Frequency and Prognostic Relevance of Volumetric MRI Changes in Contrast- and Non-Contrast-Enhancing Tumor Compartments between Surgery and Radiotherapy of IDHwt Glioblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Magnetic Resonance Imaging
2.3. Neuropathological Diagnosis
2.4. Statistical Analysis
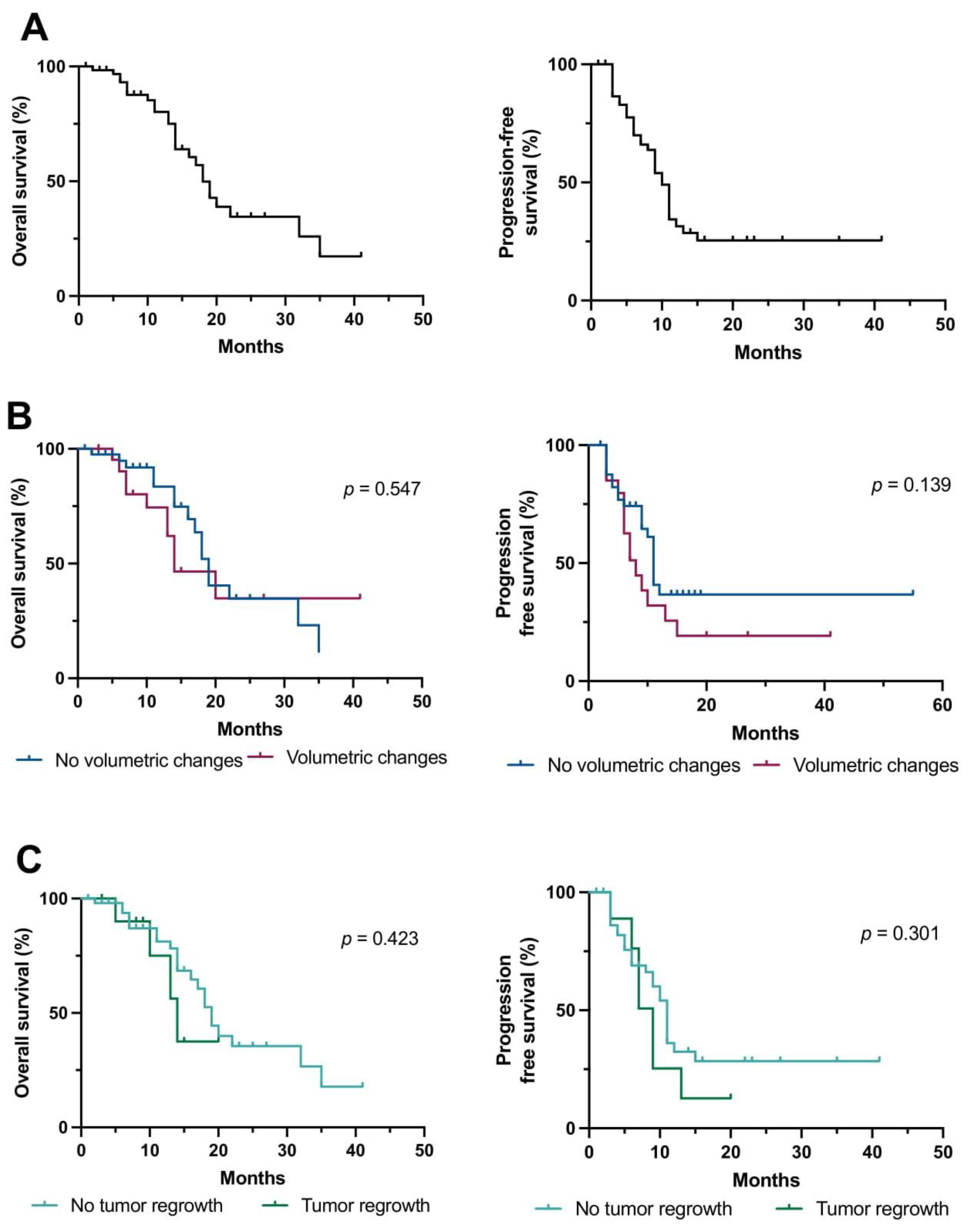
3. Results
3.1. Patient Characteristics and Adjuvant Therapies
3.2. MRI and Volumetric Analysis
3.3. Predictors of Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Sciortino, T.; Bruno, F.; Juenger, S.T.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro-Oncology 2022, noac193. [Google Scholar] [CrossRef] [PubMed]
- Waqar, M.; Roncaroli, F.; Lehrer, E.J.; Palmer, J.D.; Villanueva-Meyer, J.; Braunstein, S.; Hall, E.; Aznar, M.; De Witt Hamer, P.C.; D’Urso, P.I.; et al. Rapid early progression (REP) of glioblastoma is an independent negative prognostic factor: Results from a systematic review and meta-analysis. Neurooncol. Adv. 2022, 4, vdac075. [Google Scholar] [CrossRef]
- Pirzkall, A.; McGue, C.; Saraswathy, S.; Cha, S.; Liu, R.; Vandenberg, S.; Lamborn, K.R.; Berger, M.S.; Chang, S.M.; Nelson, S.J. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology 2009, 11, 842–852. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Lakomy, R.; Kazda, T.; Selingerova, I.; Poprach, A.; Pospisil, P.; Belanova, R.; Fadrus, P.; Smrcka, M.; Vybihal, V.; Jancalek, R.; et al. Pre-Radiotherapy Progression after Surgery of Newly Diagnosed Glioblastoma: Corroboration of New Prognostic Variable. Diagnostics 2020, 10, 676. [Google Scholar] [CrossRef]
- Wee, C.W.; Kim, E.; Kim, T.M.; Park, C.K.; Kim, J.W.; Choi, S.H.; Yoo, R.E.; Lee, S.T.; Kim, I.H. Impact of interim progression during the surgery-to-radiotherapy interval and its predictors in glioblastoma treated with temozolomide-based radiochemotherapy. J. Neurooncol. 2017, 134, 169–175. [Google Scholar] [CrossRef]
- Niyazi, M.; Brada, M.; Chalmers, A.J.; Combs, S.E.; Erridge, S.C.; Fiorentino, A.; Grosu, A.L.; Lagerwaard, F.J.; Minniti, G.; Mirimanoff, R.O.; et al. ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother. Oncol. 2016, 118, 35–42. [Google Scholar] [CrossRef]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; Degroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Teske, N.; Karschnia, P.; Weller, J.; Siller, S.; Dorostkar, M.M.; Herms, J.; von Baumgarten, L.; Tonn, J.C.; Thon, N. Extent, pattern, and prognostic value of MGMT promotor methylation: Does it differ between glioblastoma and IDH-wildtype/TERT-mutated astrocytoma? J. Neurooncol. 2021, 156, 317–327. [Google Scholar] [CrossRef]
