The Influence of Obesity on Melanoma and Sentinel Lymph Node Diagnosis: A Retrospective Monocentric Study in 1001 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analysis
3. Results
3.1. Demographic Characteristics
3.2. Analysis of BMI Groups
3.3. Metastases in the Sentinel Lymph Node Biopsy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robert Koch Institute. 3 Results 3.0 Overview of Incident Cancer Cases and Cancer Deaths Non-Hodgkin Lymphoma 3.6% Ovaries 3.1% Stomach 2.4% Kidney 2.4% Leukaemia 2.3% Bladder 2.0 % Oral Cavity and Pharynx 1.9% Cervix 1.9% Thyroid Gland 1.8% Vulva 1.4% Central Nervous System 1.3% Liver 1.2% Multiple Myeloma 1.2% Soft Tissue without Mesothelioma 0. 2017. Available online: www.krebsdaten.de/all_cancers (accessed on 4 October 2022).
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar]
- Oh, S.W.; Yoon, Y.S.; Shin, S.-A. Effects of Excess Weight on Cancer Incidences Depending on Cancer Sites and Histologic Findings Among Men: Korea National Health Insurance Corporation Study. J. Clin. Oncol. 2005, 23, 4742–4754. [Google Scholar] [CrossRef]
- Samanic, C.; Gridley, G.; Chow, W.-H.; Lubin, J.; Hoover, R.N.; Fraumeni, J.F., Jr. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control 2004, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Samanic, C.; Chow, W.-H.; Gridley, G.; Jarvholm, B.; Fraumeni, J.F., Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006, 17, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Dennis, L.K.; Lowe, J.B.; Lynch, C.F.; Alavanja, M.C. Cutaneous Melanoma and Obesity in the Agricultural Health Study. Ann. Epidemiol. 2008, 18, 214–221. Available online: http://www.aghealth.org/questionnaires.html (accessed on 6 October 2022). [CrossRef]
- Gallus, S.; Naldi, L.; Martin, L.; Martinelli, M. Anthropometric measures and risk of cutaneous malignant melanoma: A case–control study from Italy. Melanoma Res. 2006, 16, 83–87. [Google Scholar] [CrossRef]
- Kirkpatrick, C.S.; White, E.; Lee, J.A. Case-Control Study of Malignant Melanoma in Washington State. II. Diet, alcohol, and obesity. Am. J. Epidemiol. 1994, 139, 869–880. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Cassano, N.; Caccavale, S.; Vena, G.A.; Argenziano, G. Body Mass Index and Melanoma Prognosis. Dermatol. Pr. Concept. 2021, e2021106. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.