Simple Summary
The occurrence of second primary cancers (SPCs) in patients with pleural mesothelioma (PM) included in the Lombardy Mesothelioma Registry (Italy) was investigated, with the aim of assessing its prognostic implications. The results of our study showed that the presence of an SPC in a patient’s history did not significantly impact survival in the overall PM population; however, patients with non-epithelioid PM had a worse prognosis when an SPC was diagnosed. Further studies, including next-generation sequencing of cancer susceptibility genes on germline DNA, are needed to clarify the role of SPCs as markers of genetic susceptibility in mesothelioma.
Abstract
Background: The presence of a second primary cancer (SPC) in patients with pleural mesothelioma (PM) may impact overall survival and suggest a common mechanism of carcinogenesis or an underlying germline genetic alteration. Methods: We evaluated the occurrence of SPCs within PM cases collected from 2000 to 2018 by the Lombardy Mesothelioma Registry and their prognostic implications. Kaplan–Meier analysis was performed to estimate median survival times, together with univariate and multivariate Cox regression models to estimate hazard ratios (HR) and 95% confidence intervals (CI) of death. Results: The median overall survival (OS) of the entire study population (N = 6646) was 10.9 months (95% CI: 10.4–11.2); patient age and histotype were the strongest prognostic factors. No substantial survival difference was observed by the presence of an SPC (10.5 months in 1000 patients with an SPC vs. 10.9 months in 5646 patients in the non-SPC group, HR 1.03, p = 0.40). Shorter OS in the SPC group was only observed in 150 patients with the non-epithelioid subtype (median OS of 5.4 vs. 7.1 months, HR 1.21, p = 0.03). Conclusions: The diagnosis of an SPC did not influence the outcome of PM patients in the overall study population but was associated with shorter OS in non-epithelioid cases. Further studies are needed to clarify the role of SPCs as markers of genetic susceptibility in mesothelioma.
1. Introduction
Mesothelioma is an aggressive tumor arising from the lining membrane of the serous cavities of the body, including the pleura, peritoneum, pericardium, and vaginal tunic of the testicles; pleural mesothelioma (PM) accounts for nearly 90% of diagnosed patients [1]. Most cases of mesothelioma are due to asbestos exposure [2], although other uncommon etiologic factors have been reported [3]. The evidence of familial cases [4] and the occurrence of mesothelioma in patients with no identifiable history of exposure to asbestos or asbestos-like fibers have suggested the possibility of an underlying genetic susceptibility [5,6]. Namely, several studies have demonstrated that carriers of BRCA1-associated protein 1 (BAP1) germline mutations can develop a variety of tumor types, including mesothelioma, uveal and cutaneous melanoma, and renal cell carcinoma; less frequently, breast cancer, different types of skin carcinomas, and other neoplasms have been observed [7]. Interestingly, mesothelioma in patients carrying BAP1 germline mutations is usually diagnosed earlier in life and is much less aggressive than mesotheliomas in the general population [8].
Second primary cancers (SPCs) or multiple cancers have been proposed as possible markers of genetic or familial clustering [9]; indeed, in a study of next-generation sequencing of 85 cancer susceptibility genes on germline DNA from 198 patients with pleural, peritoneal, and tunica vaginalis mesotheliomas, having a diagnosis of SPC significantly increased the odds of carrying a BAP1 germline mutation [5]. Identifying mesothelioma patients with SPCs may therefore have a relevant impact on both prognostic information and familial counseling in cases of an underlying germline alteration. Moreover, investigation of SPCs may help generate hypotheses on the carcinogenic mechanisms of mesothelioma [10].
In this study, we investigated the occurrence of SPCs in the Lombardy Mesothelioma Registry (Registro Mesoteliomi Lombardia, RML), a region in northern Italy that has among the highest number of mesothelioma cases in our country. We focused on PM cases, exploring a possible correlation between SPCs and gender, histological mesothelioma subtype, asbestos exposure, and other demographic variables.
2. Methods
2.1. The Lombardy Mesothelioma Registry
The Lombardy Mesothelioma Registry is a regional operating center of the National Mesothelioma Registry (Registro Nazionale Mesoteliomi, ReNaM). It opened in 2000 and collects all newly diagnosed cases of mesothelioma among Lombardy residents [11]. Although mesothelioma reporting is compulsory by law (277/1991 and 81/2008), for a complete recognition of mesothelioma incidence, an active search is performed by exploiting several databases, including hospital admissions and mortality.
