Reproductive Health Outcomes among Adolescent and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods
2.2. Study Screening and Inclusion
2.3. Data Extraction and Quality Assessment
2.4. Analysis
3. Results
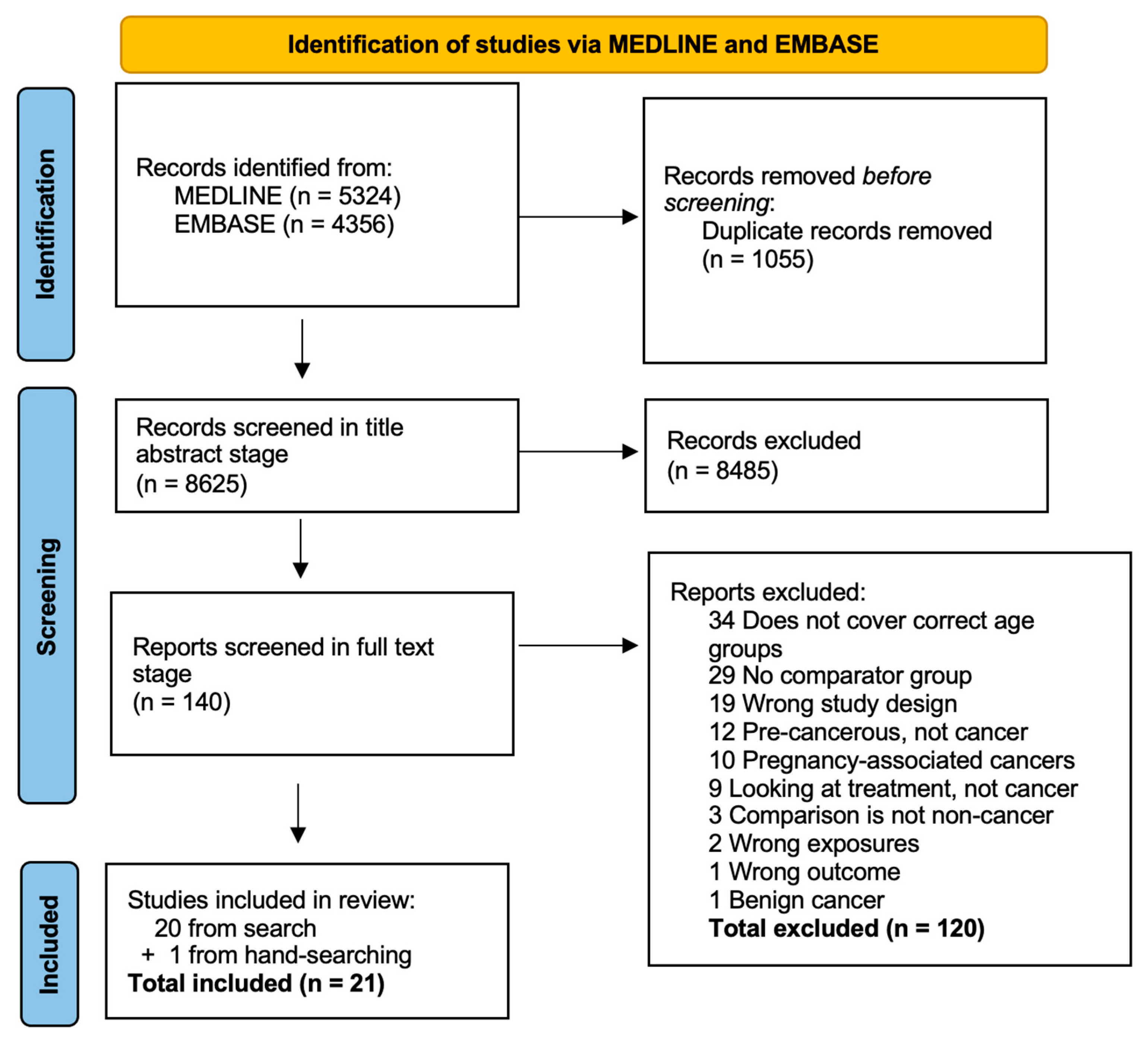
3.1. Search Results
3.2. Study Characteristics
3.3. Reproductive Health Outcomes
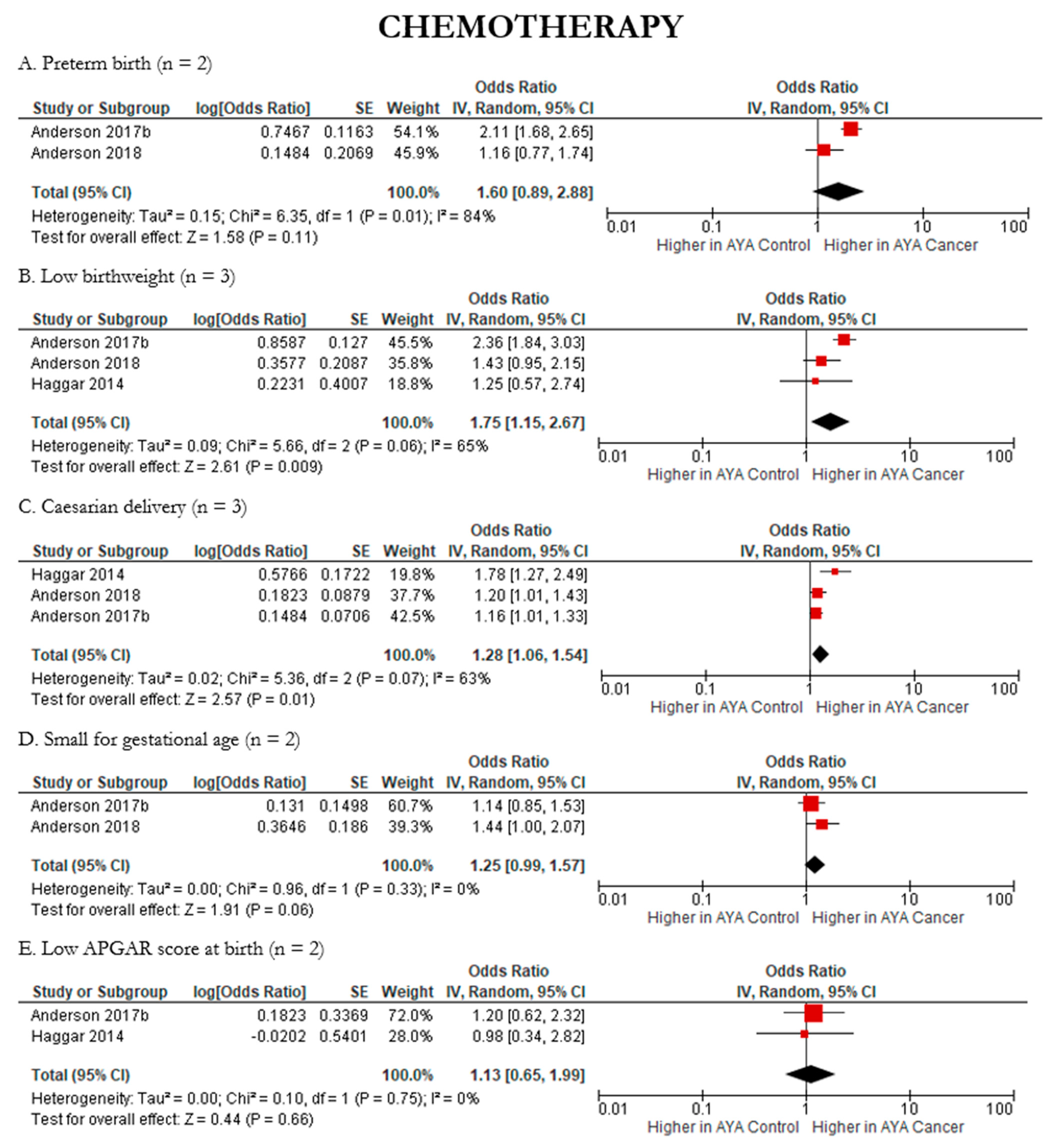
3.4. Meta-Analysis
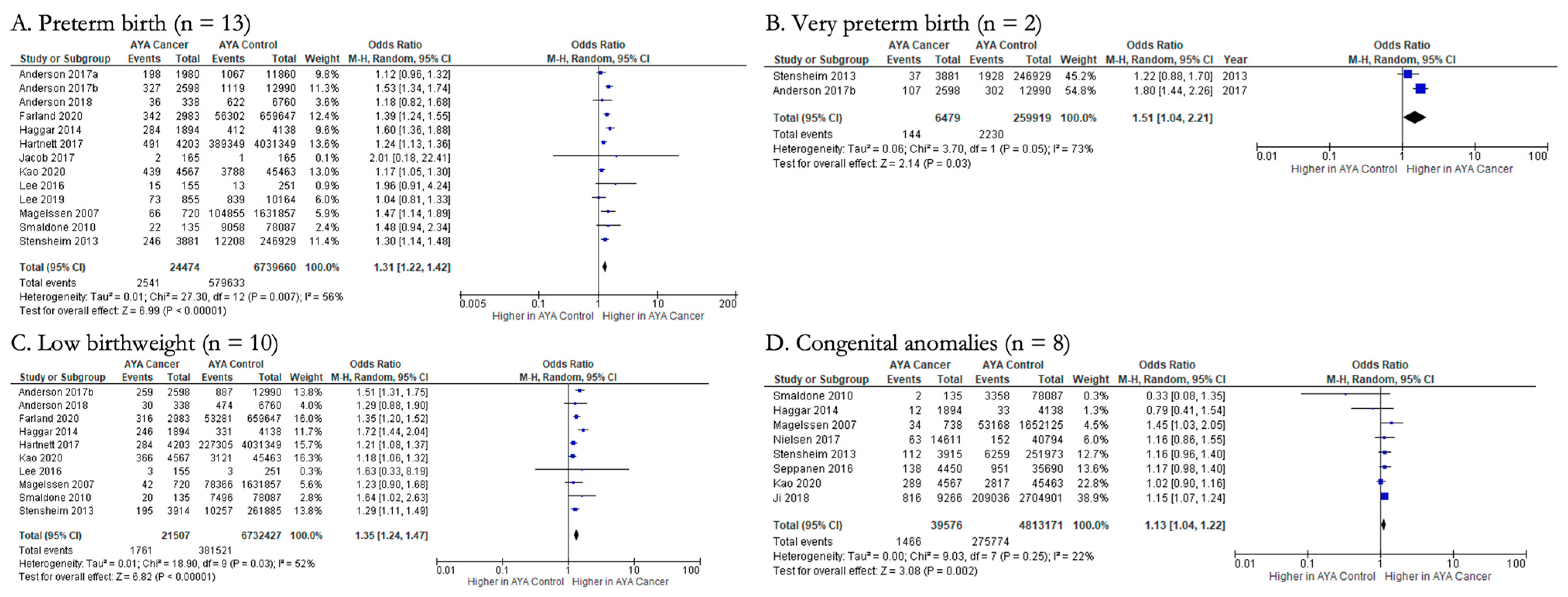
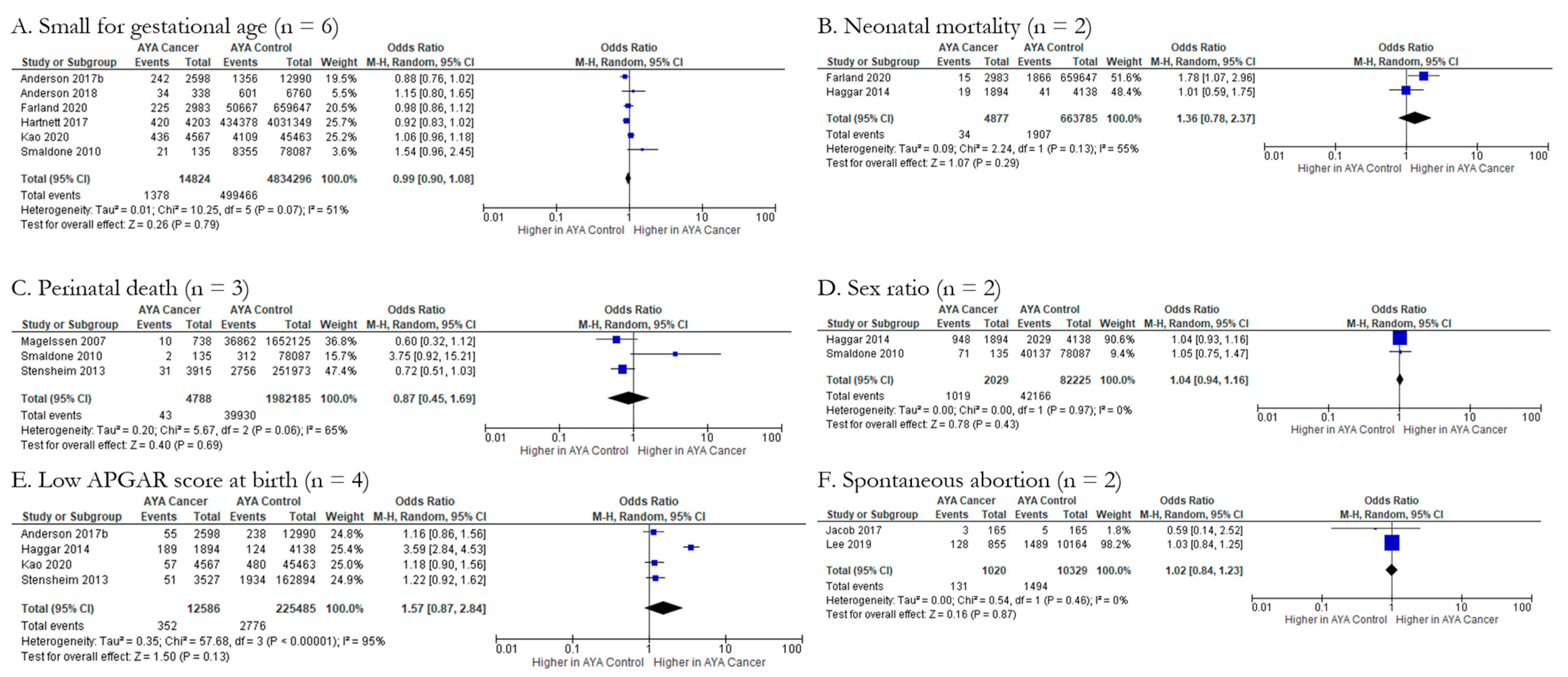
3.4.1. Fetal/Neonatal Outcomes (n = 10)
3.4.2. Maternal Outcomes (n = 3)
3.4.3. Fetal/Neonatal-Maternal Outcomes (n = 3)
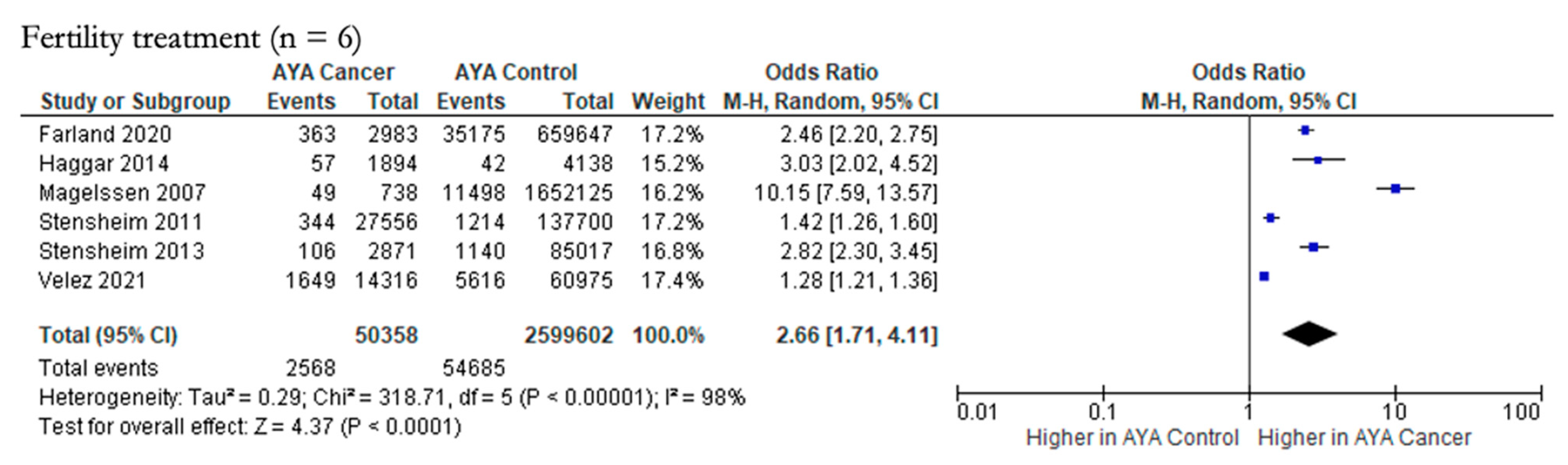
3.4.4. Maternal-Paternal Outcomes (n = 1)
3.4.5. Impact of Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scott, A.R.; Stoltzfus, K.C.; Tchelebi, L.T.; Trifiletti, D.M.; Lehrer, E.J.; Rao, P.; Bleyer, A.; Zaorsky, N.G. Trends in Cancer Incidence in US Adolescents and Young Adults, 1973–2015. JAMA Netw. Open 2020, 3, e2027738. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Vitrano, V.; Catania, V. Sexual issues in early and late stage cancer: A review. Support. Care Cancer 2010, 18, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Abbott-Anderson, K.; Kwekkeboom, K.L. A systematic review of sexual concerns reported by gynecological cancer survivors. Gynecol. Oncol. 2012, 124, 477–489. [Google Scholar] [CrossRef]
- Geue, K.; Brahler, E.; Faller, H.; Harter, M.; Schulz, H.; Weis, J.; Koch, U.; Wittchen, H.U.; Mehnert, A. Prevalence of mental disorders and psychosocial distress in German adolescent and young adult cancer patients (AYA). Psychooncology 2018, 27, 1802–1809. [Google Scholar] [CrossRef]
- Barnett, M.; McDonnell, G.; DeRosa, A.; Schuler, T.; Philip, E.; Peterson, L.; Touza, K.; Jhanwar, S.; Atkinson, T.M.; Ford, J.S. Psychosocial outcomes and interventions among cancer survivors diagnosed during adolescence and young adulthood (AYA): A systematic review. J. Cancer Surviv. 2016, 10, 814–831. [Google Scholar] [CrossRef]
- Zucchetti, G.; Bellini, S.; Bertolotti, M.; Bona, F.; Biasin, E.; Bertorello, N.; Tirtei, E.; Fagioli, F. Body Image Discomfort of Adolescent and Young Adult Hematologic Cancer Survivors. J. Adolesc. Young Adult Oncol. 2017, 6, 377–380. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Wen, Y.; Wang, H.; Sun, H.; Liang, W.; Zhang, B.; Humphris, G. Fear of cancer recurrence in adolescent and young adult cancer survivors: A systematic review of the literature. Psychooncology 2019, 28, 675–686. [Google Scholar] [CrossRef] [PubMed]
- van Dorp, W.; Haupt, R.; Anderson, R.A.; Mulder, R.L.; van den Heuvel-Eibrink, M.M.; van Dulmen-den Broeder, E.; Su, H.I.; Winther, J.F.; Hudson, M.M.; Levine, J.M.; et al. Reproductive Function and Outcomes in Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Review. J. Clin. Oncol. 2018, 36, 2169–2180. [Google Scholar] [CrossRef]
- Chao, C.; Bhatia, S.; Xu, L.; Cannavale, K.L.; Wong, F.L.; Huang, P.S.; Cooper, R.; Armenian, S.H. Chronic Comorbidities among Survivors of Adolescent and Young Adult Cancer. J. Clin. Oncol. 2020, 38, 3161–3174. [Google Scholar] [CrossRef]
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.; Myklebust, T.A.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Wee, M.; Dau, H.; Gastonguay, L. How do individuals with colorectal cancer perceive the term “cancer survivor”: A cross-sectional survey. J. Cancer Surviv. 2022, 16, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gerstl, B.; Sullivan, E.; Chong, S.; Chia, D.; Wand, H.; Anazodo, A. Reproductive Outcomes after a Childhood and Adolescent Young Adult Cancer Diagnosis in Female Cancer Survivors: A Systematic Review and Meta-analysis. J. Adolesc. Young Adult Oncol. 2018, 7, 627–642. [Google Scholar] [CrossRef] [PubMed]
- DePauw, S.; Rae, C.; Schacter, B.; Rogers, P.; Barr, R.D. Evolution of adolescent and young adult oncology in Canada. Curr. Oncol. 2019, 26, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- How to Integrate Sex and Gender into Research. Available online: https://cihr-irsc.gc.ca/e/50836.html (accessed on 1 March 2022).
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef]
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 1 January 2022).
- StataCorp. Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Reproductive Health. Available online: https://www.niehs.nih.gov/health/topics/conditions/repro-health/index.cfm (accessed on 1 January 2022).
- Wells, G.A.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch; Losos, M.; Tugwell, P.; Ga, S.W.; et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 January 2022).
