A Successful Bridge Therapy Combining Hypomethylating Agents with Venetoclax for Adult Patients with Newly Diagnosed or Relapsed/Refractory Acute Myeloid Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
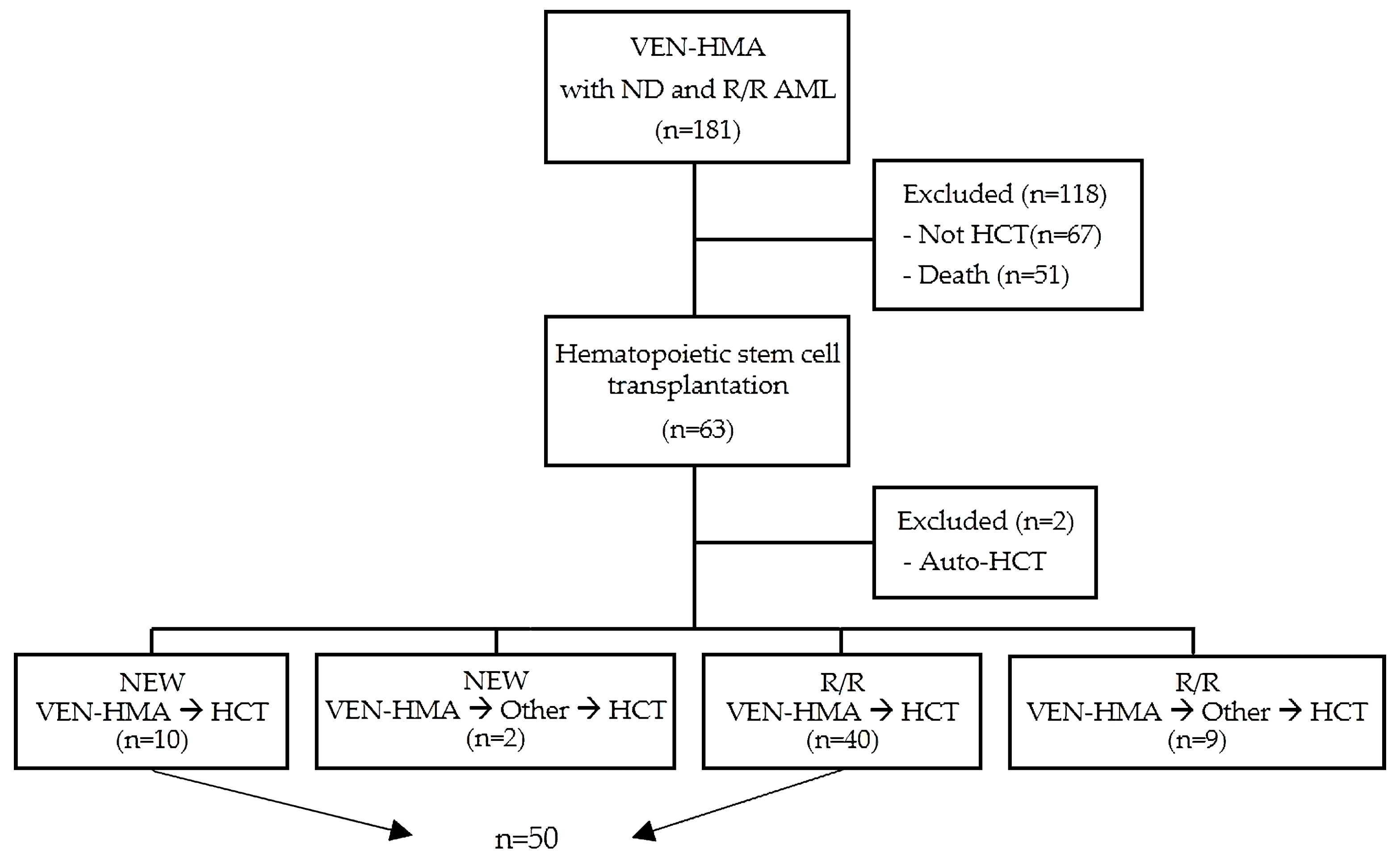
2.1. Study Population and Data Selection
2.2. VEN-HMA Treatment and Response Assessment
2.3. Transplant Procedure
2.4. CMV Prophylaxis
2.5. Definition and Assessment of Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient’s Characteristics
3.2. Response to VEN-HMA before Allo-HCT and Transplant Characteristics
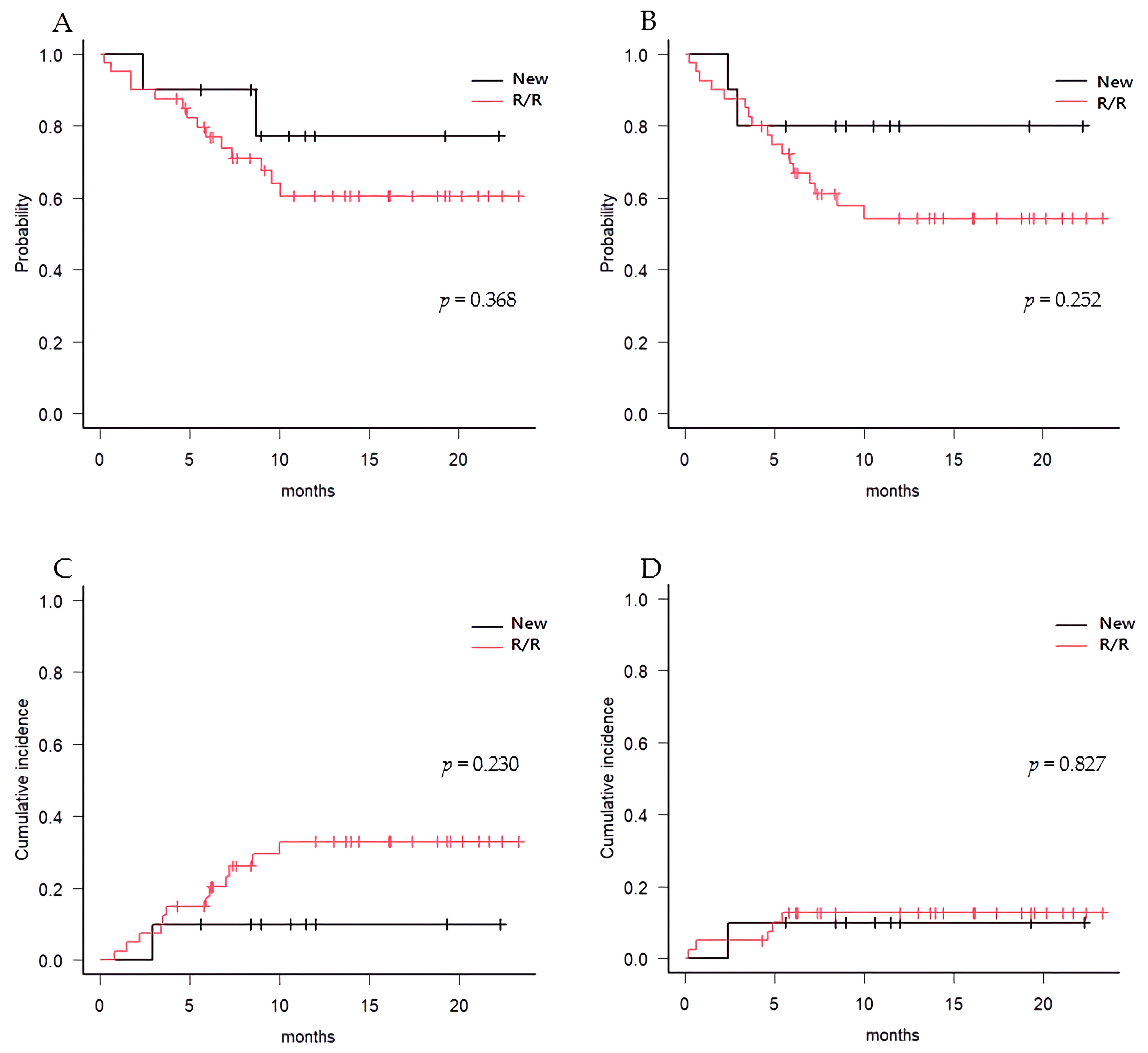
3.3. Post-Transplant Survival and Relapse Outcomes
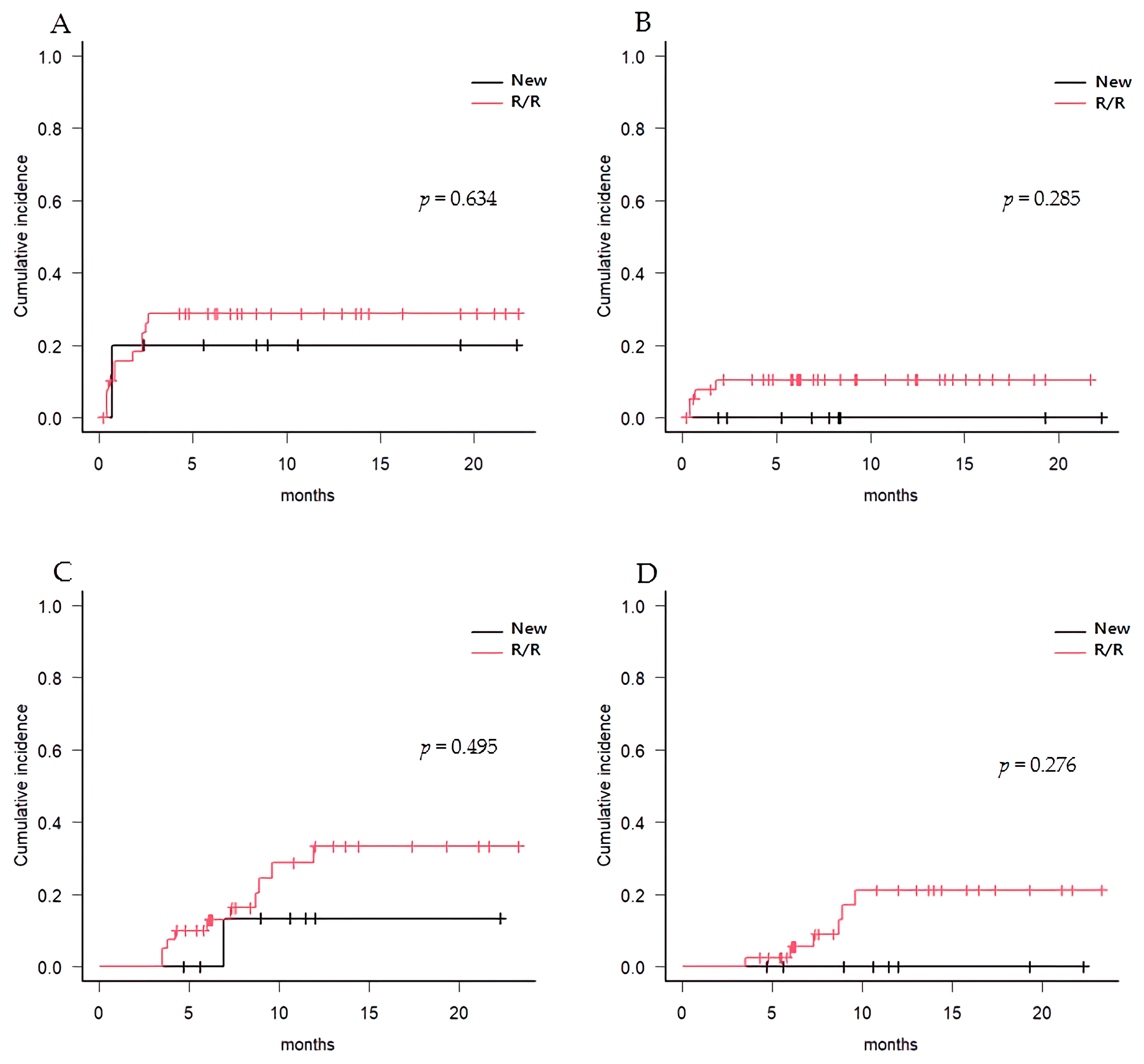
3.4. Post-Transplant Complications and Mortality
3.5. Predicting Factors for Post-Transplant Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, J.H.; Cho, B.S.; Kim, H.J.; Kim, J.H.; Shin, S.H.; Yahng, S.A.; Lee, S.E.; Eom, K.S.; Kim, Y.J.; Lee, S.; et al. Outcomes of elderly de novo acute myeloid leukemia treated by a risk-adapted approach based on age, comorbidity, and performance status. Am. J. Hematol. 2013, 88, 1074–1081. [Google Scholar] [CrossRef]
- Kantarjian, H.; O’Brien, S.; Cortes, J.; Giles, F.; Faderl, S.; Jabbour, E.; Garcia-Manero, G.; Wierda, W.; Pierce, S.; Shan, J.; et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 2006, 106, 1090–1098. [Google Scholar] [CrossRef]