Mesothelioma diagnosis was assessed after examining medical records and classified according to ReNaM guidelines as “definite” (histological diagnosis, possibly with immunohistochemical confirmation and imaging), “probable” (cytology plus imaging), “possible” (positive imaging), or non-mesothelioma. Qualified personnel interviews confirmed mesothelioma cases or their next-of-kin using a standardized questionnaire to collect lifetime occupational and residential history and occupational history of family members. Following ReNaM guidelines, a group of experts classified asbestos exposure as either occupational (certain, probable, and possible) or extra-occupational. Subjects without any evidence of asbestos exposure were considered unexposed [11].
The presence of a second primary cancer (SPC) was retrieved from the medical records at the time of inclusion in the registry database. The registry collected information on cancer type and date of diagnosis when available. SPCs were recorded according to the International Classification of Diseases, 10th version (ICD-10) [12].
2.2. Statistical Analysis
Mesothelioma collection was completed through 2021. For the purpose of this study, all cases of PM diagnosed from 2000 to 2018 were extracted to ensure at least 3 years of mortality follow-up. Kaplan–Meier analysis was conducted to evaluate 3-year survival by the presence of one or more SPC, overall and stratified by PM histological subtype.
Univariate and multivariable Cox regression models were fitted to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for SPCs adjusted for gender, age (<55, 55–64, 65–74, 75+), year of diagnosis (2000–2004, 2005–2009, 2010–2014, and 2015–2018), diagnostic certainty (“definite”, “probable”, and “possible”), histotype (epithelioid, sarcomatoid, or biphasic—the last two grouped as “non-epithelioid”), asbestos exposure (ever, never), and tobacco smoking history (never, former, current). All analyses were performed using Stata 17 (Stata Corp. 2021, College Station, TX, USA) [13].
3. Results
A total of 6688 PM patients registered in the RML from 2000 to 2018 were included in the analysis. Of them, 1005 (15.0%) had a recorded SPC; in the remaining 5683 (85.0%), no SPC was reported. The main characteristics of SPC and non-SPC PM cases are listed in Table 1. In both groups, the majority of patients were males, aged ≥ 75 years, had ever been exposed to asbestos, and were never smokers. Most had a definite diagnosis, with epithelioid histological subtype reported in nearly 60% of cases. The only difference we observed between the two groups was an increasing frequency of PM with SPCs over time.

Table 1.
Characteristics of pleural mesothelioma patients by presence/absence of a second primary cancer (SPC), Lombardy, 2000–2018.
Of the 1005 patients with an SPC, 103 (10.2%), 12 (1.2%), and 2 (0.2%) had 2, 3, and 4 malignancies, respectively, in addition to PM, for a total of 1138 SPCs. Table 2 reports the distribution of SPCs overall and by gender. Prostate cancer (38.0%), colorectal cancer (11.6%), bladder cancer (10.4%), cutaneous basal cell carcinoma (7.1%), and renal cell cancer (5.1%) were the most frequent neoplasms among men. Among women, the most frequent malignancies were breast cancer (42.2%), uterine carcinoma (8.6%), non-melanoma skin cancer (8.1%), colorectal cancer (6.4%), cutaneous melanoma (5.3%), and thyroid cancer (5.3%). The year of SPC diagnosis was available in 915 out of 1005 cases, for a total of 1042 cancers. Almost all SPCs occurred before their PM diagnosis, but 56 (5.5%) were diagnosed concomitantly with PM (in the same year). Of these, the most frequent were prostate cancer (23.2%), colorectal cancer (14.3%), non-melanoma cutaneous cancers (10.7%), and kidney cancer (7.1%). The distribution of SPCs was similar for epithelioid and non-epithelioid PMs (Table 3), except for breast cancer, which was more frequently associated with epithelioid PM (17.1% vs. 9.3% of total SPCs, p = 0.02).

Table 2.
Distribution of second primary cancer (SPC) overall and by gender, Lombardy, 2000–2018.

Table 3.