- Biczok, A.; Kraus, T.; Suchorska, B.; Terpolilli, N.A.; Thorsteinsdottir, J.; Giese, A.; Tonn, J.C.; Schichor, C. TERT promoter mutation is associated with worse prognosis in WHO grade II and III meningiomas. J. Neuro-Oncol. 2018, 139, 671–678. [Google Scholar] [CrossRef]
- Neumann, J.E.; Dorostkar, M.M.; Korshunov, A.; Mawrin, C.; Koch, A.; Giese, A.; Schüller, U. Distinct Histomorphology in Molecular Subgroups of Glioblastomas in Young Patients. J. Neuropathol. Exp. Neurol. 2016, 75, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: The Heidelberg experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Skardelly, M.; Kaltenstadler, M.; Behling, F.; Mäurer, I.; Schittenhelm, J.; Bender, B.; Paulsen, F.; Hedderich, J.; Renovanz, M.; Gempt, J.; et al. A Continuous Correlation Between Residual Tumor Volume and Survival Recommends Maximal Safe Resection in Glioblastoma Patients: A Nomogram for Clinical Decision Making and Reference for Non-Randomized Trials. Front. Oncol. 2021, 11, 748691. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Molinaro, A.M.; Hervey-Jumper, S.; Morshed, R.A.; Young, J.; Han, S.J.; Chunduru, P.; Zhang, Y.; Phillips, J.J.; Shai, A.; Lafontaine, M.; et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non–Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol. 2020, 6, 495–503. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Jost, S.; Aghi, M.K.; Heimberger, A.B.; Sampson, J.H.; Wen, P.Y.; Macdonald, D.R.; Van den Bent, M.J.; Chang, S.M. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery 2012, 70, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kläsner, B.; Buchmann, N.; Gempt, J.; Ringel, F.; Lapa, C.; Krause, B.J. Early [18F]FET-PET in Gliomas after Surgical Resection: Comparison with MRI and Histopathology. PLoS ONE 2015, 10, e0141153. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Albert, N.L.; Unterrainer, M.; la Fougere, C.; Egensperger, R.; Schüller, U.; Lutz, J.; Kreth, S.; Tonn, J.C.; Kreth, F.W.; et al. Dynamic 18F-FET PET is a powerful imaging biomarker in gadolinium-negative gliomas. Neuro-Oncology 2019, 21, 274–284. [Google Scholar] [CrossRef]