- McPheeters, M.L.; Kripalani, S.; Peterson, N.B.; Idowu, R.T.; Jerome, R.N.; Potter, S.A.; Andrews, J.C. Closing the quality gap: Revisiting the state of the science (vol. 3: Quality improvement interventions to address health disparities). Evid. Rep. Technol. Assess. (Full Rep.) 2012, 2083, 1–475. [Google Scholar]
- Herzog, R.; Alvarez-Pasquin, M.J.; Diaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; (updated February 2022); Cochrane, Ed.; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- World Bank Country and Lending Groups—World Bank Data Help Desk. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 26 July 2022).
- Velez, M.P.; Richardson, H.; Baxter, N.N.; McClintock, C.; Greenblatt, E.; Barr, R.; Green, M. Risk of infertility in female adolescents and young adults with cancer: A population-based cohort study. Hum. Reprod. 2021, 36, 1981–1988. [Google Scholar] [CrossRef]
- Kao, W.H.; Kuo, C.F.; Chiou, M.J.; Liu, Y.C.; Wang, C.C.; Hong, J.H.; Hsu, J.T.; Chiang, Y.J.; Chuang, Y.F. Adverse birth outcomes in adolescent and young adult female cancer survivors: A nationwide population-based study. Br. J. Cancer 2020, 122, 918–924. [Google Scholar] [CrossRef]
- Lee, B.C.; Yen, R.F.; Lin, C.L.; Liang, J.A.; Lin, M.C.; Kao, C.H. Pregnancy Incidence in Female Nasopharyngeal Carcinoma Survivors of Reproductive Age: A Population-Based Study. Medicine 2016, 95, e3729. [Google Scholar] [CrossRef]
- Magelssen, H.; Melve, K.K.; Skjaerven, R.; Fossa, S.D. Parenthood probability and pregnancy outcome in patients with a cancer diagnosis during adolescence and young adulthood. Hum. Reprod. 2008, 23, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Stensheim, H.; Cvancarova, M.; Moller, B.; Fossa, S.D. Pregnancy after adolescent and adult cancer: A population-based matched cohort study. Int. J. Cancer 2011, 129, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Stensheim, H.; Klungsoyr, K.; Skjaerven, R.; Grotmol, T.; Fossa, S.D. Birth outcomes among offspring of adult cancer survivors: A population-based study. Int. J. Cancer 2013, 133, 2696–2705. [Google Scholar] [CrossRef]
- Seppanen, V.I.; Artama, M.S.; Malila, N.K.; Pitkaniemi, J.M.; Rantanen, M.E.; Ritvanen, A.K.; Madanat-Harjuoja, L.M. Risk for congenital anomalies in offspring of childhood, adolescent and young adult cancer survivors. Int. J. Cancer 2016, 139, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.F.; Schmidt, A.A.; Mulvihill, J.J.; Frederiksen, K.; Tawn, E.J.; Stovall, M.; Johansen, C.; Boice, J.D., Jr.; Winther, J.F. Chromosomal Abnormalities in Offspring of Young Cancer Survivors: A Population-Based Cohort Study in Denmark. J. Natl. Cancer Inst. 2018, 110, 534–538. [Google Scholar] [CrossRef]
- Jacob, L.; Kalder, M.; Arabin, B.; Kostev, K. Impact of prior breast cancer on mode of delivery and pregnancy- associated disorders: A retrospective analysis of subsequent pregnancy outcomes. J. Cancer Res. Clin. Oncol. 2017, 143, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, B.W.; Park, S.; Park, S.; Lee, J.E.; Choi, Y.J.; Kim, S.Y.; Woo, S.U.; Youn, H.J.; Lee, I. Childbirth in young Korean women with previously treated breast cancer: The SMARTSHIP study. Breast Cancer Res. Treat. 2019, 176, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Haggar, F.A.; Pereira, G.; Preen, D.; Holman, C.D.; Einarsdottir, K. Adverse obstetric and perinatal outcomes following treatment of adolescent and young adult cancer: A population-based cohort study. PLoS ONE 2014, 9, e113292. [Google Scholar] [CrossRef]
- Ji, J.; Sundquist, J.; Sundquist, K. Congenital malformation in offspring of female cancer survivors: A national cohort study. Eur. J. Cancer Prev. 2018, 27, 274–278. [Google Scholar] [CrossRef]
- Medica, A.C.O.; Stark, S.S.; Hadnott, T.N.; Dietz, A.C.; Romero, S.A.D.; Natarajan, L.; Martinez, E.; Whitcomb, B.W.; Su, H.I. Use of emergency contraception among female young adult cancer survivors. Fertil. Steril. 2018, 109, 1114–1120.e1111. [Google Scholar] [CrossRef]
- Anderson, C.; Engel, S.M.; Anders, C.K.; Nichols, H.B. Live birth outcomes after adolescent and young adult breast cancer. Int. J. Cancer 2018, 142, 1994–2002. [Google Scholar] [CrossRef]
- Smaldone, G.M.; Krohn, M.A.; McGee, E.A. Cervical cancer and risk for delivery of small-for-gestational age neonates. J. Womens Health 2010, 19, 969–974. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Mertens, A.C.; Kramer, M.R.; Lash, T.L.; Spencer, J.B.; Ward, K.C.; Howards, P.P. Pregnancy after cancer: Does timing of conception affect infant health? Cancer 2018, 124, 4401–4407. [Google Scholar] [CrossRef]
- Anderson, C.; Engel, S.M.; Mersereau, J.E.; Black, K.Z.; Wood, W.A.; Anders, C.K.; Nichols, H.B. Birth Outcomes Among Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017, 3, 1078–1084. [Google Scholar] [CrossRef]