Distribution of second primary cancer (SPC) by histotype, Lombardy, 2000–2018.
Considering cancers for which there is an established correlation with asbestos exposure, there were 33 (2.9%) cases of laryngeal cancer, 18 (1.6%) cases of lung cancer, and 8 (0.7%) cases of ovary cancer. When we focused on tumors potentially associated with BAP1 syndrome [7], we found 84 (7.4%) cases of cutaneous basal cell carcinoma, 49 (4.3%) of renal cell cancer, 46 (4.0%) of melanoma (42 cutaneous melanoma, 4 uveal melanoma), and 11 (1.0%) of hepatocellular carcinoma.
In survival analyses, 42 cases were excluded because the dates of diagnosis and death were coincident, leaving 6646 subjects in the analysis. The median overall survival (OS) was 10.9 months (95% CI: 10.4–11.2) (Figure 1). Histology, age at diagnosis (particularly age ≥ 75 years), year of diagnosis (with a reduced risk of death from 2005 onward), and level of certainty of diagnosis were the strongest prognostic factors (Table 4). Histologic subtype was confirmed as a paramount prognostic factor, with longer survival observed in patients with epithelioid histotype (Figure 2). Females showed slightly better survival in the adjusted model. Patients with a missing history of asbestos exposure had an increased risk of death.
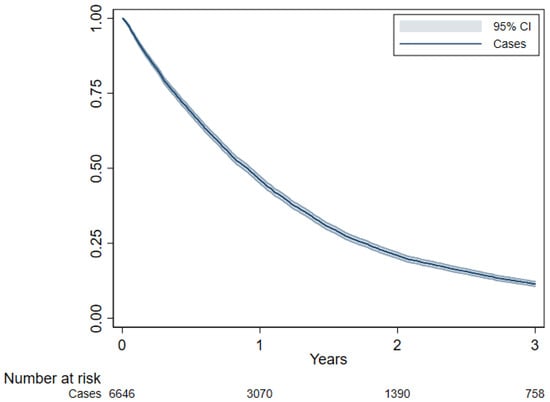
Figure 1.
Overall survival estimated using Kaplan–Meier curve, Lombardy, 2000–2018. Follow-up period 2000–2021.

Table 4.
Prognostic factor analysis in the entire study population, Lombardy 2000–2018. Follow-up period 2000–2021.
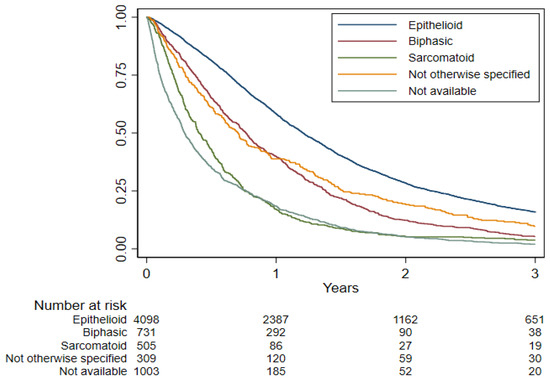
Figure 2.
Overall survival by histotype estimated using Kaplan–Meier curve, Lombardy, 2000–2018. Follow-up period 2000–2021.
No substantial OS difference was observed in patients with an SPC (median OS 10.5 months; 3-year OS 11.1%) compared to patients without SPCs (median OS 10.9 months; 3-year OS 11.5%; HR 1.03, p = 0.40) (Figure 3; Table 5). Among patients with the non-epithelioid (biphasic or sarcomatoid) phenotype, the OS of patients with SPCs was slightly worse (OS 5.4 months vs. 7.1 months in patients without SPCs; HR 1.21, p = 0.03), while no difference between the SPC and no SPC groups was observed in epithelioid patients (Figure 4; Table 5).
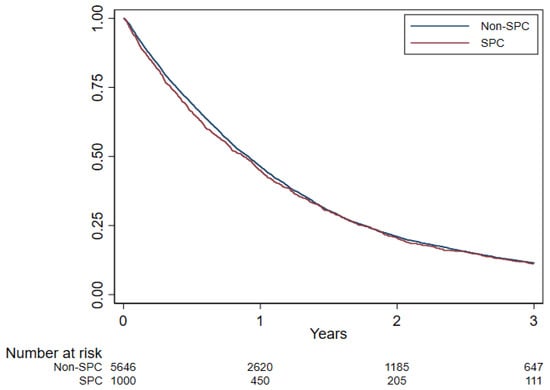
Figure 3.
