Do Biliary Stents Affect EUS-Guided Tissue Acquisition (EUS-TA) in Solid Pancreatic Lesions Determining Biliary Obstruction? A Literature Review with Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Strategy
2.3. Outcomes
2.4. Statistical Analysis
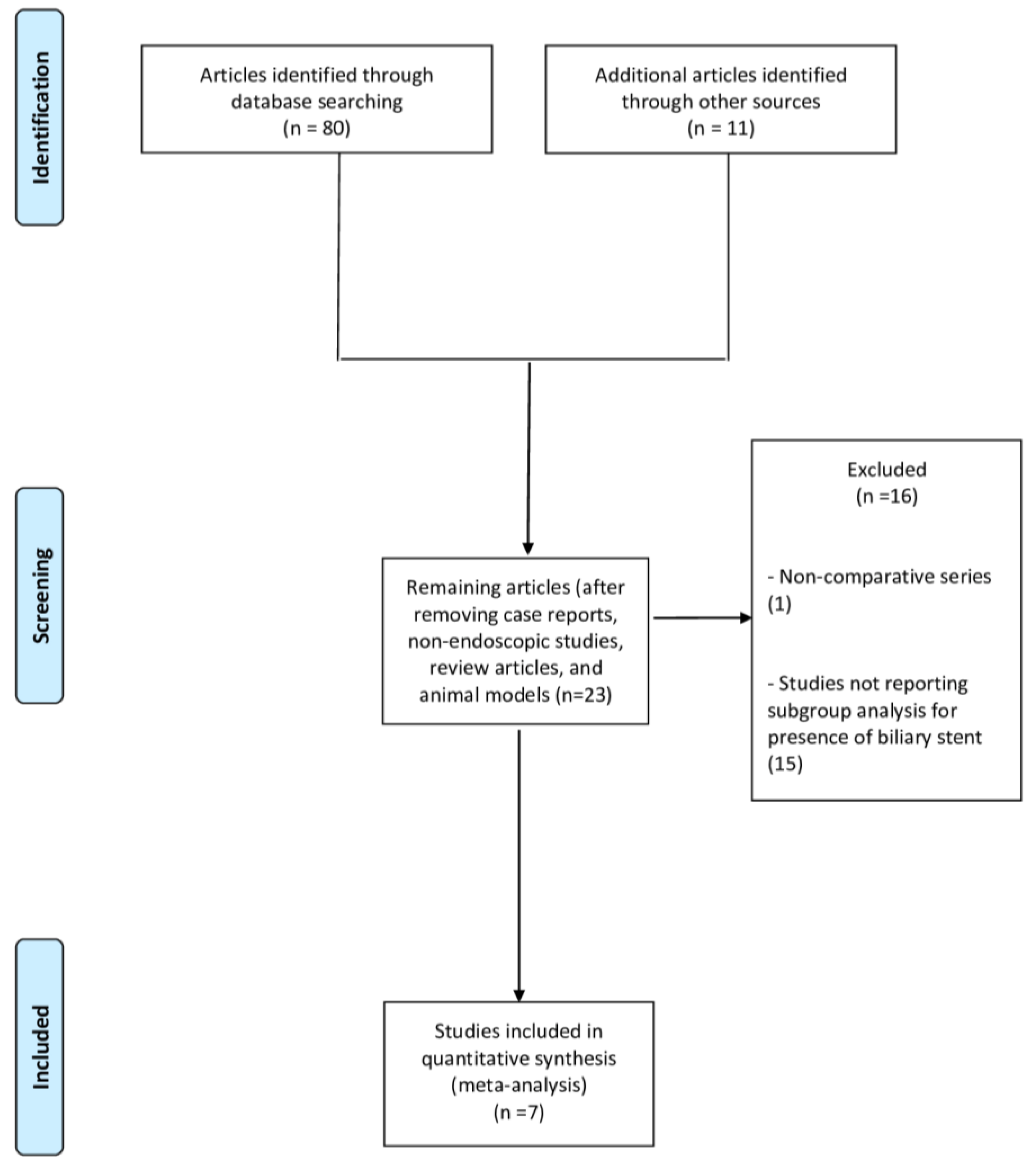
3. Results
3.1. Included Studies
3.2. Diagnostic Accuracy
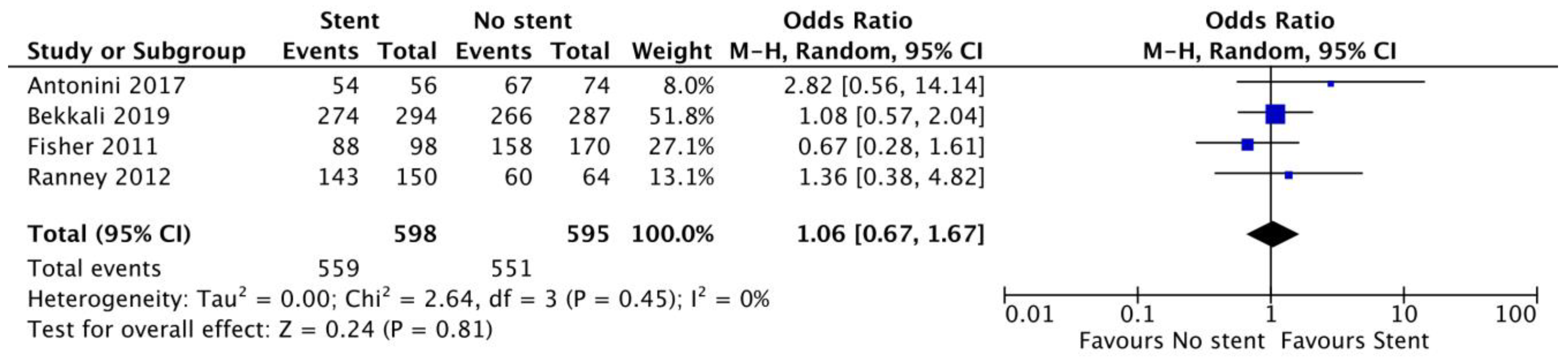
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gkolfakis, P.; Crinò, S.F.; Tziatzios, G.; Ramai, D.; Papaefthymiou, A.; Papanikolaou, I.S.; Triantafyllou, K.; Arvanitakis, M.; Lisotti, A.; Fusaroli, P.; et al. Comparative Diagnostic Performance of End-cutting Fine-needle Biopsy Needles for Endoscopic Ultrasound Tissue Sampling of Solid Pancreatic Masses: A Network Meta-analysis. Gastrointest Endosc. 2022, 95, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Rota, R.; Valenti, M.; Elio, G.; Crinò, S.F.; Manfrin, E.; Parisi, A.; Cingarlini, S.; D’Onofrio, M.; et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: A retrospective analysis of 110 cases. Endoscopy 2020, 52, 988–994. [Google Scholar] [CrossRef]
- Crinó, S.F.; Brandolese, A.; Vieceli, F.; Paiella, S.; Conti Bellocchi, M.C.; Manfrin, E.; Bernardoni, E.; Sina, S.; D’Onofrio, M.; Marchegiani, G.; et al. Endoscopic Ultrasound Features Associated with Malignancy and Aggressiveness of Nonhypovascular Solid Pancreatic Lesions: Results from a Prospective Observational Study. Ultraschall Med. 2021, 42, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.; Mullady, D.; Early, D.S.; Rastogi, A.; Collins, B.; Wang, J.F.; Marshall, C.; Sams, S.B.; Yen, R.; Rizeq, M.; et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: A prospective multicenter randomized controlled trial. Am. J. Gastroenterol. 2015, 110, 1429–1439. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Di Mitri, R.; Nguyen, N.Q.; Tarantino, I.; de Nucci, G.; Deprez, P.H.; Carrara, S.; Kitano, M.; Shami, V.M.; Fernández-Esparrach, G.; et al. Endoscopic Ultrasound-guided Fine-needle Biopsy With or Without Rapid On-site Evaluation for Diagnosis of Solid Pancreatic Lesions: A Randomized Controlled Non-Inferiority Trial. Gastroenterology 2021, 161, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Estrada, J.D.; Rossi, F.; Dziura, J.; Jamidar, P.A.; Siddiqui, U.D. Endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for obstructing pancreas head masses: Combined or separate procedures? J. Clin. Gastroenterol. 