiang, H.; Li, Q.; Wang-Gillam, A.; Yu, J.; Head, R.; Liu, J.; Ruzinova, M.B.; Lim, K.-H. Tumor–Stroma IL1β-IRAK4 Feedforward Circuitry Drives Tumor Fibrosis, Chemoresistance, and Poor Prognosis in Pancreatic CancerTumor-Stromal IRAK4 Drives Pancreatic Cancer. Cancer Res. 2018, 78, 1700–1712. [Google Scholar] [CrossRef]
- Shi, J.; Song, X.; Traub, B.; Luxenhofer, M.; Kornmann, M. Involvement of IL-4, IL-13 and their receptors in pancreatic cancer. Int. J. Mol. Sci. 2021, 22, 2998. [Google Scholar] [CrossRef]
- Mace, T.A.; Shakya, R.; Pitarresi, J.R.; Swanson, B.; McQuinn, C.W.; Loftus, S.; Nordquist, E.; Cruz-Monserrate, Z.; Yu, L.; Young, G. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018, 67, 320–332. [Google Scholar] [CrossRef]
- Feng, L.; Qi, Q.; Wang, P.; Chen, H.; Chen, Z.; Meng, Z.; Liu, L. Serum levels of IL-6, IL-8, and IL-10 are indicators of prognosis in pancreatic cancer. J. Int. Med. Res. 2018, 46, 5228–5236. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Jiang, X.; Guha, P.; Lausted, C.; Carter, J.A.; Hsu, C.; Labadie, K.P.; Kohli, K.; Kenerson, H.L.; Daniel, S.K. Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases. Gut 2022, 72, 325–337. [Google Scholar] [CrossRef]
- Ahmed, A.; Köhler, S.; Klotz, R.; Giese, N.; Lasitschka, F.; Hackert, T.; Springfeld, C.; Zörnig, I.; Jäger, D.; Halama, N. Peripheral blood and tissue assessment highlights differential tumor-circulatory gradients of IL2 and MIF with prognostic significance in resectable pancreatic ductal adenocarcinoma. OncoImmunology 2021, 10, 1962135. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Köhler, S.; Klotz, R.; Giese, N.; Hackert, T.; Springfeld, C.; Zörnig, I.; Jäger, D.; Halama, N. Tertiary lymphoid structures and their association to immune phenotypes and circulatory IL2 levels in pancreatic ductal adenocarcinoma. OncoImmunology 2022, 11, 2027148. [Google Scholar] [CrossRef] [PubMed]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and gender equity in research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef]

| Male | Female | Total | p-Value | ||
|---|---|---|---|---|---|
| Characteristic | (n = 26) | (n = 26) | (n = 52) | ||
| Age (years) | 64.5 ± 1.8 | 66.5 ± 2.2 | 65.5 ± 1.4 | - | |
| >/≤Average age (n/n) | 13/13 | 16/10 | - | 0.412 | |
| Body weight (kg) | 75.4 ± 2.4 | 66.6 ± 2.1 | 71.5 ± 1.8 | - | |
| >/≤Body weight (n/n) | 13/13 | 5/21 | - | 0.019 | |
| BMI | 23.9 ± 2.3 | 25.2 ± 3.0 | 24.5 ± 1.8 | - | |
| >/≤BMI (n/n) | 7/19 | 9/17 | - | 0.557 | |
| Diabetes mellitus (n) | 7 | 8 | 15 | 0.765 | |
| Glucocorticoid or immunosuppressive drug intake (n) | 1 | 0 | 1 | 0.322 | |
| Cardiovascular comorbidities (n) | 10 | 17 | 27 | 0.053 | |
| Pulmonary comorbidities (n) | 2 | 3 | 5 | 0.646 | |
| Renal comorbidities (n) | 1 | 2 | 3 | 0.561 | |
| Hepatic comorbidities (n) | 0 | 0 | 0 | n.a. | |
| Autoimmune comorbidities (n) | 1 | 1 | 2 | >0.999 | |
| T | T3 (n) | 25 | 26 | 51 | 0.322 |
| T4 (n) | 1 | 0 | 1 | ||
| N | N0 (n) | 4 | 5 | 9 | 0.491 |
| N1 (n) | 22 | 21 | 43 | ||
| M | M0 (n) | 23 | 25 | 48 | 0.307 |
| M1 (n) | 3 | 1 | 4 | ||
| Grade | G2 (n) | 15 | 18 | 33 | 0.397 |
| G3 (n) | 11 | 8 | 19 | ||
| R a | R0 (n) | 4 | 2 | 6 | 0.367 |
| R1 (n) | 21 | 24 | 45 | ||
| Survival days | 573 ± 98 | 738 ± 131 | 655 ± 82 | 0.619 | |
| >/≤ Survival days (n/n) | 7/19 | 9/17 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.; Köhler, S.; Klotz, R.; Giese, N.; Hackert, T.; Springfeld, C.; Jäger, D.; Halama, N. Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients. Cancers 2023, 15, 1815. https://doi.org/10.3390/cancers15061815
Ahmed A, Köhler S, Klotz R, Giese N, Hackert T, Springfeld C, Jäger D, Halama N. Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients. Cancers. 2023; 15(6):1815. https://doi.org/10.3390/cancers15061815
Chicago/Turabian StyleAhmed, Azaz, Sophia Köhler, Rosa Klotz, Nathalia Giese, Thilo Hackert, Christoph Springfeld, Dirk Jäger, and Niels Halama. 2023. "Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients" Cancers 15, no. 6: 1815. https://doi.org/10.3390/cancers15061815
APA StyleAhmed, A., Köhler, S., Klotz, R., Giese, N., Hackert, T., Springfeld, C., Jäger, D., & Halama, N. (2023). Sex Differences in the Systemic and Local Immune Response of Pancreatic Cancer Patients. Cancers, 15(6), 1815. https://doi.org/10.3390/cancers15061815