- Anderson, C.; Smitherman, A.B.; Engel, S.M.; Nichols, H.B. Modifiable and non-modifiable risk factors for preterm delivery among adolescent and young adult cancer survivors. Cancer Causes Control 2018, 29, 289–295. [Google Scholar] [CrossRef]
- Farland, L.V.; Stern, J.E.; Hwang, S.S.; Liu, C.L.; Cabral, H.; Knowlton, R.; Gershman, S.T.; Coddington, C.C.; Missmer, S.A. Early-life cancer, infertility, and risk of adverse pregnancy outcomes: A registry linkage study in Massachusetts. Cancer Causes Control. 2021, 32, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, K.P.; Ward, K.C.; Kramer, M.R.; Lash, T.L.; Mertens, A.C.; Spencer, J.B.; Fothergill, A.; Howards, P.P. The risk of preterm birth and growth restriction in pregnancy after cancer. Int J Cancer. 2017, 141, 2187–2196. [Google Scholar] [CrossRef]
- Mitani, A.A.; Haneuse, S. Small Data Challenges of Studying Rare Diseases. JAMA Netw. Open 2020, 3, e201965. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, D.; Trott, M.; Grabovac, I.; Yang, L.; Barnett, Y.; Parris, C.; McDermott, D.T.; Veronese, N.; Kronbichler, A.; Ghayda, R.A.; et al. Ejaculation Disorders in Male Patients with Cancer: A Systematic Review and Meta-Analysis of Prevalence. J. Urol. 2021, 206, 1361–1372. [Google Scholar] [CrossRef]
- Kenney, L.B.; Antal, Z.; Ginsberg, J.P.; Hoppe, B.S.; Bober, S.L.; Yu, R.N.; Constine, L.S.; van Santen, H.M.; Skinner, R.; Green, D.M. Improving Male Reproductive Health After Childhood, Adolescent, and Young Adult Cancer: Progress and Future Directions for Survivorship Research. J. Clin. Oncol. 2018, 36, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of female survivors of childhood cancer: A report from the childhood cancer survivor study. J. Clin. Oncol. 2009, 27, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Nolan, V.G.; Kawashima, T.; Stovall, M.; Donaldson, S.S.; Srivastava, D.; Leisenring, W.; Robison, L.L.; Sklar, C.A. Decreased fertility among female childhood cancer survivors who received 22-27 Gy hypothalamic/pituitary irradiation: A report from the Childhood Cancer Survivor Study. Fertil. Steril. 2011, 95, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Safer, J.D.; Coleman, E.; Feldman, J.; Garofalo, R.; Hembree, W.; Radix, A.; Sevelius, J. Barriers to healthcare for transgender individuals. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Mprah, A. Sexual and Reproductive Health needs of LGBT. Afr. J. Reprod. Health 2016, 20, 16–20. [Google Scholar] [CrossRef]
- Quinn, G.P.; Sanchez, J.A.; Sutton, S.K.; Vadaparampil, S.T.; Nguyen, G.T.; Green, B.L.; Kanetsky, P.A.; Schabath, M.B. Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA Cancer J. Clin. 2015, 65, 384–400. [Google Scholar] [CrossRef]
- Richards, N.; Pandolfelli, L.; Bouziani, B.; Ofosu-Baadu, B.; Carter, K. The role of administrative data in gender statistics: Supporting inclusive development for women and girls. J. Int. Dev. 2022, 34, 349–378. [Google Scholar] [CrossRef]





| Search Line | Search Term | Hits |
|---|---|---|
| 1 | Young adult/ | 442,421 |
| 2 | Adolescent/ | 1,642,976 |
| 3 | (young adult* or teen* or adolescen* or youth*).ti,ab,kw. | 609,445 |
| 4 | 1 or 2 or 3 | 2,127,993 |
| 5 | exp Neoplasm/or exp Cancer Radiotherapy/or exp Antineoplastic agent/ or exp Early cancer diagnosis/or exp cancer chemotherapy/ | 6,205,318 |
| 6 | (Chemotherap* or “cancer treatment*” or radiation or brachytherap* or “antineoplastic agent*” or “antitumor* drug*” or “antitumor* agent*” or antineoplastics* or “anticancer* agent**” or “anticancer* drug*” or “early detection of cancer” or “oncolog* surger*”).ti,ab,kw. | 1,297,180 |
| 7 | 5 or 6 | 6,527,287 |
| 8 | exp reproductive health/or exp spontaneous abortion/or exp stillbirth/or exp birth weight/ or exp small for date infant/or exp prematurity/ or exp obstetric delivery/or exp cesarean section/or exp forceps delivery/or exp vacuum extraction/or exp pregnancy diabetes mellitus/or exp maternal hypertension/ or exp “eclampsia and preeclampsia”/or exp preeclampsia/or exp HELLP syndrome/ or exp postnatal depression/or exp labor complication/or exp perinatal death/or exp perinatal mortality/ or exp fetus death/or exp pregnancy complication/or drug induced malformation/or radiation induced malformation/ | 603,279 |