-J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final Version of 2009 AJCC Melanoma Staging and Classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Skowron, F.; Berard, F.; Balme, B.; Maucort-Boulch, D. Role of obesity on the thickness of primary cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Montella, M.; Ayala, F.; Benedetto, L.; Rossi, C.R.; Vecchiato, A.; Corradin, M.T.; De Giorgi, V.; Queirolo, P.; Zannetti, G.; et al. Sun exposure and melanoma prognostic factors. Oncol. Lett. 2016, 11, 2706–2714. [Google Scholar] [CrossRef] [PubMed]
- de Giorgi, V.; Gori, A.; Papi, F.; Grazzini, M.; Rossari, S.; Verdelli, A.; Crocetti, E.; Savarese, I.; Giorgi, L.; Massi, D. Excess body weight and increased Breslow thickness in melanoma patients. Eur. J. Cancer Prev. 2013, 22, 480–485. [Google Scholar] [CrossRef]
- Stenehjem, J.; Veierød, M.; Nilsen, L.; Ghiasvand, R.; Johnsen, B.; Grimsrud, T.; Babigumira, R.; Støer, N.; Rees, J.; Robsahm, T. Anthropometric factors and Breslow thickness: Prospective data on 2570 cases of cutaneous melanoma in the population-based Janus Cohort. Br. J. Dermatol. 2018, 179, 632–641. [Google Scholar] [CrossRef]
- von Schuckmann, L.A.; Smith, D.; Hughes, M.C.B.; Malt, M.; van der Pols, J.C.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Associations of Statins and Diabetes with Diagnosis of Ulcerated Cutaneous Melanoma. J. Investig. Dermatol. 2017, 137, 2599–2605. [Google Scholar] [CrossRef]
- Newton-Bishop, J.A.; Davies, J.R.; Latheef, F.; Randerson-Moor, J.; Chan, M.; Gascoyne, J.; Waseem, S.; Haynes, S.; O’Donovan, C.; Bishop, D.T. 25-Hydroxyvitamin D2/D3 levels and factors associated with systemic inflammation and melanoma survival in the Leeds Melanoma Cohort. Int. J. Cancer 2015, 136, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Shreckengost, C.S.H.; Tariq, M.; Farley, C.R.; Zhang, C.; Delman, K.A.; Kudchadkar, R.R.; Lowe, M.C. The Impact of Obesity on Surgically Treated Locoregional Melanoma. Ann. Surg. Oncol. 2021, 28, 6140–6151. [Google Scholar] [CrossRef]
- Robert Koch Institute. Übergewicht und Adipositas bei Erwachsenen. J. Health Monit. 2017, 2, 21–28. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and Cancer Mechanisms: Cancer Metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Brandon, E.L.; Gu, J.-W.; Cantwell, L.; He, Z.; Wallace, G.; Hall, J.E. Obesity promotes melanoma tumor growth: Role of leptin. Cancer Biol. Ther. 2009, 8, 1871–1879. [Google Scholar] [CrossRef]