- Farace, P.; Amelio, D.; Ricciardi, G.K.; Zoccatelli, G.; Magon, S.; Pizzini, F.; Alessandrini, F.; Sbarbati, A.; Amichetti, M.; Beltramello, A. Early MRI changes in glioblastoma in the period between surgery and adjuvant therapy. J. Neurooncol. 2013, 111, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Bhamidipati, D.; Shukla, G.; Sharma, D.; Glass, J.; Kim, L.; Evans, J.J.; Judy, K.; Farrell, C.; Andrews, D.W.; et al. Rapid Early Tumor Progression is Prognostic in Glioblastoma Patients. Am. J. Clin. Oncol. 2019, 42, 481–486. [Google Scholar] [CrossRef]
- Merkel, A.; Soeldner, D.; Wendl, C.; Urkan, D.; Kuramatsu, J.B.; Seliger, C.; Proescholdt, M.; Eyupoglu, I.Y.; Hau, P.; Uhl, M. Early postoperative tumor progression predicts clinical outcome in glioblastoma-implication for clinical trials. J. Neurooncol. 2017, 132, 249–254. [Google Scholar] [CrossRef]
- Karschnia, P.; Young, J.S.; Dono, A.; Häni, L.; Juenger, S.T.; Sciortino, T.; Bruno, F.; Teske, N.; Morshed, R.A.; Haddad, A.F.; et al. TERT promotor status does not add prognostic information in IDH-wildtype glioblastomas fulfilling other diagnostic WHO criteria: A report of the RANO resect group. Neuro-Oncol. Adv. 2022, 4, vdac158. [Google Scholar] [CrossRef]

| Characteristics | Categories | Patients without Volumetric Changes | Patients with (+) Volumetric Changes | Total | p-Value |
|---|---|---|---|---|---|
| Overall, n (%) | 42 (66%) | 22 (34%) | 64 (100%) | ||
| Age, years (%) | Mean | 61.6 | 57.2 | 60.1 | 0.123 |
| 18–50 | 8 (19%) | 4 (18%) | 12 (19%) | ||
| 51–65 | 16 (38%) | 13 (59%) | 29 (45%) | ||
| >65 | 18 (43%) | 5 (23%) | 23 (36%) | ||
| Sex, n (%) | Female | 11 (26%) | 10 (45%) | 21 (33%) | 0.163 |
| Male | 31 (74%) | 12 (55%) | 43 (67%) | ||
| Clinical performance | Pre-op KPS, median (range) | 80 (60–100%) | 90 (20–100%) | 85 (20–100%) | 0.133 |
| Post-op KPS, median (range) | 80 (50–100%) | 90 (50–90%) | 80 (50–100%) | 0.394 | |
| New postoperative deficit, n (%) | 3 (7%) | 5 (23%) | 8 (13%) | 0.111 | |
| MGMT promotor, n (%) | Methylated | 12 (29%) | 10 (45%) | 22 (34%) | 0.268 |
| Non-methylated | 30 (71%) | 12 (55%) | 42 (66%) | ||
| TERT promotor, n (%) | Wildtype | 10 (24%) | 0 (0%) | 10 (16%) | * 0.012 |
| Mutated | 32 (76%) | 22 (100%) | 54 (84%) | ||
| Tumor localization, n (%) | (Sub)-cortical | 38 (90%) | 18 (82%) | 56 (88%) | 0.430 |
| Multifocal | 1 (2%) | 4 (18%) | 5 (8%) | ||
| Deep-seated | 2 (5%) | 0 (0%) | 2 (3%) | ||
| Cerebellar | 1 (2%) | 0 (0%) | 1 (2%) | ||