- Itzykson, R.; Gardin, C.; Pautas, C.; Thomas, X.; Turlure, P.; Raffoux, E.; Terre, C.; Fenaux, P.; Castaigne, S.; Dombret, H.; et al. Impact of post-remission therapy in patients aged 65–70 years with de novo acute myeloid leukemia: A comparison of two concomitant randomized ALFA trials with overlapping age inclusion criteria. Haematologica 2011, 96, 837–844. [Google Scholar] [CrossRef]
- Pasvolsky, O.; Shimony, S.; Ram, R.; Shimoni, A.; Shargian, L.; Avni, B.; Wolach, O.; Shochat, T.; Yerushalmi, R.; Amit, O.; et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first complete remission after 5-azacitidine and venetoclax: A multicenter retrospective study. Ann. Hematol. 2022, 101, 379–387. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Dohner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef]
- Garciaz, S.; Hospital, M.A.; Alary, A.S.; Saillard, C.; Hicheri, Y.; Mohty, B.; Rey, J.; D’Incan, E.; Charbonnier, A.; Villetard, F.; et al. Azacitidine Plus Venetoclax for the Treatment of Relapsed and Newly Diagnosed Acute Myeloid Leukemia Patients. Cancers 2022, 14, 2025. [Google Scholar] [CrossRef]
- Aldoss, I.; Yang, D.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M.; Mei, M.; Salhotra, A.; Khaled, S.; Nakamura, R.; et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 2018, 103, e404–e407. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef]
- Park, S.; Kwag, D.; Kim, T.Y.; Lee, J.H.; Lee, J.Y.; Min, G.J.; Park, S.S.; Yahng, S.A.; Jeon, Y.W.; Shin, S.H.; et al. A retrospective comparison of salvage intensive chemotherapy versus venetoclax-combined regimen in patients with relapsed/refractory acute myeloid leukemia (AML). Ther. Adv. Hematol. 2022, 13, 20406207221081637. [Google Scholar] [CrossRef]