- Karimi, K.; Lindgren, T.H.; Koch, C.A.; Brodell, R.T. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev. Endocr. Metab. Disord. 2016, 17, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Vijayakumar, M.V.; Ajay, A.K.; Malvi, P.; Bhat, M.K. Diet-induced obesity increases melanoma progression: Involvement of Cav-1 and FASN. Int. J. Cancer 2011, 130, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Veierød, M.B.; Thelle, D.S.; Laake, P. Diet and risk of cutaneous malignant melanoma: A prospective study of 50,757 Norwegian men and women. Int. J. Cancer 1997, 71, 600–604. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Muller, C.; Nieto, L. Obesity and melanoma: Could fat be fueling malignancy? Pigment Cell Melanoma Res. 2017, 30, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Maione, V.; Buligan, C.; Linussio, M.; Serraino, D.; Stinco, G. BsmI (rs1544410) and FokI (rs2228570) vitamin D receptor polymorphisms, smoking, and body mass index as risk factors of cutaneous malignant melanoma in northeast Italy. Cancer Biol. Med. 2017, 14, 302–318. [Google Scholar] [CrossRef]
- Li, X.; Liang, L.; Zhang, M.; Song, F.; Nan, H.; Wang, L.-E.; Wei, Q.; Lee, J.E.; Amos, C.I.; Qureshi, A.A.; et al. Obesity-related genetic variants, human pigmentation, and risk of melanoma. Hum. Genet. 2013, 132, 793–801. [Google Scholar] [CrossRef]
- Risica, P.M.; Weinstock, M.A.; Rakowski, W.; Kirtania, U.; Martin, R.A.; Smith, K.J. Body Satisfaction: Effect on thorough Skin Self-Examination. Am. J. Prev. Med. 2008, 35, 68–72. [Google Scholar] [CrossRef]
- Derossis, A.M.; Fey, J.V.; Cody, H.S.; Borgen, P.I. Obesity influences outcome of sentinel lymph node biopsy in early-stage breast cancer. J. Am. Coll. Surg. 2003, 197, 896–901. [Google Scholar] [CrossRef]
- Chagpar, A.B.; Martin, R.C.; Scoggins, C.R.; Carlson, D.J.; Laidley, A.L.; El-Eid, S.E.; McGlothin, T.Q.; Noyes, R.D.; Ley, P.B.; McMasters, K.M.; et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery 2005, 138, 56–63. [Google Scholar] [CrossRef]
- Bandera, E.V.; Fay, S.H.; Giovannucci, E.; World Cancer Research Fund International Continuous Update Project Panel. The use and interpretation of anthropometric measures in cancer epidemiology: A perspective from the World Cancer Research Fund international continuous update project. Int. J. Cancer 2016, 139, 2391–2397. [Google Scholar] [CrossRef]
- van der Kooy, K.; Leenen, R.; Seidell, J.C.; Deurenberg, P.; Droop, A.; Bakker, C.J. Waist-hip ratio is a poor predictor of changes in visceral fat. Am. J. Clin. Nutr. 1993, 57, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Mccrory, M.A.; Gomez, T.D.; Bernauer, E.M.; Mol, P.A. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995, 27, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-W.; Heymsfield, S.; Gallagher, D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int. J. Obes. 2002, 26, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef]