Overall survival by second primary cancer (SPC) estimated using Kaplan–Meier curve, Lombardy, 2000–2018. Follow-up period 2000–2021.

Table 5.
Overall survival by second primary cancers (SPC), histotype, and by SPC/histotype jointly, Lombardy 2000–2018. Follow-up period 2000–2021.
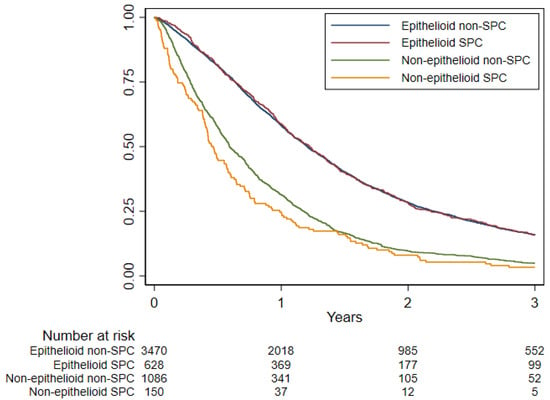
Figure 4.
Overall survival among non-SPC and SPC for epithelioid and non-epithelioid histotypes estimated using Kaplan–Meier curve, Lombardy, 2000–2018. Follow-up period 2000–2021.
4. Discussion
The risk of SPC in association with a first malignancy is well known [9,14], particularly with melanoma [15,16,17], skin cancers [18], breast cancer [19], thyroid cancer [20], liver cancer [21], head and neck cancer [22], and mesothelioma [23,24,25,26,27]. In this study, we evaluated the occurrence of SPCs in patients with PM included in a large population-based registry and its possible prognostic role.
Among PM cases collected in the RML, a diagnosis of SPC was reported in 15% of patients. This percentage is far higher than previously reported in other Italian studies on the general cancer population at the national and regional levels [28,29], probably due to different methodologies in retrieving SPC data. In our study, the presence of an SPC was retrieved from the medical records at the time of inclusion in the registry database and also asked about during the interview carried out to collect asbestos exposure history. We observed no gender difference in distribution among the SPC and non-SPC groups; there were approximately twice as many males as females in the analyzed series. As expected, SPCs were more frequent in older patients and in former smokers. Patients with a “possible” PM diagnosis, i.e., without cytologic or histologic confirmation, had a higher rate of SPCs, probably reflecting worse clinical conditions and diagnostic challenges, with difficulty in achieving an adequate bioptic sample in a patient with a previous or concomitant diagnosis of another cancer. Finally, we observed an increase in SPC cases over the years.
Many studies have focused on genetic mutations [5,30] or epigenetic mechanisms [31] of mesothelioma and found common patterns with other cancers. Several findings have suggested a potential role for BAP1 in the carcinogenesis of numerous malignancies [32,33,34,35]. BAP1 is an oncosuppressor protein that acts as a deubiquitinase and is involved in gene expression and chromatin regulation [34,35,36]; it is commonly inactivated in mesotheliomas [7,37,38,39,40]. Germline BAP1 mutations underpin the BAP1 tumor predisposition syndrome (BAP1-TPDS). Some recent studies have suggested that BAP1 mutations may interact with exposure to carcinogens, such as asbestos. This gene–environment interaction is a possible carcinogenic mechanism [34,36,41,42]. Therefore, we evaluated the frequency of asbestos-related and BAP1 syndrome-related malignancies among SPC patients. Interestingly, we found that they comprised nearly a quarter of the entire group of SPCs; among them, the majority were cancers potentially related to BAP1-TPDS.