- Vasu, S.; Kohlschmidt, J.; Mrozek, K.; Eisfeld, A.K.; Nicolet, D.; Sterling, L.J.; Becker, H.; Metzeler, K.H.; Papaioannou, D.; Powell, B.L.; et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv. 2018, 2, 1645–1650. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Colditz, G.A.; DiPersio, J.F. Outcomes of Allogeneic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia: A Systematic Review and Meta-analysis. Biol. Blood Marrow Transplant. 2016, 22, 651–657. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Dadwal, S.; Yang, D.; Mei, M.; Palmer, J.; Salhotra, A.; Al Malki, M.; Aribi, A.; Ali, H.; Khaled, S.; et al. Outcome of Allogeneic Hematopoietic Cell Transplantation after Venetoclax and Hypomethylating Agent Therapy for Acute Myelogenous Leukemia. Biol. Blood Marrow Transplant. 2020, 26, e322–e327. [Google Scholar] [CrossRef]
- Kennedy, V.E.; Hui, G.; Azenkot, T.; Gaut, D.; Wieduwilt, M.J.; Oliai, C.; Jonas, B.A.; Mittal, V.; Logan, A.C.; Muffly, L.S.; et al. Outcomes of allogeneic transplantation after hypomethylating agents with venetoclax in acute myeloid leukemia. Am. J. Hematol. 2022, 97, E191–E194. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Winters, A.; McMahon, C.; Schwartz, M.; Jordan, C.T.; Rabinovitch, R.; Abbott, D.; Smith, C.A.; Gutman, J.A. Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transplant. 2022, 57, 160–166. [Google Scholar] [CrossRef]