| 9 | (“Reproductive health outcome*” or “pregnancy loss*” or miscarriage* or “spontaneous abortion*” or stillbirth* or “still birth*” or “fetal death*” or “Perinatal death*” or “low birth weight*” or “low birthweight*” or “neonatal underweight” or “small for gestational age*” or “premature birth*” or “preterm birth*” or prematur* or pre-matur* or “pre-term birth*” or “pre-mature birth*” or C-section* or “cesarean section*” or “vaginal deliver*” or “forceps deliver*” or “vacuum extraction*” or “natural deliver*” or “gestational hypertens*” or “pregnancy-induced hypertens*” or “pregnancy transient hypertens*” or “pregnancy-induced diabetes” or “gestational diabetes” or “pre eclampsia” or “preeclampsia” or “pregnancy toxemia” or “pre-eclampsia” or “HELLP syndrome” or “hemolysis elevated liver enzymes and low platelets syndrome” or “postnatal depression” or “postpartum depression” or “postpartum anxiety” or “postnatal anxiety” or “pregnancy anxiety” or “pregnancy depression” or “perinatal anxiety” or “perinatal depression” or “pregnancy complication” or “Congenital Abnormalit*” or “Congenital malformation*” or “Congenital Defect*” or “Fetal Malformation*” or “Fetal Anomal*” or “Birth defect*” or “Congenital anomal*” or “Development anomal*” or “Obstetric Labor Complication*” or “Labor Complication*”).ti,ab,kw. | 537,559 |
| 10 | 8 or 9 | 844,485 |
| 11 | 4 and 7 and 10 | 7022 |
| 12 | limit 11 to yr = “2000–Current” | 6226 |
| 13 | limit 12 to “humans only (removes records about animals)” | 6165 |
| 14 | limit 13 to embase | 4356 |
| Search Line | Search Term | Hits |
|---|---|---|
| 1 | Young adult/ | 975,082 |
| 2 | Adolescent/ | 2,154,258 |
| 3 | (young adult* or teen* or adolescen* or youth*).ti,ab,kw. | 486,342 |
| 4 | 1 or 2 or 3 | 2,751,081 |
| 5 | exp Neoplasms/or exp Radiotherapy/or exp Antineoplastic agents/ or exp “Early Detection of Cancer”/ | 4,271,519 |
| 6 | (Chemotherap* or “cancer treatment*” or radiation or brachytherap* or “antineoplastic agent*” or “antitumor* drug*” or “antitumor* agent*” or antineoplastics* or “anticancer* agent**” or “anticancer* drug*” or “early detection of cancer” or “oncolog* surger*”).ti,ab,kw. | 905,198 |
| 7 | 5 or 6 | 4,604,154 |
| 8 | exp Reproductive Health/or exp Spontaneous Abortion/or exp Stillbirth/or exp Birth Weight/or exp Infant, Small for Gestational Age/or exp Premature Birth/or exp Premature Infant/or exp Infant, Extremely Premature/or exp Delivery, Obstetric/or exp Cesarean Section/or exp Extraction, Obstetrical/or exp Vacuum extraction, Obstetrical/or exp Obstetrical Forceps/or exp Diabetes, Gestational/ or exp Hypertension, pregnancy-induced/or exp Pre-Eclampsia/or exp HELLP Syndrome/or exp Depression, post-partum/or exp Obstetric Labor Complications/or exp perinatal death/or exp pregnancy complications/or abnormalities, drug-induced/or abnormalities, radiation-induced/ | 586,641 |
| 9 | (“Reproductive health outcome*” or “pregnancy loss*” or miscarriage* or “spontaneous abortion*” or stillbirth* or “still birth*” or “fetal death*” or “Perinatal death*” or “low birth weight*” or “low birthweight*” or “neonatal underweight” or “small for gestational age*” or “premature birth*” or “preterm birth*” or prematur* or pre-matur* or “pre-term birth*” or “pre-mature birth*” or C-section* or “cesarean section*” or “vaginal deliver*” or “forceps deliver*” or “vacuum extraction*” or “natural deliver*” or “gestational hypertens*” or “pregnancy-induced hypertens*” or “pregnancy transient hypertens*” or “pregnancy-induced diabetes” or “gestational diabetes” or “pre eclampsia” or “preeclampsia” or “pregnancy toxemia” or “pre-eclampsia” or “HELLP syndrome” or “hemolysis elevated liver enzymes and low platelets syndrome” or “postnatal depression” or “postpartum depression” or “postpartum anxiety” or “postnatal anxiety” or “pregnancy anxiety” or “pregnancy depression” or “perinatal anxiety” or “perinatal depression” or “pregnancy complication” or “Congenital Abnormalit*” or “Congenital malformation*” or “Congenital Defect*” or “Fetal Malformation*” or “Fetal Anomal*” or “Birth defect*” or “Congenital anomal*” or “Development anomal*” or “Obstetric Labor Complication*” or “Labor Complication*”).ti,ab,kw. | 399,110 |
| 10 | 8 or 9 | 798,565 |
| 11 | 4 and 7 and 10 | 8746 |
| 12 | limit 11 to yr = “2000–Current” | 5382 |
| 13 | limit 12 to “humans only (removes records about animals)” | 5324 |
| Study | Country | Study Design | Follow-Up Timeline (Years) | Sex (% Female) | Data Cancer a | Type of Cancer b | AYA N | AYA Age at Diagnosis (yr) | AYA Age at Study (yr) | Quality Assessment c |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson 2017a [43] | United States | Cohort | 14 | 100 | North Carolina Central Cancer Registry | Any | 1980 | Average years between diagnosis and birth = 3.5 ± 2.4 | 31.2 ± 5.3 | 7 = Good |
| Anderson 2017b [42] | United States | Cohort | 14 | 100 | North Carolina Central Cancer Registry | Any | 2598 | 28.1 ± 5.5 | 31.1 ± 5.3 | 8 = Good |
| Anderson 2018 [39] | United States | Cohort | 14 | 100 | North Carolina Central Cancer Registry | Breast cancer | 338 | 35 ± 3.7 | 35.1 ± 4.3 | 8 = Good |