No difference in survival was found between patients with or without an SPC. However, among PM patients with the non-epithelioid histotype, OS was worse in the SPC subgroup. Our study confirmed the well-established prognostic role of histologic subtype and age at diagnosis in PM [1,3]. Patients with the epithelioid histotype had better survival than non-epithelioid cases; in particular, patients with the sarcomatoid phenotype showed an almost three-fold higher risk of death compared to those with the epithelioid phenotype. Interestingly, patients with unavailable histology had poor survival, likely related to their worse clinical conditions, as discussed above. As already reported [43], patients aged 75 and older showed a worse prognosis, almost a two-fold higher risk of death. Year of diagnosis and degree of diagnostic certainty were other significant prognostic factors in our series. Patients diagnosed before 2005 had an increased risk of death. The improved outcomes of patients diagnosed with PM in more recent years, and particularly after 2005, are at least partially related to the availability of more effective therapies for PM, namely pemetrexed-based chemotherapy [44,45], which represented the cornerstone of PM treatment until the introduction of immunotherapy [46]. The poor outcomes of patients with “possible” PM are likely explained by their worse clinical conditions, which might have precluded an accurate and timely diagnosis of PM. As expected, no difference in survival was observed in association with asbestos exposure [47]. Conversely, patients with missing asbestos or smoking histories had a high risk of death. The majority had no interviews or proxy interviews (with a family member) due to sudden death or serious clinical conditions. Hence, missing information, like missing histotype, is simply an indicator of a severe prognosis.
The main strength of our investigation is the large dataset. The RML is an operative regional center part of a surveillance network, the National Mesothelioma Registry, and has been active since 2000. The registry systematically collects mesothelioma cases throughout Lombardy using standard criteria for the classification of diagnosis and asbestos exposure. Furthermore, RML retrieves information as complete as possible about lifetime occupational and residential history using standard and structured questionnaires [11].
Of course, our study also has some limitations. First, genetic information is not routinely assessed at the time of diagnosis, so we could not perform an analysis of germline mutations related to BAP1 syndrome. Second, the registry retrospectively collects clinical data, including information on SPCs, from medical records, but full clinical data are not always available. Furthermore, in a minority of patients, their inability to sustain a timely interview due to poor clinical conditions or even early death caused a loss of information regarding asbestos exposure, smoking habits, and occupational and residential history. In these cases, close relatives (wife/husband, sons/daughters, siblings) were interviewed.
5. Conclusions
In conclusion, in our large population-based series of PM patients, SPCs were common. However, their presence was not associated with a significant change in survival, except for patients with non-epithelioid PM. Consistent percentages of SPCs were potentially asbestos-related and BAP1 syndrome-related; however, genetic analysis is mandatory to demonstrate any germline component in the pathogenesis of these tumors. Non-epithelioid histology and older age were confirmed to be negative prognostic factors in PM.
Author Contributions
Conceptualization, C.M., G.L.C. and S.S.; methodology, D.C.; software, D.C. and S.S.; formal analysis, D.C. and S.S.; data curation, C.M., B.D., S.R. and S.S.; writing—original draft preparation, G.L.C., C.M. and S.S.; writing—review and editing, G.L.C., C.M., A.C.P. and D.C.; supervision, C.M. and D.C.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially founded by the Istituto Nazionale per l’Assicurazione contro gli Infortuni sul Lavoro (INAIL), Rome, Italy: BRiC 55/2019.
Institutional Review Board Statement
Reporting malignant mesothelioma to registries is compulsory by Italian law (277/1991 and 81/2008), therefore ethics approval is not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
C.M. and D.C. served the court as consultants in litigations concerning asbestos-related diseases. G.L.C. reports personal fees for speaker engagements from Bristol Myers Squibb, Merck Sharp & Dohme, Novocure, AstraZeneca, Bayer, and Astellas and personal fees for advisory roles from Novocure and AstraZeneca. All the other authors report no conflict of interest.