2011, 45, 711–713. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Fein, M.; Kowalski, T.E.; Loren, D.E.; Eloubeidi, M.A. Comparison of the influence of plastic and fully covered metal biliary stents on the accuracy of EUS-FNA for the diagnosis of pancreatic cancer. Dig. Dis. Sci. 2012, 57, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Bekkali, N.L.H.; Nayar, M.K.; Leeds, J.S.; Thornton, L.; Johnson, S.J.; Haugk, B.; Darné, A.; Howard-Tripp, N.; Charnley, R.M.; Bassett, P.; et al. Impact of metal and plastic stents on endoscopic ultrasound-guided aspiration cytology and core histology of head of pancreas masses. Endoscopy 2019, 51, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Conti Bellocchi, M.C.; Antonini, F.; Macarri, G.; Carrara, S.; Lamonaca, L.; Di Mitri, R.; Conte, E.; Fabbri, C.; Binda, C.; et al. Impact of biliary stents on the diagnostic accuracy of EUS-guided fine-needle biopsy of solid pancreatic head lesions: A multicenter study. Endosc. Ultrasound 2021, 10, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ranney, N.; Phadnis, M.; Trevino, J.; Ramesh, J.; Wilcox, C.M.; Varadarajulu, S. Impact of biliary stents on EUS-guided FNA of pancreatic mass lesions. Gastrointest Endosc. 2012, 76, 76–83. [Google Scholar] [CrossRef]
- Kim, J.J.; Walia, S.; Lee, S.H.; Patel, B.; Vetsa, M.; Zhao, Y.; Srikureja, W.; Laine, L. Lower yield of endoscopic ultrasound-guided fine-needle aspiration in patients with pancreatic head mass with a biliary stent. Dig. Dis. Sci. 2015, 60, 543–549. [Google Scholar] [CrossRef]
- Oppong, K.W.; Nayar, M.K.; Bekkali, N.L.H.; Maheshwari, P.; Haugk, B.; Darne, A.; Manas, D.M.; French, J.J.; White, S.; Sen, G.; et al. Impact of prior biliary stenting on diagnostic performance of endoscopic ultrasound for mesenteric vascular staging in patients with head of pancreas and periampullary malignancy. BMJ Open Gastroenterol. 2022, 9, e000864. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle—Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 March 2022).