| Chao 2020 [9] | United States | Cohort | 2 | 65 | Kaiser Permanente Southern California SEER d affiliated cancer registry | Any | 6778 | 31.3 ± 6.5 | Age: Number of participants (%): 15–19: 521 (7.7%) 20–29: 1706 (25.2%) 30–39: 4551 (67.1%) | 8 = Good |
| Farland 2020 [44] | United States | Cohort | 9 | 100 | Massachusetts Cancer Registry | Any | 2983 | Age: Number of participants (%) = <15: 54 (2.2%) 15–26: 802 (33.1%) >26: 1566 (64.7%) | 33.6 ± 5.2 | 7 = Good |
| Haggar 2014 [36] | Australia | Cohort | 25 | 100 | Western Australian Data Linkage System | Any | 1894 | Age: Number of participants (%) = 15–19: 739 (39%) 20–29: 98 (52%) 30–39: 170 (9%) | Age: Number of participants (%): 15–19: 193 (10%) 20–29: 841 (44%) 30–34: 550 (29%) ≥35: 310 (16%) | 8 = Good |
| Hartnett 2017 [45] | United States | Cohort | Georgia: 18 North Carolina: 14 Tennessee: 9 | 100 | Cancer registries in the states of Georgia, North Carolina, and Tennessee | Any | 4203 | Between 20–45 | Age: Number of participants (%): 20–24: 250 (5.9%) 25–29: 1084 (26%) 30–34: 1479 (35%) 35–39: 1091 (26%) 40–45: 299 (7.1%) | 8 = Good |
| Hartnett 2018 [41] | United States | Cohort | Georgia: 18 North Carolina: 14 Tennessee: 9 | 100 | Cancer registries in the states of Georgia, North Carolina, and Tennessee. Subset of participants from Furthering Understanding of Cancer, Health, and Survivorship in Adult (FUCHSIA) Women’s Study | Any | 4203 | Age: Number of participants (%) = 20–24: 910 (22%) 25–29: 1412 (34%) 30–34: 1283 (31%) 35–39: 532 (13%) 40–45: 66 (2%) | Age: Number of participants (%): 20–24: 251 (6%) 25–29: 1084 (26%) 30–34: 1480 (35%) 35–39: 1089 (26%) 40–45: 299 (7%) | 8 = Good |
| Jacob 2017 [34] | Germany | Cohort | 14 | 100 | Disease Analyzer database (IMS Health) | Breast cancer | 165 | Interval between breast cancer diagnosis and first pregnancy was 18 months, with a minimum of 6 months and a maximum of 10 yrs | 34.6 ± 5.2 | 7 = Good |
| Ji 2018 [37] | Sweden | Cohort | 52 | 100 | Swedish Cancer Registry | Any | 9266 | Not reported | Median (range): 33 (16–46) | 6 = Good |
| Kao 2020 [27] | Taiwan | Cohort | 10 | 100 | Taiwan Birth Reporting System and National Health Insurance database | Any | 3531 | Median: 27.1 | Age: Number of participants (%): 15–24: 148 (3.3) 25–34: 28820 (63.4) ≥35 (max. 48): 1517 (33.4) | 8 = Good |
| Lee 2016 [28] | Taiwan | Cohort | 12 | 100 | Taiwan National Health Insurance Research database | Nasopharyngeal carcinoma | 155 | Not reported | Age: Number of participants/overall: 15–24: 21/155 25–34: 95/155 35–44: 37/155 ≥45: 2/155 | 8 = Good |
| Lee 2019 [35] | South Korea | Cohort | 6 | 100 | National Health Information Database from the Korean National Health Insurance Service | Breast cancer | 855 | 34.9 ± 3.8 | Age: Number of participants (%): 20–29: 745 (87.1) 30–39: 110 (12.9) | 8 = Good |
| Magelssen 2007 [29] | Norway | Cohort | Substudy 1: 11 Substudy 2: 37 | 38 | Cancer Registry in Norway | Any | 747 | Substudy 1: Male: 22 (15–30) Female: 22 (15–31) Substudy 2: Group 1: Male: 25 (15–35) Female: 24 (15–35) Group 2: Male: 29 (21–35) Female: 28 (19–36) | Male: 27 (17–36) Female: 25 (17–35) | 6 = Good |
| Medica 2018 [38] | United States | Cross-sectional | Not applicable | 100 | Reproductive Window Study and National Survey of Family Growth (2006–2010) | Any | 616 | Mean (SD) years since cancer diagnosis: 7.5 ± 5.3 | Age: Number of participants (%): 18–24: 35 (5.8%) 25–30: 138 (23%) 31–35: 215 (35.8%) 36–40: 213 (35.4%) | 3 = Poor |
| Nielsen 2017 [33] | Denmark | Cohort | 34 | 44 | Danish Cancer Registry | Any | 8945 | Age (%): <35: 80.3% ≥35: 19.7% | Not reported | 8 = Good |
| Seppanen 2016 [32] | Finland | Cohort | 51 | 49 | Finnish Cancer Registry | Any | 6862 | 0–34 | Age: Number of participants (%) <20: 718 (5.1%) 20–24: 3604 (25.4%) 25–29: 5221 (36.7%) 30–34: 3389 (23.8%) 35+: 1275 (9%) | 8 = Good |
| Smaldone 2010 [40] | United States | Cohort | 17 | 100 | University of Pittsburgh Medical Center Network Cancer Registry | Cervical | 135 | Not reported | Age: Number of participants (%) <24: 17725 (22.7%) 25–29: 19834 (25.4%) 30–34: 24831 (31.8%) ≥35: 15617 (20%) | 7 = Good |
| Stensheim 2011 [30] | Norway | Cohort | 37 | 58 | Cancer Registry of Norway | Any | 27556 | Median: Male: 32 Female: 36 | Median observation (range): Male: 6.2 (0–29.8) Female: 5.0 (0–29.8) | 8 = Good |
| Stensheim 2013 [31] | Norway | Cohort | 37 | 47 | Cancer Registry of Norway | Any | 3915 | Female: Nulliparous: 24.0 ± 5.1 Primiparous: 27.3 ± 4.5 | Female: Nulliparous: 29.1 ± 4.9 Primiparous: 31.1 ± 4.4 | 8 = Good |