References
- Janes, S.M.; Alrifai, D.; Fennell, D.A. Perspectives on the treatment of malignant pleural mesothelioma. N. Engl. J. Med. 2021, 385, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Rasko, J.E.J.; George, A.M.; Frank, A.L.; Reid, G. The silent malignant mesothelioma epidemic: A call to action. Lancet Oncol. 2022, 23, 1245–1248. [Google Scholar] [CrossRef]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bonomi, A.; Bueno, R.; Felley-Bosco, E.; Galateau-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, D.; Neri, M.; Ceppi, M.; Cesario, A.; Dianzani, I.; Filiberti, R.; Germignani, F.; Landi, S.; Magnani, C.; Mutti, L.; et al. Genetic susceptibility to malignant mesothelioma and exposure to asbestos: The influence of the familial factor. Mutat. Res. 2008, 658, 162–171. [Google Scholar] [CrossRef]
- Panou, V.; Gadiraju, M.; Wolin, A.; Weipert, C.M.; Skarda, E.; Husain, A.N.; Patel, J.D.; Rose, B.; Zhang, S.R.; Weatherly, M.; et al. Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J. Clin. Oncol. 2018, 36, 2863–2871. [Google Scholar] [CrossRef]
- Betti, M.; Aspesi, A.; Sculco, M.; Matullo, G.; Magnani, C.; Dianzani, I. Genetic predisposition for malignant mesothelioma: A concise review. Mutat. Res. Rev. Mutat. Res. 2019, 781, 1–10. [Google Scholar] [CrossRef]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef]
- Carbone, M.; Pass, H.I.; Ak, G.; Alexander, H.R., Jr.; Bass, P.; Baumann, F.; Blakely, A.M.; Bueno, R.; Bzura, A.; Cardillo, G.; et al. Medical and surgical care of patients with mesothelioma and their relatives carrying germline BAP1 mutations. J. Thorac. Oncol. 2022, 17, 873–889. [Google Scholar] [CrossRef]
- Chen, T.; Kharazmi, E.; Lou, J.; Zhang, X.; Sundquist, K.; Hemminki, K. Risk of second primary cancers after malignant mesothelioma and vice versa. Cancer Lett. 2016, 379, 94–99. [Google Scholar] [CrossRef]
- Liu, Y.L.; Cadoo, K.A.; Mukherjee, S.; Khurram, A.; Tkachuk, K.; Kemel, Y.; Maio, A.; Belhadj, S.; Carlo, M.I.; Latham, A.; et al. Multiple primary cancers in patients undergoing tumor-normal sequencing define novel associations. Cancer Epidemiol. Biomarkers Prev. 2022, 31, 362–371. [Google Scholar] [CrossRef]
- Mensi, C.; De Matteis, S.; Catelan, D.; Dallari, B.; Riboldi, L.; Pesatori, A.C.; Consonni, D. Geographical patterns of mesothelioma incidence and asbestos exposure in Lombardy, Italy. Med. Lav. 2016, 107, 340–355. [Google Scholar]
- WHO. International Classification of Diseases, 10th Revision, Fifth Edition, 2016. Available online: https://apps.who.int/iris/handle/10665/246208 (accessed on 10 January 2023).
- StataCorp. Stata Statistical Software: Release 17, StataCorp LLC: College Station, TX, USA, 2021.
- Tanjak, P.; Suktitipat, B.; Vorasan, N.; Juengwiwattanakitti, P.; Thiengtrong, P.; Songjang, C.; Therasakvichya, S.; Laiteraapong, S.; Chinswangwatanakul, V. Risks and cancer associations of metachronous and synchronous multiple primary cancers: A 25-year retrospective study. BMC Cancer 2021, 21, 1045. [Google Scholar] [CrossRef]
- Caini, S.; Boniol, M.; Botteri, E.; Tosti, G.; Bazolli, B.; Russell-Edu, W.; Giusti, F.; Testori, A.; Gandini, S. The risk of developing a second primary cancer in melanoma patients: A comprehensive review of the literature and meta-analysis. Dermatol. Sci. 2014, 75, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Radice, D.; Tosti, G.; Spadola, G.; Cocorocchio, E.; Ferrucci, P.F.; Testori, A.; Pennacchioli, E.; Fargnoli, M.C.; Palli, D.; et al. Risk of second primary malignancies among 1537 melanoma patients and risk of second primary melanoma among 52354 cancer patients in Northern Italy. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Frank, C.; Sundquist, J.; Hemminki, A.; Hemminki, K. Risk of other cancers in families with melanoma: Novel familial links. Sci. Rep. 2017, 7, 42601. [Google Scholar] [CrossRef] [PubMed]