| Dominant hemisphere | 20 (48%) | 10 (45%) | 30 (47%) | 0.999 | |
| Tumor volumes, cm3; mean ± SEM | Pre-op CE | 30.9 ± 3.8 | 34.1 ± 5.3 | 32.0 ± 3.1 | 0.624 |
| Pre-op non-CE | 47.7 ± 5.4 | 66.0 ± 6.2 | 53.9 ± 4.2 | * 0.009 | |
| Post-op CE | 0.5 ± 0.2 | 2.3 ± 1.2 | 1.1 ± 0.4 | 0.258 | |
| Post-op non-CE | 2.2 ± 0.6 | 12.2 ± 4.1 | 5.7 ± 1.6 | * 0.007 | |
| Pre-RT CE | 0.5 ± 0.2 | 6.2 ± 2.5 | 2.4 ± 0.9 | * 0.001 | |
| Pre-RT non-CE | 2.2 ± 0.6 | 19.8 ± 5.4 | 8.3 ± 2.1 | * 0.001 | |
| RANO categories of EOR in glioblastoma, n (%) | RANO class 1 | 28 (67%) | 8 (36%) | 36 (56%) | * 0.033 |
| RANO class 2 | 6 (14%) | 9 (41%) | 15 (23%) | ||
| RANO class 3 | 8 (19%) | 5 (23%) | 13 (20%) | ||
| Adjuvant therapy | EORTC-26981/22981 | 28 (67%) | 19 (86%) | 47 (73%) | 0.137 |
| RT alone | 9 (21%) | 1 (5%) | 10 (16%) | ||
| EORTC-26981/22981 + study drug | 3 (7%) | 1 (5%) | 4 (6%) | ||
| CeTeG | 2 (5%) | 1 (5%) | 3 (5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teske, N.; Teske, N.C.; Niyazi, M.; Belka, C.; Thon, N.; Tonn, J.-C.; Forbrig, R.; Karschnia, P. Frequency and Prognostic Relevance of Volumetric MRI Changes in Contrast- and Non-Contrast-Enhancing Tumor Compartments between Surgery and Radiotherapy of IDHwt Glioblastoma. Cancers 2023, 15, 1745. https://doi.org/10.3390/cancers15061745
Teske N, Teske NC, Niyazi M, Belka C, Thon N, Tonn J-C, Forbrig R, Karschnia P. Frequency and Prognostic Relevance of Volumetric MRI Changes in Contrast- and Non-Contrast-Enhancing Tumor Compartments between Surgery and Radiotherapy of IDHwt Glioblastoma. Cancers. 2023; 15(6):1745. https://doi.org/10.3390/cancers15061745
Chicago/Turabian StyleTeske, Nico, Nina C. Teske, Maximilian Niyazi, Claus Belka, Niklas Thon, Joerg-Christian Tonn, Robert Forbrig, and Philipp Karschnia. 2023. "Frequency and Prognostic Relevance of Volumetric MRI Changes in Contrast- and Non-Contrast-Enhancing Tumor Compartments between Surgery and Radiotherapy of IDHwt Glioblastoma" Cancers 15, no. 6: 1745. https://doi.org/10.3390/cancers15061745
APA StyleTeske, N., Teske, N. C., Niyazi, M., Belka, C., Thon, N., Tonn, J.-C., Forbrig, R., & Karschnia, P. (2023). Frequency and Prognostic Relevance of Volumetric MRI Changes in Contrast- and Non-Contrast-Enhancing Tumor Compartments between Surgery and Radiotherapy of IDHwt Glioblastoma. Cancers, 15(6), 1745. https://doi.org/10.3390/cancers15061745