- Wani, S.; Muthusamy, V.R.; McGrath, C.M.; Sepulveda, A.R.; Das, A.; Messersmith, W.; Kochman, M.L.; Shah, J. AGA White Paper: Optimizing Endoscopic Ultrasound-Guided Tissue Acquisition and Future Directions. Clin. Gastroenterol. Hepatol. 2018, 16, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE Working Group. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Antonini, F.; Fuccio, L.; Giorgini, S.; Fabbri, C.; Frazzoni, L.; Scarpelli, M.; Macarri, G. Biliary plastic stent does not influence the accuracy of endoscopic ultrasound-guided sampling of pancreatic head masses performed with core biopsy needles. Dig. Liver Dis. 2017, 49, 898–902. [Google Scholar] [CrossRef]
- Fisher, J.M.; Gordon, S.R.; Gardner, T.B. The impact of prior biliary stenting on the accuracy and complication rate of endoscopic ultrasound fine-needle aspiration for diagnosing pancreatic adenocarcinoma. Pancreas 2011, 40, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, A.; Plotogea, O.M.; Stan-Ilie, M.; Ciurea, T.; Gheonea, D.I.; Ungureanu, B.; Bălan, G.; Rinja, E.; Panic, N.; Şandru, V.; et al. Impact of biliary stenting in endoscopic ultrasound-guided tissue acquisition among patients with pancreatic cancer. J. Clin. Ultrasound 2022, 50, 844–849. [Google Scholar] [CrossRef]
- Nakai, Y.; Isayama, H.; Wang, H.P.; Rerknimitr, R.; Khor, C.; Yasuda, I.; Kogure, H.; Moon, J.H.; Lau, J.; Lakhtakia, S.; et al. International consensus statements for endoscopic management of distal biliary stricture. J. Gastroenterol. Hepatol. 2020, 35, 967–979. [Google Scholar] [CrossRef]
- Arya, N.; Wyse, J.M.; Jayaraman, S.; Ball, C.G.; Lam, E.; Paquin, S.C.; Lightfoot, P.; Sahai, A.V. A proposal for the ideal algorithm for the diagnosis, staging, and treatment of pancreas masses suspicious for pancreatic adenocarcinoma: Results of a working group of the Canadian Society for Endoscopic Ultrasound. Endosc. Ultrasound 2020, 9, 154–161. [Google Scholar]
- Tol, J.A.; van Hooft, J.E.; Timmer, R.; Kubben, F.J.; van der Harst, E.; de Hingh, I.H.J.T.; Vleggaar, F.P.; Molenaar, I.Q.; Keulemans, Y.C.A.; Boerma, D.; et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut 2016, 65, 1981–1987. [Google Scholar] [CrossRef]
- Moss, A.C.; Morris, E.; Mac Mathuna, P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst. Rev. 2006, 2006, CD004200. [Google Scholar]