- Borghi, A.; Corazza, M.; Chiaranda, G.; Michiara, M.; Mangone, L.; Caruso, B.; Falcini, F.; Maestri, I.; Ferretti, S. Second primary malignancies in patients with non-melanoma skin cancer: Results from a cancer registry–based study in Emilia Romagna, north-east Italy. Cancer Epidemiol. 2019, 61, 176–184. [Google Scholar] [CrossRef]
- Ricceri, F.; Fasanelli, F.; Giraudo, M.T.; Sieri, S.; Tumino, R.; Mattiello, A.; Vagliano, L.; Masala, G.; Quirós, J.R.; Travier, N.; et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC). Int. J. Cancer 2015, 137, 940–948. [Google Scholar] [CrossRef]
- Crocetti, E.; Mattioli, V.; Buzzoni, C.; Franceschi, S.; Serraino, D.; Vaccarella, S.; Ferretti, S.; Busco, S.; Fedeli, U.; Varvarà, M.; et al. for AIRTUM working group. Risk of thyroid as a first or second primary cancer. A population-based study in Italy, 1998–2012. Cancer Med. 2021, 10, 6855–6867. [Google Scholar] [CrossRef]
- Zheng, G.; Sundquist, K.; Sundquist, J.; Chen, T.; Försti, A.; Hemminki, A.; Liska, V.; Hemminki, K. Second primary cancers after liver, gallbladder and bile duct cancers, and these cancers as second primary cancers. Clin. Epidemiol. 2021, 13, 683–691. [Google Scholar] [CrossRef]
- Priante, A.V.M.; Castilho, E.C.; Kowalski, L.P. Second primary tumors in patients with head and neck cancer. Curr. Oncol. Rep. 2011, 13, 132–137. [Google Scholar] [CrossRef]
- Attanoos, R.L.; Thomas, D.H.; Gibbs, A.R. Synchronous diffuse malignant mesothelioma and carcinomas in asbestos-exposed individuals. Histopathology 2003, 43, 387–392. [Google Scholar] [CrossRef]
- Özbudak, I.H.; Özbudak, Ö.; Arslan, G.; Erdoğan, A.; Özbılım, G. Metachronous malignant mesothelioma and pulmonary adenocarcinoma. Turk. Patoloji Derg. 2013, 29, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Vanni, I.; Coco, S.; Bonfiglio, S.; Cittaro, D.; Genova, C.; Biello, F.; Mora, M.; Rossella, V.; Dal Bello, M.G.; Truini, A.; et al. Whole exome sequencing of independent lung adenocarcinoma, lung squamous cell carcinoma, and malignant peritoneal mesothelioma: A case report. Medicine 2016, 95, e5447. [Google Scholar] [CrossRef] [PubMed]
- Soni, T.P.; Soni, S.; Somani, N.; Batthacharya, T.; Kucha, N. Prostate carcinoma and pleural mesothelioma: An extremely rare co-occurrence. Cureus 2019, 11, e4655. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki-Ushiku, A.; Kohsaka, S.; Kage, H.; Oda, K.; Miyagawa, K.; Nakajima, H.; Aburatani, H.; Mano, H.; Ushiku, T. Genomic profiling of multiple primary cancers including synchronous lung adenocarcinoma and bilateral malignant mesotheliomas: Identification of a novel BAP1 germline variant. Pathol. Int. 2020, 70, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Buiatti, E.; Falini, P.; Italian Multiple Primary Cancers Working Group. Multiple primary cancer incidence in Italy. Eur. J. Cancer 2001, 37, 2449–2456. [Google Scholar] [CrossRef]
- Ragusa, R.; Torrisi, A.; Di Prima, A.A.; Torrisi, A.A.; Ippolito, A.; Ferrante, M.; Madeddu, A.; Guardabasso, V. Cancer prevention for survivors: Incidence of second primary cancers and sex differences. A population-based study from an Italian cancer registry. Int. J. Environ. Res. Public Health 2022, 19, 12201. [Google Scholar] [CrossRef]
- Quetel, L.; Meiller, C.; Assié, J.-B.; Blum, Y.; Imbeaud, S.; Montagne, F.; Tranchant, R.; de Wolf, J.; Caruso, S.; Copin, M.-C.; et al. Genetic alterations of malignant pleural mesothelioma: Association with tumor heterogeneity and overall survival. Mol. Oncol. 2020, 14, 1207–1223. [Google Scholar] [CrossRef]