- Kavazović, I.; Krapić, M.; Beumer-Chuwonpad, A.; Polić, B.; Wensveen, T.T.; Lemmermann, N.A.; van Gisbergen, K.P.; Wensveen, F.M. Hyperglycemia and Not Hyperinsulinemia Mediates Diabetes-Induced Memory CD8 T-Cell Dysfunction. Diabetes 2022, 71, 706–721. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef]
- Rutkowski, P.; Indini, A.; De Luca, M.; Merelli, B.; Mariuk-Jarema, A.; Teterycz, P.; Rogala, P.; Lugowska, I.; Cybulska-Stopa, B.; Labianca, A.; et al. Body mass index (BMI) and outcome of metastatic melanoma patients receiving targeted therapy and immunotherapy: A multicenter international retrospective study. J. Immunother. Cancer 2020, 8, e001117. [Google Scholar] [CrossRef]
- Richtlinie des Gemeinsamen Bundesausschusses über die Früherkennung von Krebserkrankungen (Krebsfrüherkennungs-Richtlinie/KFE-RL). Available online: https://www.g-ba.de/richtlinien/17/ (accessed on 26 November 2022).
- Onkologie, L. S3-Leitlinie zur Diagnostik, Therapie und Nachsorge des Melanoms. Available online: https://register.awmf.org/de/leitlinien/detail/032-024OL (accessed on 25 April 2022).
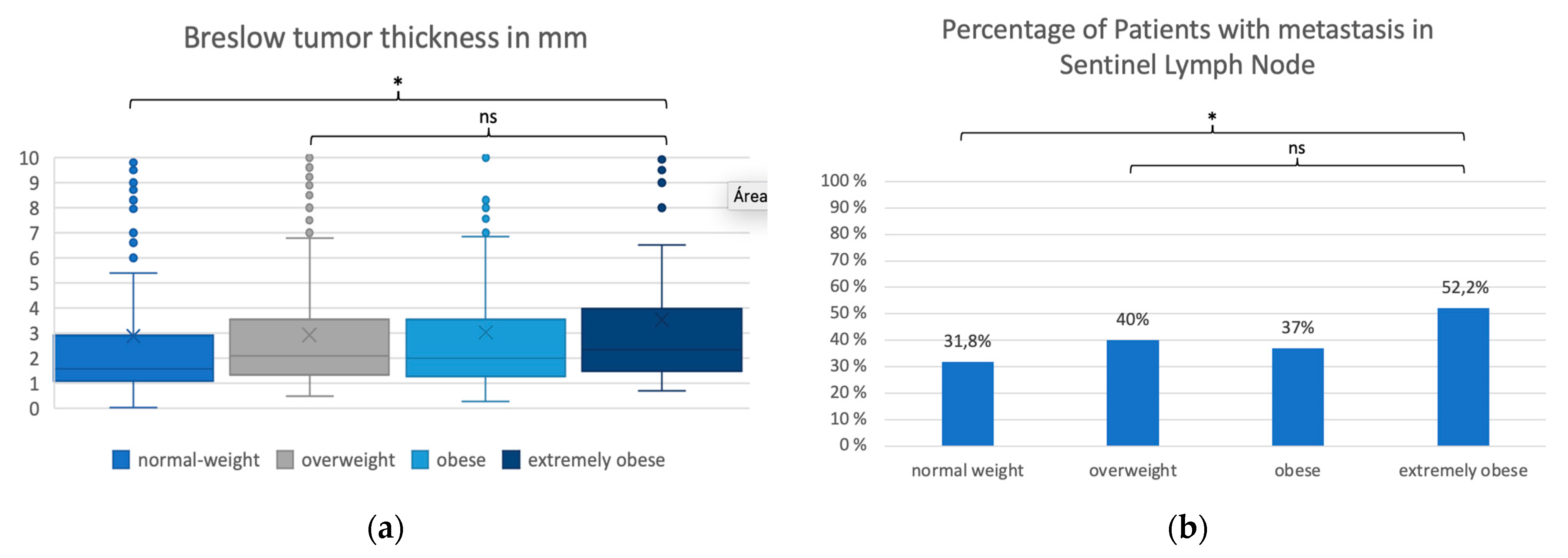
| BMI Group | n | Mw | SD | Median | Min-Max | p-Value * (Global Test) | |
|---|---|---|---|---|---|---|---|
| Age | Normal weight (>=18.5 and <25) | 336 | 53.8 | 16.9 | 54.0 | 22.0–92.0 | |
| Overweight (>=25 and <30) | 402 | 60.0 | 14.6 | 60.0 | 21.0–95.0 | ||
| Obese (>=30 and <35) | 173 | 60.0 | 13.2 | 60.0 | 24.0–89.0 | ||
| Extremely obese (>= 35) | 90 | 57.8 | 12.5 | 58.0 | 30.0–81.0 | ||
| Total | 1001 | 57.7 | 15.3 | 58.0 | 21.0–95.0 | <0.001 | |
| Breslow | Normal weight (>=18.5 and <25) | 336 | 2.9 | 5.9 | 1.6 | 0.04–75.0 | |
| tumor | Overweight (>=25 and <30) | 402 | 2.9 | 2.5 | 2.1 | 0.5–17.0 | |
| thickness | Obese (>=30 and <35) | 173 | 3.0 | 3.2 | 2.0 | 0.3–32.0 | |
| Extremely obese (>=35) | 90 | 3.5 | 3.2 | 2.3 | 0.7–14.2 | ||
| Total | 1001 | 3.0 | 4.1 | 1.9 | 0.04–75.0 | <0.001 | |
| S100 value | Normal weight (>=18.5 and <25) | 306 | 0.1 | 0.1 | 0.1 | 0.0–1.5 | |
| Overweight (>=25 and <30) | 370 | 0.1 | 0.1 | 0.1 | 0.0–0.8 | ||
| Obese (>=30 and <35) | 159 | 0.1 | 0.1 | 0.1 | 0.0–0.8 | ||
| Extremely obese (>= 5) | 81 | 0.1 | 0.1 | 0.1 | 0.0–0.6 | ||
| Total | 916 | 0.1 | 0.1 | 0.1 | 0.0–1.5 | 0.139 |
| Patients (n = 1001) | Normal Weight (BMI >= 18.5 and <25) | Overweight (BMI >= 25 and <30) | Obese (BMI >= 30 and <35) | Extremely Obese (BMI >= 35) | p-Value * (Global Test) | ||