| Male: Nulliparous: 25.1 ± 5.0 Primiparous: 28.9 ± 5.0 | Male: Nulliparous: 30.7 ± 4.9 Primiparous: 32.7 ± 5.0 | |||||||||
| Velez 2021 [26] | Canada | Cohort | 9 | 100 | Ontario Cancer Registry | Any | 14316 | 31.4 ± 6.3 | Median follow-up time (SD): 13.1 ± 0.08 | 8 = Good |
| Outcome | Study | Crude Event Rates Reported? (Y/N) | Crude Estimate (95% Confidence Interval) | Adjusted Estimate (95% Confidence Interval) |
|---|---|---|---|---|
| MATERNAL HEALTH OUTCOMES (n = 11 outcomes) | ||||
| Before pregnancy (n = 3 outcomes) | ||||
| Emergency contraception use | ||||
| 1 | Medica 2018 [38] | Y | OR 2.09 (1.82, 1.39) | - b |
| Known or suspected abnormality of pelvic organs | ||||
| 1 | Jacob 2017 [34] | Y | OR 1.00 (0.41, 2.47) | - |
| Premature ovarian failure | - | |||
| 1 | Chao 2020 [9] | Y | OR 3.12 (1.70, 5.72) a | IRR 2.87 (1.56, 5.28) |
| During pregnancy (n = 4 outcomes) | ||||
| Preeclampsia | ||||
| 1 | Farland 2020 [44] | Y | RR 1.08 (0.95, 1.22) | RR 1.05 (0.92, 1.19) |
| 2 | Haggar 2014 [36] | Y | OR 1.37 (1.01, 1.86) a | RR 1.44 (1.13, 1.87) |
| 3 | Jacob 2017 [34] | Y | OR 2.54 (0.49, 13.32) | - |
| 4 | Lee 2016 [28] | Y | OR 3.27 (0.29, 36.3) | OR 3.48 (0.31, 39.1) |
| 5 | Lee 2019 [35] | Y | OR 0.64 (0.28, 1.46) a | OR 0.61 (0.27, 1.40) |
| 6 | Stensheim 2013 [31] | Y | OR 1.55 (1.34, 1.80) a | - |
| Gestational diabetes | ||||
| 1 | Farland 2020 [44] | Y | RR 1.29 (1.13, 1.48) | RR 1.08 (0.94, 1.23) |
| 2 | Haggar 2014 [36] | Y | OR 2.75 (2.05, 3.70) a | RR 1.38 (1.09, 2.98) |
| 3 | Jacob 2017 [34] | Y | OR 1.48 (0.61, 3.57) | - |
| 4 | Kao 2020 [27] | Y | OR 1.13 (0.96, 1.34) a | - |
| 5 | Lee 2016 [28] | Y | OR 0.80 (0.27, 2.40) | OR 0.79 (0.26, 2.40) |
| 6 | Smaldone 2010 [40] | Y | RR 0.61 (0.15, 2.46) | - |
| Gestational hypertension | ||||
| 1 | Lee 2016 [28] | Y | OR 3.33 (0.82, 13.5) | OR 3.30 (0.79, 13.8) |
| 2 | Smaldone 2010 [40] | Y | RR 0.95 (0.52, 1.72) | - |
| Maternal anemia | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.18 (0.69, 2.00) a | RR 1.31 (0.71, 2.19) |
| After delivery (n = 4 outcomes) | ||||
| Postpartum hemorrhage | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.05 (0.81, 1.34) a | RR 1.08 (0.82, 1.56) |
| Retained placenta | ||||
| 1 | Haggar 2014 [36] | Y | OR 0.97 (0.71, 1.33) a | RR 0.98 (0.73, 1.34) |
| Postpartum length of stay >5 days | ||||
| 1 | Haggar 2014 [36] | Y | OR 2.85 (2.33, 3.48) | RR 3.01 (1.72, 5.58) |
| Genito-urinary tract infections | ||||
| 1 | Jacob 2017 [34] | Y | OR 0.53 (0.19, 1.46) | - |
| FETAL/NEONATAL HEALTH OUTCOMES (n = 26 outcomes) | ||||
| Intrauterine (n = 3 outcomes) | ||||
| Intrauterine growth restriction | ||||
| 1 | Haggar 2014 [36] | Y | OR 2.88 (2.19, 3.80) | RR 1.21 (0.97, 2.06) |
| Intrauterine death | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.03 (0.70, 1.51) a | RR 1.07 (0.86, 1.65) |
| Suspected poor fetal growth | ||||
| 1 | Jacob 2017 [34] | Y | OR 2.11 (0.88–5.07) | - |
| Delivery (n = 2 outcomes) | ||||
| Low APGAR score at birth | ||||
| 1 | Anderson 2017b [42] | Y | OR 1.16 (0.86, 1.56) a | PR 1.18 (0.87, 1.61) |
| 2 | Haggar 2014 [36] | Y | OR 3.59 (2.84, 4.53) | RR 2.83 (2.28, 3.56) |
| 3 | Hartnett 2017 [45] | N | Could not pool | - |
| 4 | Kao 2020 [27] | Y | OR 1.19 (0.90, 1.57) | OR 1.14 (0.86, 1.51) |
| 5 | Stensheim 2013 [31] | Y | OR 1.22 (0.92, 1.62) a | - |
| Resuscitation | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.83 (1.48, 2.26) | RR 1.66 (1.27, 2.19) |
| After delivery (n = 21 outcomes) | ||||
| Preterm birth | ||||
| 1 | Anderson 2017a [43] | Y | RR 1.17 (1.01, 1.35) | RR 1.24 (1.07, 1.43) |
| 2 | Anderson 2017b [42] | Y | OR 1.53 (1.34, 1.74) a | PR 1.52 (1.34, 1.71) |
| 3 | Anderson 2018 [39] | Y | OR 1.18 (0.82, 1.68) a | PR 1.10 (0.78, 1.54) |
| 4 | Farland 2020 [44] | Y | RR 1.30 (1.16, 1.46) | RR 1.19 (1.07, 1.32) |
| 5 | Haggar 2014 [36] | Y | OR 1.60 (1.36, 1.88) a | RR 1.68 (1.21, 2.08) |
| 6 | Hartnett 2017 [45] | Y | OR 1.24 (1.13, 1.36) a | - |
| 7 | Hartnett 2018 [41] | N | Could not pool | - |
| 8 | Jacob 2017 [34] | Y | OR 2.01 (0.18, 22.41) | - |
| 9 | Kao 2020 [27] | Y | OR 1.16 (1.04, 1.29) | OR 1.12 (1.00, 1.25) |
| 10 | Lee 2016 [28] | Y | OR 1.96 (0.91, 4.24) | OR 2.03 (0.92, 4.45) |
| 11 | Lee 2019 [35] | Y | OR 1.04 (0.81, 1.33) a | OR 1.02 (0.80, 1.31) |
| 12 | Magelssen 2007 [29] | Y | OR 1.47 (1.14, 1.89) a | - |
| 13 | Smaldone 2010 [40] | Y | RR 1.48 (0.94, 2.34) | - |
| 14 | Stensheim 2013 [31] | Y | OR 1.30 (1.14, 1.48) a | - |
| Low birthweight | ||||
| 1 | Anderson 2017b [42] | Y | OR 1.51 (1.31, 1.75) a | PR 1.59 (1.38, 1.83) |
| 2 | Anderson 2018 [39] | Y | OR 1.29 (0.88, 1.90) a | PR 1.11 (0.77, 1.61) |
| 3 | Farland 2020 [44] | Y | RR 1.27 (1.13, 1.43) | RR 1.19 (1.071.32) |