- Tan, Y.; Sementino, E.; Cheung, M.; Peri, S.; Menges, C.W.; Kukuyan, A.-M.; Zhang, T.; Khazak, V.; Fox, L.A.; Ross, E.A.; et al. Somatic epigenetic silencing of RIPK3 inactivates necroptosis and contributes to chemoresistance in malignant mesothelioma. Clin. Cancer Res. 2021, 27, 1200–1213. [Google Scholar] [CrossRef]
- Pilarski, R.; Carlo, M.I.; Cebulla, C.; Abdel-Rahman, M. BAP1 tumor predisposition syndrome. In GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Rai, K.; Pilarski, R.; Cebulla, C.M.; Abdel-Rahman, M.H. Comprehensive review of BAP1 tumor predisposition syndrome with report of two new cases. Clin. Gene 2016, 89, 285–294. [Google Scholar] [CrossRef]
- Masclef, L.; Ahmed, O.; Estavoyer, B.; Larrivée, B.; Labrecque, N.; Nijnik, A.; Affar, E.B. Roles and mechanisms of BAP1 deubiquitinase in tumor suppression. Cell Death Differ. 2021, 28, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Sculco, M.; La Vecchia, M.; Aspesi, A.; Clavenna, M.G.; Salvo, M.; Borgonovi, G.; Pittaro, A.; Witel, G.; Napoli, F.; Listì, A.; et al. Diagnostics of BAP1-tumor predisposition syndrome by a multitesting approach: A ten-year-long experience. Diagnostics 2022, 12, 1710. [Google Scholar] [CrossRef] [PubMed]
- Caporali, S.; Butera, A.; et Amelio, I. BAP1 in cancer: Epigenetic stability and genome integrity. Discov. Oncol. 2022, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.; Brevet, M.; Taylor, B.S.; Shimizu, S.; Ito, T.; Wang, L.; Creaney, J.; Lake, R.A.; Zakowski, M.F.; Reva, B.; et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat. Genet. 2011, 43, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Bindal, N.; Bamford, S.; Cole, C.; Kok, C.Y.; Beare, D.; Jia, M.; Sheperd, R.; Leung, K.; Menzies, A.; et al. COSMIC: Mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic. Acids Res. 2011, 39, D945–D950. [Google Scholar] [CrossRef]
- Alakus, H.; Yost, S.E.; Woo, B.; French, R.; Lin, G.Y.; Jepsen, K.; Frazer, K.A.; Lowy, A.M.; Harismendy, O. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J. Transl. Med. 2015, 13, 122. [Google Scholar] [CrossRef]
- Nasu, M.; Emi, M.; Pastorino, S.; Tanji, A.; Powers, A.; Luk, H.; Baumann, F.; Zhang, Y.-A.; Gazdar, A.; Kanodia, S.; et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J. Thorac. Oncol. 2015, 10, 565–576. [Google Scholar] [CrossRef]
- Cheung, M.; Kadariya, Y.; Talarchek, J. Germline BAP1 mutation in a family with high incidence of multiple primary cancers and a potential gene-environment interaction. Cancer Lett. 2015, 369, 261–265. [Google Scholar] [CrossRef]
- Gaudino, G.; Xue, J.; Yang, H. How asbestos and other fibers cause mesothelioma. Transl. Lung Cancer Res. 2020, 9 (Suppl. S1), S39–S46. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Grosso, F.; Zucali, P.A.; Mencoboni, M.; Pasello, G.; Ripa, C.; Degiovanni, D.; Simonelli, M.; Bruzzone, A.; Dipietrantonj, C.; et al. Prognostic factors in elderly patients with malignant pleural mesothelioma: Results of a multicenter survey. Br. J. Cancer 2014, 111, 220–226. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.E.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Ceresoli, G.L.; Zucali, P.A.; Favaretto, A.G.; Grossi, F.; Bidoli, P.; Del Conte, G.; Ceribelli, A.; Bearz, A.; Morenghi, E.; Cavina, R.; et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J. Clin. Oncol. 2006, 24, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 2021, 30, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, P.G.; Consonni, D.; Somigliana, A. Asbestos lung burden does not predict survival in malignant pleural mesothelioma: A necropsy-based study of 185 cases. J. Thorac. Oncol. 2022, 8, 1042–1049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).