|---|---|---|---|---|---|---|---|
| Gender | Male | 483 (48.3%) | 114 (33.9%) | 241 (60.0%) | 94 (54.3%) | 34 (37.8%) | |
| Female | 518 (51.7%) | 222 (66.1%) | 161 (40.0%) | 79 (45.7%) | 56 (62.2%) | p < 0.001 | |
| Sentinel | Negative | 622 (62.1%) | 229 (68.2%) | 241 (60.0%) | 109 (63.0%) | 43 (47.8%) | |
| Lymph Node | Positive | 379 (37.9%) | 107 (31.8%) | 161 (40.0%) | 64 (37.0%) | 47 (52.2%) | p = 0.003 |
| Extracapsular | No | 901 (90.0%) | 306 (91.1%) | 367 (91.3%) | 149 (86.1%) | 79 (87.8%) | |
| spread | Yes | 100 (10.0%) | 30 (8.9%) | 35 (8.7%) | 24 (13.9%) | 11 (12.2%) | p = 0.204 |
| Tumor | Body trunk | 415 (41.5%) | 140 (41.7%) | 169 (42.0%) | 76 (43.9%) | 30 (33.3%) | |
| location | Upper extremity | 223 (22.3%) | 73 (21.7%) | 85 (21.1%) | 37 (21.4%) | 28 (31.1%) | |
| Lower extremity | 363 (36.3%) | 123 (36.6%) | 148 (36.8%) | 60 (34.7%) | 32 (35.6%) | p = 0.495 | |
| Ulceration | No | 780 (78.0%) | 263 (78.3%) | 312 (77.8%) | 134 (77.5%) | 71 (78.9%) | |
| Yes | 220 (22.0%) | 73 (21.7%) | 89 (22.2%) | 39 (22.5%) | 19 (21.1%) | p = 0.993 |
| Age | Breslow Tumor Thickness | S100 Value | |||
|---|---|---|---|---|---|
| Normal weight | vs. | overweight | <0.001 # | <0.001 # | 0.127 |
| Normal weight | vs. | obese | <0.001 # | 0.002 # | 0.098 |
| Normal weight | vs. | extremely obese | 0.039 | <0.001 # | 0.057 |
| Overweight | vs. | obese | 0.800 | 0.823 | 0.563 |
| Overweight | vs. | extremely obese | 0.119 | 0.173 | 0.273 |
| Obese | vs. | extremely obese | 0.200 | 0.192 | 0.596 |
| Gender | Metastases in SLN | Extracapsular Spread | Location | Ulceration | |||
|---|---|---|---|---|---|---|---|
| Normal weight | vs. | overweight | <0.001 # | 0.021 | 1.000 | 0.986 | 0.929 |
| Normal weight | vs. | obese | <0.001 # | 0.276 | 0.095 | 0.878 | 0.822 |
| Normal weight | vs. | extremely obese | 0.534 | 0.001 # | 0.420 | 0.145 | 1.000 |
| Overweight | vs. | obese | 0.231 | 0.515 | 0.072 | 0.891 | 0.913 |
| Overweight | vs. | extremely obese | <0.001 # | 0.044 | 0.317 | 0.106 | 0.889 |
| Obese | vs. | extremely obese | 0.013 # | 0.025 | 0.849 | 0.146 | 0.876 |
| SLN | N | Mw | SD | Median | Min-Max | OR (95%-CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| BMI | Negative | 622 | 27.2 | 5.0 | 26.6 | 18.6–55.5 | ||
| Positive | 379 | 28.1 | 5.4 | 27.3 | 18.6–49.9 | 1.04 (1.01–1.06) | 0.005 | |
| Age | Negative | 622 | 57.0 | 15.2 | 58.0 | 21.0–89.0 | ||
| (Years) | Positive | 379 | 58.9 | 15.3 | 60.0 | 21.0–95.0 | 1.01 (1.00–1.02) | 0.057 |