| 4 | Haggar 2014 [36] | Y | OR 1.72 (1.44, 2.04) a | RR 1.51 (1.23, 2.12) |
| 5 | Hartnett 2017 [45] | Y | OR 1.21 (1.08, 1.37) a | - |
| 6 | Hartnett 2018 [41] | N | Could not pool | - |
| 7 | Kao 2020 [27] | Y | OR 1.19 (1.06, 1.34) | OR 1.15 (1.02, 1.30) |
| 8 | Lee 2016 [28] | Y | OR 1.63 (0.33, 8.19) | OR 1.71 (0.33, 8.89) |
| 9 | Magelssen 2007 [29] | Y | OR 1.23 (0.90, 1.68) a | - |
| 10 | Smaldone 2010 [40] | Y | RR 1.65 (1.03, 2.65) | - |
| 11 | Stensheim 2013 [31] | Y | OR 1.29 (1.11, 1.49) a | - |
| Small for gestational age | ||||
| 1 | Anderson 2017b [42] | Y | OR 0.88 (0.76, 1.02) | PR 0.97 (0.85, 1.11) |
| 2 | Anderson 2018 [39] | Y | OR 1.15 (0.80, 1.65) a | PR 1.02 (0.72, 1.45) |
| 3 | Farland 2020 [44] | Y | RR 0.97 (0.85, 1.10) | RR 1.02 (0.89, 1.16) |
| 4 | Hartnett 2017 [45] | Y | OR 0.92 (0.83, 1.02) a | - |
| 5 | Hartnett 2018 [41] | N | Could not pool | - |
| 6 | Kao 2020 [27] | Y | OR 1.08 (0.97, 1.20) | OR 1.07 (0.96, 1.19) |
| 7 | Smaldone 2010 [40] | Y | RR 1.54 (1.00, 2.46) | - |
| Congenital anomalies | ||||
| 1 | Haggar 2014 [36] | Y | OR 0.79 (0.41, 1.54) a | RR 0.78 (0.41, 1.37) |
| 2 | Ji 2018 [37] | Y | OR 1.15 (1.07, 1.24) | OR 1.11 (1.04, 1.20) |
| 3 | Kao 2020 [27] | Y | OR 1.03 (0.91, 1.18) | OR 1.01 (0.89, 1.15) |
| 4 | Magelssen 2007 [29] | Y | OR 1.02 (0.90, 1.16) a | - |
| 5 | Nielsen 2017 [33] | Y | OR 1.16 (0.86, 1.56) | OR 0.99 (0.67, 1.44) |
| 6 | Seppanen 2016 [32] | Y | OR 1.17 (0.98, 1.40) a | PR 1.01 (0.83, 1.23) |
| 7 | Smaldone 2010 [40] | Y | RR 0.33 (0.08, 1.35) | - |
| 8 | Stensheim 2013 [31] | Y | OR 1.17 (0.98, 1.40) a | - |
| Low birthweight at term | ||||
| 1 | Hartnett 2017 [45] | N | Could not pool | - |
| 2 | Hartnett 2018 [41] | N | Could not pool | - |
| 3 | Stensheim 2013 [31] | Y | OR 1.28 (0.92, 1.47) | - |
| Very preterm birth | ||||
| 1 | Anderson 2017b [42] | Y | OR 1.80 (1.44, 2.26) a | PR 2.03 (1.62, 2.55) |
| 2 | Hartnett 2017 [45] | N | Could not pool | - |
| 3 | Stensheim 2013 [31] | Y | OR 1.22 (0.88, 1.70) a | - |
| Perinatal death | ||||
| 1 | Magelssen 2007 [29] | Y | OR 0.60 (0.32, 1.12) a | - |
| 2 | Smaldone 2010 [40] | Y | RR 3.63 (0.90, 14.7) | - |
| 3 | Stensheim 2013 [31] | Y | OR 0.72 (0.51, 1.03) a | - |
| Neonatal mortality | ||||
| 1 | Farland 2020 [44] | Y | RR 1.55 (0.86, 2.79) | RR 1.30 (0.75, 2.25) |
| 2 | Haggar 2014 [36] | Y | OR 1.01 (0.59, 1.75) a | RR 1.03 (0.54, 1.71) |
| Admission to special/intensive care | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.44 (1.11, 1.86) a | RR 1.44 (1.13, 1.78) |
| 2 | Hartnett 2017 [45] | N | Could not pool | - |
| Sex ratio | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.04 (0.93, 1.16) a | RR 1.05 (0.98, 1.10) |
| 2 | Smaldone 2010 [40] | Y | RR 0.92 (0.66, 1.30) | - |
| Large for gestational age | ||||
| 1 | Kao 2020 [27] | Y | OR 1.03 (0.93, 1.14) | OR 1.03 (0.93, 1.14) |
| Stillbirth | ||||
| 1 | Kao 2020 [27] | Y | OR 1.05 (0.76, 1.45) | OR 1.01 (0.74, 1.40) |
| High birthweight | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.25 (1.04, 1.50) a | RR 1.33 (0.99, 1.71) |
| Very low birthweight | ||||
| 1 | Hartnett 2017 [45] | Y | OR 1.65 (1.34, 2.04) a | - |
| Neonatal prolonged hospital stay | ||||
| 1 | Farland 2020 [44] | Y | RR 1.19 (1.03, 1.38) | RR 1.16 (1.01, 1.34) |
| Infectious disease conditions | ||||
| 1 | Farland 2020 [44] | Y | RR 1.12 (0.86, 1.46) | RR 1.04 (0.81, 1.33) |
| Cardiovascular disease conditions | ||||
| 1 | Farland 2020 [44] | Y | RR 1.09 (0.85, 1.39) | RR 0.90 (0.71, 1.14) |
| Respiratory conditions | ||||
| 1 | Farland 2020 [44] | Y | RR 1.19 (1.07, 1.33) | RR 1.04 (0.94, 1.14) |
| Gastrointestinal conditions | ||||
| 1 | Farland 2020 [44] | Y | RR 1.43 (1.22, 1.68) | RR 1.17 (1.02, 1.35) |
| Neurologic conditions | ||||
| 1 | Farland 2020 [44] | Y | RR 1.03 (0.84, 1.26) | RR 1.06 (0.87, 1.29) |
| Hematologic conditions | ||||
| 1 | Farland 2020 [44] | Y | RR 1.10 (0.93, 1.30) | RR 0.98 (0.84, 1.14) |
| FETAL/NEONATAL-MATERNAL HEALTH OUTCOMES (n = 23 outcomes) | ||||
| During pregnancy (n = 13 outcomes) | ||||
| Antepartum hemorrhage | ||||
| 1 | Haggar 2014 [36] | Y | OR 0.91 (0.51, 1.60) a | RR 0.92 (0.59, 1.78) |
| 2 | Lee 2016 [28] | Y | OR 0.81 (0.20, 3.27) | OR 1.07 (0.25, 4.55) |
| Spontaneous abortion | ||||
| 1 | Jacob 2017 [34] | Y | OR 0.59 (0.14, 2.52) | - |
| 2 | Lee 2019 [35] | Y | OR 1.03 (0.84, 1.25) a | OR 1.05 (0.86, 1.27) |
| Post-term pregnancy | ||||
| 1 | Haggar 2014 [36] | Y | OR 0.78 (0.64, 0.95) a | OR 1.04 (0.94, 1.56) |
| Obstetric hemorrhage | ||||
| 1 | Lee 2019 [35] | Y | OR 0.99 (0.74, 1.33) a | OR 1.00 (0.75, 1.34) |
| Hydroaminos/Oligo | ||||