- Almadi, M.A.; Barkun, A.; Martel, M. Plastic vs. Self-Expandable Metal Stents for Palliation in Malignant Biliary Obstruction: A Series of Meta-Analyses. Am. J. Gastroenterol. 2017, 112, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Cirocchi, R.; Partelli, S.; Petrone, M.C.; Muffatti, F.; Renzi, C.; Falconi, M.; Arcidiacono, P.G. Systematic review and meta-analysis of metal versus plastic stents for preoperative biliary drainage in resectable periampullary or pancreatic head tumors. Eur. J. Surg. Oncol. 2016, 42, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative Diagnostic Performance of Different Techniques for Endoscopic Ultrasound-Guided Fine-Needle Biopsy of Solid Pancreatic Masses: A Network Meta-analysis. Gastrointest Endosc. 2023, in press. [CrossRef]
- Facciorusso, A.; Gkolfakis, P.; Tziatzios, G.; Ramai, D.; Papanikolaou, I.S.; Triantafyllou, K.; Lisotti, A.; Fusaroli, P.; Mangiavillano, B.; Chandan, S.; et al. Comparison between EUS-guided fine-needle biopsy with or without rapid on-site evaluation for tissue sampling of solid pancreatic lesions: A systematic review and meta-analysis. Endosc. Ultrasound 2022, 11, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Di Maso, M.; Serviddio, G.; Vendemiale, G.; Spada, C.; Costamagna, G.; Muscatiello, N. Factors Associated With Recurrence of Advanced Colorectal Adenoma After Endoscopic Resection. Clin. Gastroenterol. Hepatol 2016, 14, 1148–1154. [Google Scholar] [CrossRef]
- Facciorusso, A.; Bajwa, H.S.; Menon, K.; Buccino, V.R.; Muscatiello, N. Comparison between 22G aspiration and 22G biopsy needles for EUS-guided sampling of pancreatic lesions: A meta-analysis. Endosc. Ultrasound 2020, 9, 167–174. [Google Scholar] [CrossRef]
| Study (Country) Period/Design | Study Group | Age | Gender Male | Lesion Size (mm) | Type of Stent (Plastic/Metal) | Needle | Needle Caliper and Time between ERCP and EUS | ROSE |
|---|---|---|---|---|---|---|---|---|
| Crinò, 2021 [11] Italy 2017–2019/ Retrospective | Stent: 347 No stent: 495 | 68 (57.5–76) 70 (64–78) | 202 (58.2%) 271 (58.8%) | 29.3 ± 8.9 31.7 ± 8.7 | 217 (62.5%)/130 (37.5%) | Side-fenestrated FNB 335/end-cutting FNB 507 | 101(29.1%) 25G/217(62.5%) 22G/29(8.4%) 20G 129 (26.1%) 25G/294 (59.4%) 22G/72 (14.5%) 20G 11 days (3–30) | 41 (11.8%) 70 (14.1%) |
| Antonini, 2017 [18] Italy 2013–2015/Retrospective | Stent: 56 No stent: 74 | 69 ± 9.9 70.3 ± 10.3 | 33 (58.9%) 46 (62.2%) | 30.7 ± 12.6 30.8 ± 8.5 | 56 (100%)/0 (0%) | Side-fenestrated FNB 100% | 29 (51.8%) 22G/27 (48.2%) 25G 29 (39.2%) 22G/45 (60.8%) 25G <48 h: 10 (17.8%) | 16 (28.5%) 14 (18.9%) |