| Breslow tumor | Negative | 622 | 2.3 | 3.5 | 1.6 | 0.04–75.0 | ||
| Thickness (mm) | Positive | 379 | 4.1 | 4.9 | 2.9 | 0.2–70.0 | 1.11 (1.07–1.14) | <0.001 |
| S100 value | Negative | 573 | 0.1 | 0.1 | 0.1 | 0.0–1.4 | ||
| Positive | 343 | 0.1 | 0.1 | 0.1 | 0.0–1.5 | 1.45 (0.39–5.45) | 0.105 |
| n | SLN Negative | SLN Positive | OR (95%-CI) | p-Value | ||
|---|---|---|---|---|---|---|
| Gender | Male | 483 | 283 (58.6%) | 200 (41.4%) | ||
| Female | 518 | 339 (65.4%) | 179 (34.6%) | 0.75 (0.58–0.97) | 0.027 | |
| BMI | Normal weight (>=18.5 and <25) | 336 | 229 (68.2%) | 107 (31.8%) | ||
| Overweight (>=25 and <30) | 402 | 241 (60.0%) | 161 (40.0%) | 1.43 (1.05–1.94) | 0.021 | |
| Obese (>=30 and <35) | 173 | 109 (63.0%) | 64 (37.0%) | 1.03 (0.73–1.45) | 0.861 | |
| Extremely obese (>=35) | 90 | 43 (47.8%) | 47 (52.2%) | 1.91 (1.23–2.95) | 0.004 | |
| Tumor | Upper Extrem. | 223 | 154 (69.1%) | 69 (30.9%) | ||
| location | Trunk | 415 | 251 (60.5%) | 164 (39.5%) | 1.46 (1.03–2.06) | 0.038 |
| Lower Extrem. | 363 | 217 (59.8%) | 146 (40.2%) | 1.50 (1.05–2.14) | 0.027 | |
| Ulceration | No | 780 | 523 (67.1%) | 257 (32.9%) | ||
| Yes | 220 | 98 (44.5%) | 122 (55.5%) | 2.53 (1.86–3.46) | <0.001 |
| Full Model | Stepwise Selection | ||
|---|---|---|---|
| OR (95%-CI), p-Value | OR (95%-CI), p-Value | ||
| Gender | Female vs. male | 0.79 (0.58–1.08), p = 0.142 | |
| BMI | >=18.5 and <25 | Ref | Ref |
| >=25 and <30 | 1.20 (0.85–1.70), p = 0.299 | 1.26 (0.90–1.77), p = 0.173 | |
| >=30 and <35 | 1.09 (0.70–1.69), p = 0.698 | 1.12 (0.73–1.72), p = 0.610 | |
| >=35 | 2.10 (1.20–3.65), p = 0.009 | 1.99 (1.15–3.42), p = 0.013 | |
| Location | Upper extremity | Ref | |
| Trunk | 1.52 (1.04–2.23), p = 0.032 | ||
| Lower extremity | 1.36 (0.91–2.04), p = 0.131 | ||
| Ulceration (yes vs. no) | 1.76 (1.16–2.67), p = 0.008 | 1.79 (1.18–2.70), p = 0.006 | |
| Age (years) | 1.00 (0.99–1.01), p = 0.619 | ||
| Breslow tumor thickness (mm) | 1.18 (1.00–1.40), p = 0.050 | 1.18 (1.01–1.38), p = 0.040 |
| Patients (n = 122) | SLN without Metastases | SLN with Metastases | OR (95%-KI) | p-Value | ||
|---|---|---|---|---|---|---|
| Gender | Male | 46 | 36 (78.3%) | 10 (21.7%) | ||
| Female | 76 | 61 (80.3%) | 15 (19.7%) | 0.89 (0.36–2.19) | 0.820 | |
| BMI | >=18.5 and <25 | 61 | 47 (77.0%) | 14 (23.0%) | ||
| >=25 and <30 | 36 | 30 (83.3%) | 6 (16.7%) | 0.67 (0.23–1.96) | 0.605 | |
| >=30 and <35 | 19 | 17 (89.5%) | 2 (10.5%) | 0.45 (0.10–2.15) | 0.522 | |
| >=35 | 6 | 3 (50.0%) | 3 (50.0%) | 4.27 (0.78–23.31) | 0.100 | |