| 1 | Lee 2019 [35] | Y | OR 1.11 (0.81, 1.53) | OR 1.15 (0.83, 1.58) |
| Placental previa | ||||
| 1 | Lee 2016 [28] | Y | OR 1.22 (0.27, 5.52) | OR 1.55 (0.33, 7.25) |
| Plural birth | ||||
| 1 | Lee 2019 [35] | Y | OR 0.80 (0.50, 1.28) a | OR 0.83 (0.52, 1.33) |
| Threatened abortion | ||||
| 1 | Haggar 2014 [36] | Y | OR 2.04 (1.49, 2.80) a | RR 2.09 (1.51, 2.74) |
| Threatened preterm labor | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.31 (0.93, 1.84) a | RR 1.28 (0.88, 1.88) |
| Medical abortion | ||||
| 1 | Jacob 2017 [34] | Y | OR 1.12 (0.44, 2.83) | - |
| Unspecified abortion | ||||
| 1 | Jacob 2017 [34] | Y | OR 0.40 (0.21, 0.76) | - |
| Hemorrhage in early pregnancy without fetal loss | ||||
| 1 | Jacob 2017 [34] | Y | OR 0.53 (0.27, 1.05) | - |
| Preterm contractions without preterm birth | ||||
| 1 | Jacob 2017 [34] | Y | OR 0.43 (0.22, 0.84) | - |
| Delivery (n = 9 outcomes) | ||||
| Caesarean delivery | ||||
| 1 | Anderson 2017b [42] | Y | OR 1.12 (1.02, 1.22) a | PR 1.08 (1.01, 1.14) |
| 2 | Anderson 2018 [39] | Y | OR 1.27 (1.02, 1.59) a | PR 1.14 (1.00, 1.31) |
| 3 | Farland 2020 [44] | Y | RR 1.17 (1.11, 1.23) | RR 1.05 (1.00, 1.11) |
| 4 | Haggar 2014 [36] | Y | OR 2.95 (2.50, 3.48) a | RR 2.62 (2.22, 3.04) |
| 5 | Hartnett 2017 [45] | N | Could not pool | - |
| 6 | Hartnett 2018 [41] | N | Could not pool | - |
| 7 | Jacob 2017 [34] | Y | OR 0.85 (0.49, 1.49) | - |
| 8 | Kao 2020 [27] | Y | OR 1.20 (1.12, 1.28) | OR 1.18 (1.10, 1.27) |
| 9 | Smaldone 2010 [40] | Y | RR 1.01 (0.66, 1.53) | - |
| 10 | Stensheim 2013 [31] | Y | OR 1.89 (1.74, 2.07) a | - |
| Premature ruptured membranes | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.05 (0.82, 1.34) a | RR 0.99 (0.83, 1.31) |
| 2 | Lee 2019 [35] | Y | OR 0.85 (0.70, 1.03) a | OR 0.83 (0.68, 1.01) |
| 3 | Smaldone 2010 [40] | Y | RR 1.19 (0.49, 2.90) | - |
| Failure to progress | ||||
| 1 | Haggar 2014 [36] | Y | OR 1.50 (0.95, 2.35) a | RR 1.51 (0.97, 2.37) |
| Fetal malpresentation | ||||
| 1 | Jacob 2017 [34] | Y | OR 0.77 (0.34, 1.75) | - |
| Preterm labour | ||||
| 1 | Lee 2019 [35] | Y | OR 1.36 (1.09, 1.69) a | OR 1.33 (1.06, 1.65) |
| Fetal distress | ||||
| 1 | Kao 2020 [27] | Y | OR 1.14 (0.99, 1.31) | OR 1.14 (0.99, 1.31) |
| Spontaneous delivery | ||||
| 1 | Jacob 2017 [34] | Y | OR 1.96 (1.26, 3.05) | - |
| Full-term delivery | ||||
| 1 | Lee 2019 [35] | Y | OR 0.78 (0.68, 0.90) a | OR 0.78 (0.68, 0.90) |
| Successful delivery | ||||
| 1 | Lee 2016 [28] | Y | OR 2.57 (1.69, 3.90) | OR 2.85 (1.83, 4.43) |
| After delivery (n = 1 outcome) | ||||
| Disorders of breast and lactation associated with childbirth | ||||
| 1 | Jacob 2017 [34] | Y | OR 1.77 (0.68, 4.62) | - |
| MATERNAL/PATERNAL HEALTH OUTCOMES (n = 2 outcomes) | ||||
| Before pregnancy (n = 1 outcome) | ||||
| Fertility treatment | ||||
| 1 | Farland 2020 [44] | Y | OR 2.46 (2.20, 2.75) a | - |
| 2 | Haggar 2014 [36] | Y | OR 3.03 (1.02, 4.53) a | RR 1.94 (1.36, 2.69) |
| 3 | Magelssen 2007 [29] | Y | OR 10.12 (7.60, 13.57) a | - |
| 4 | Stensheim 2011 [30] | Y | OR 2.82 (2.30, 3.45) a | - |
| 5 | Stensheim 2013 [31] | Y | OR 2.82 (2.30, 3.45) a | - |
| 6 | Velez 2021 [26] | Y | RR 1.30 (1.23, 1.37) | - |
| After delivery (n = 1 outcome) | ||||
| Birth rate | ||||
| 1 | Lee 2019 [35] | Y | HR 0.44 (0.41, 0.47) a | HR 0.41 (0.38, 0.44) |
| 2 | Stensheim 2011 [30] | N | Could not pool | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oveisi, N.; Cheng, V.; Ellis, U.; Peacock, S.; McTaggart-Cowan, H.; Brotto, L.A.; Loree, J.; Hanley, G.E.; Gill, S.; Rayar, M.; et al. Reproductive Health Outcomes among Adolescent and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1707. https://doi.org/10.3390/cancers15061707
Oveisi N, Cheng V, Ellis U, Peacock S, McTaggart-Cowan H, Brotto LA, Loree J, Hanley GE, Gill S, Rayar M, et al. Reproductive Health Outcomes among Adolescent and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(6):1707. https://doi.org/10.3390/cancers15061707
Chicago/Turabian StyleOveisi, Niki, Vicki Cheng, Ursula Ellis, Stuart Peacock, Helen McTaggart-Cowan, Lori A. Brotto, Jonathan Loree, Gillian E. Hanley, Sharlene Gill, Meera Rayar, and et al. 2023. "Reproductive Health Outcomes among Adolescent and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis" Cancers 15, no. 6: 1707. https://doi.org/10.3390/cancers15061707
APA StyleOveisi, N., Cheng, V., Ellis, U., Peacock, S., McTaggart-Cowan, H., Brotto, L. A., Loree, J., Hanley, G. E., Gill, S., Rayar, M., Srikanthan, A., & De Vera, M. A. (2023). Reproductive Health Outcomes among Adolescent and Young Adult Cancer Patients: A Systematic Review and Meta-Analysis. Cancers, 15(6), 1707. https://doi.org/10.3390/cancers15061707