| Bekkali, 2018 [10] UK 2010–2016/Retrospective | Stent: 294 No stent: 287 | 66.3 ± 9.4 65 ± 11.4 | 160 (54.4%) 185 (64.4%) | 35 (25–40) 31 (25–39) | 137 (46.6%)/157 (53.4%) | 290 (41.5%) FNA/228 (32.7%) side-fenestrated FNB/179 (25.6%) Fork-tip FNB | 382 (54.7%) 22G/269 (38.5%) 25G NR 22G, 25G FNA | 64 (21.7%) 49 (13.3%) |
| Fisher, 2011 [19] USA 1998–2009/Retrospective | Stent: 98 No stent: 170 | 69.2 68.2 | 44.8% 44.7% | 32.3 33.4 | 66 (67.3%)/6 (6.1%) | FNA | 77% 22G/11.5% 25G 54.1% 22G/34.7% 25G <1 day 11 (11.3%) | 100% 100% |
| Kim, 2015 [13] USA 2005–2013/Retrospective | Stent: 75 No stent: 105 | 65 ± 12 | 108 (60%) | >3 cm: 76 (42%) | 64 (85.3%)/11 (14.7%) | 75 (42%) FNA/105 (58%) side-fenestrated FNB | NR 18 days (1–247) | 81 (45%) |
| Ranney, 2012 [12] USA 2006–2010/Retrospective | Stent: 150 No stent: 64 | 68 (58–75) 69 (63–78) | 105 (49%) 32 (50%) | 30 (21–30) 30 (25–30) | 105 (70%)/45 (30%) | FNA | NR | 100% 100% |
| Constantinescu, 2022 [20] Romania 2016–2020/Retrospective | Stent: 68 No stent: 175 | 62.6 ± 12.23 61.89 ± 12.83 | 40 (58.8%) 98 (56%) | <2 cm: 9 (13.2%) <2 cm: 17 (7%) | 58 (85.3%)/10 (14.7%) | FNA or side-fenestrated FNB or Franseen FNB | 22G NR | 0% 0% |
| Variable | Subgroup | No. of Studies | No. of Patients | Odds Ratio (95% CI) p-Value | Within-Group Heterogeneity (I2) |
|---|---|---|---|---|---|
| Type of stent | Plastic | 5 | Stent: 573 No stent: 1095 | 0.89 (0.51–1.54) 0.67 | 21% |
| Metal | 4 | Stent: 342 No stent: 1021 | 0.54 (0.17–0.97) 0.05 | 17% | |
| Needle | FNB | 3 | Stent:471 No stent: 744 | 0.64 (0.43–0.95) 0.03 | 7% |
| FNA | 1 | Stent: 150 No stent: 64 | 1.36 (0.38–4.82) 0.63 | NA | |
| Availability of ROSE | Yes | 2 | Stent: 225 No stent: 169 | 0.69 (0.23–2.06) 0.51 | 34% |
| Mean number of needle passes | 2 | 2 | Stent: 218 No stent: 239 | 0.82 (0.41–1.65) 0.59 | 0% |
| >2 | 3 | Stent: 478 No stent: 674 | 0.80 (0.67–1.82) 0.63 | 25% |
| Overall Study Sample | ||||
|---|---|---|---|---|
| Outcome | No. of Studies | No. of Patients | Odds Ratio (95% CI) | Within-Group Heterogeneity (I2) |
| Sample adequacy | 4 | Stent: 598 No stent: 595 | 1.06 (0.67–1.67) p = 0.81 | 0% |
| Diagnostic sensitivity | 5 | Stent: 922 No stent: 1025 | 0.59 (0.44–0.80) p < 0.001 | 14% |
| Outcome | No. of Studies | No. of patients | Mean difference (95% CI) | Within-group heterogeneity (I2) |
| Number of needle passes | 6 | Stent: 794 No stent: 1083 | −0.09 (−0.30 to 0.11) p = 0.38 | 86% |