| Tumor | Body trunk | 19 | 15 (78.9%) | 4 (21.1%) | ||
| location | Upper extremity | 54 | 41 (75.9%) | 13 (24.1%) | 1.19 (0.33–4.26) | 1.000 |
| Lower extremity | 49 | 41 (83.7%) | 8 (16.3%) | 0.73 (0.19–2.82) | 0.727 | |
| Ulceration | No | 115 | 92 (80.0%) | 23 (20.0%) | ||
| Yes | 7 | 5 (71.4%) | 2 (28.6%) | 1.60 (0.29–8.86) | 0.631 |
| SLN | n | Mw | SD | Median | Min-Max | OR (95%-CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| BMI | Negative | 97 | 25.8 | 4.5 | 25.5 | 18.8–38.0 | ||
| Positive | 25 | 26.2 | 5.8 | 24.3 | 18.8–40.9 | 1.02 (0.93–1.11) | 0.887 | |
| Age | Negative | 97 | 49.5 | 15.1 | 50.0 | 21.0–85.0 | ||
| (Years) | Positive | 25 | 50.2 | 13.2 | 50.0 | 25.0–77.0 | 1.00 (0.97–1.03) | 0.884 |
| Breslow tumor | Negative | 97 | 0.8 | 0.2 | 0.8 | 0.04–1.0 | ||
| Thickness (mm) | Positive | 25 | 0.8 | 0.2 | 0.9 | 0.2–1.0 | 0.97 (0.07–14.09) | 0.379 |
| S100 value | Negative | 88 | 0.1 | 0.1 | 0.1 | 0.0–0.6 | ||
| Positive | 21 | 0.1 | 0.0 | 0.1 | 0.0–0.1 | 0.03 (0.00–26.07) | 0.413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida Oliveira, F.; Klose, J.; Schulze, H.-J.; Ribeiro Teixeira, M.; Dermietzel, A.; Wellenbrock, S.; Herter-Sprie, G.-S.; Hirsch, T.; Kueckelhaus, M. The Influence of Obesity on Melanoma and Sentinel Lymph Node Diagnosis: A Retrospective Monocentric Study in 1001 Patients. Cancers 2023, 15, 1806. https://doi.org/10.3390/cancers15061806
Almeida Oliveira F, Klose J, Schulze H-J, Ribeiro Teixeira M, Dermietzel A, Wellenbrock S, Herter-Sprie G-S, Hirsch T, Kueckelhaus M. The Influence of Obesity on Melanoma and Sentinel Lymph Node Diagnosis: A Retrospective Monocentric Study in 1001 Patients. Cancers. 2023; 15(6):1806. https://doi.org/10.3390/cancers15061806
Chicago/Turabian StyleAlmeida Oliveira, Filipa, Julie Klose, Hans-Joachim Schulze, Marta Ribeiro Teixeira, Alexander Dermietzel, Sascha Wellenbrock, Grit-Sophie Herter-Sprie, Tobias Hirsch, and Maximilian Kueckelhaus. 2023. "The Influence of Obesity on Melanoma and Sentinel Lymph Node Diagnosis: A Retrospective Monocentric Study in 1001 Patients" Cancers 15, no. 6: 1806. https://doi.org/10.3390/cancers15061806
APA StyleAlmeida Oliveira, F., Klose, J., Schulze, H.-J., Ribeiro Teixeira, M., Dermietzel, A., Wellenbrock, S., Herter-Sprie, G.-S., Hirsch, T., & Kueckelhaus, M. (2023). The Influence of Obesity on Melanoma and Sentinel Lymph Node Diagnosis: A Retrospective Monocentric Study in 1001 Patients. Cancers, 15(6), 1806. https://doi.org/10.3390/cancers15061806