| Plastic stent | ||||
| Outcome | No. of Studies | No. of patients | Odds ratio (95% CI) | Within-group heterogeneity (I2) |
| Sample adequacy | 3 | Stent: 298 No stent: 425 | 1.35 (0.71–2.55) p = 0.36 | 0% |
| Diagnostic sensitivity | 4 | Stent: 515 No stent: 920 | 0.68 (0.42–1.10) p = 0.12 | 45% |
| Metal stent | ||||
| Outcome | No. of Studies | No. of patients | Odds ratio (95% CI) | Within-group heterogeneity (I2) |
| Sample adequacy | 2 | Stent: 202 No stent: 351 | 1.10 (0.55–2.20) p = 0.79 | 0% |
| Diagnostic sensitivity | 3 | Stent: 332 No stent: 846 | 0.61 (0.43–0.86) p = 0.006 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facciorusso, A.; Chandan, S.; Gkolfakis, P.; Ramai, D.; Mohan, B.P.; Lisotti, A.; Conti Bellocchi, M.C.; Papanikolaou, I.S.; Mangiavillano, B.; Triantafyllou, K.; et al. Do Biliary Stents Affect EUS-Guided Tissue Acquisition (EUS-TA) in Solid Pancreatic Lesions Determining Biliary Obstruction? A Literature Review with Meta-Analysis. Cancers 2023, 15, 1789. https://doi.org/10.3390/cancers15061789
Facciorusso A, Chandan S, Gkolfakis P, Ramai D, Mohan BP, Lisotti A, Conti Bellocchi MC, Papanikolaou IS, Mangiavillano B, Triantafyllou K, et al. Do Biliary Stents Affect EUS-Guided Tissue Acquisition (EUS-TA) in Solid Pancreatic Lesions Determining Biliary Obstruction? A Literature Review with Meta-Analysis. Cancers. 2023; 15(6):1789. https://doi.org/10.3390/cancers15061789
Chicago/Turabian StyleFacciorusso, Antonio, Saurabh Chandan, Paraskevas Gkolfakis, Daryl Ramai, Babu P. Mohan, Andrea Lisotti, Maria Cristina Conti Bellocchi, Ioannis S. Papanikolaou, Benedetto Mangiavillano, Konstantinos Triantafyllou, and et al. 2023. "Do Biliary Stents Affect EUS-Guided Tissue Acquisition (EUS-TA) in Solid Pancreatic Lesions Determining Biliary Obstruction? A Literature Review with Meta-Analysis" Cancers 15, no. 6: 1789. https://doi.org/10.3390/cancers15061789
APA StyleFacciorusso, A., Chandan, S., Gkolfakis, P., Ramai, D., Mohan, B. P., Lisotti, A., Conti Bellocchi, M. C., Papanikolaou, I. S., Mangiavillano, B., Triantafyllou, K., Manthopoulou, E., Mare, R., Fusaroli, P., & Crinò, S. F. (2023). Do Biliary Stents Affect EUS-Guided Tissue Acquisition (EUS-TA) in Solid Pancreatic Lesions Determining Biliary Obstruction? A Literature Review with Meta-Analysis. Cancers, 15(6), 1789. https://doi.org/10.3390/cancers15061789