- Winters, A.C.; Bosma, G.; Abbott, D.; Minhajuddin, M.; Jordan, C.; Pollyea, D.A.; Gutman, J.A. Outcomes Are Similar after Allogeneic Hematopoietic Stem Cell Transplant for Newly Diagnosed Acute Myeloid Leukemia Patients who Received Venetoclax + Azacitidine Versus Intensive Chemotherapy. Transplant. Cell. Ther. 2022, 28, 694.e1–694.e9. [Google Scholar] [CrossRef]
- Pratz, K.W.; DiNardo, C.D.; Arellano, M.L.; Letai, A.G.; Thirman, M.; Pullarkat, V.A.; Roboz, G.J.; Becker, P.S.; Hong, W.J.; Jiang, Q.; et al. Outcomes after Stem Cell Transplant in Older Patients with Acute Myeloid Leukemia Treated with Venetoclax-Based Therapies. Blood 2019, 134, 264. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kopecky, K.J.; Buchner, T.; Willman, C.L.; Estey, E.H.; Schiffer, C.A.; Doehner, H.; Tallman, M.S.; Lister, T.A.; et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J. Clin. Oncol. 2003, 21, 4642–4649. [Google Scholar] [CrossRef]
- Cho, B.S.; Yahng, S.A.; Min, G.J.; Park, S.; Park, S.S.; Shin, S.H.; Jeon, Y.W.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; et al. Comparable Outcomes after Alternative and Matched Sibling Donor Hematopoietic Stem Cell Transplantation and the Role of Molecular Measurable Residual Disease for Acute Myeloid Leukemia in Elderly Patients. Transplant. Cell. Ther. 2021, 27, 774.e1–774.e12. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, S.; Kwag, D.; Lee, J.H.; Lee, J.; Min, G.J.; Park, S.S.; Jeon, Y.W.; Shin, S.H.; Yahng, S.A.; et al. Depth of Response to Intensive Chemotherapy Has Significant Prognostic Value among Acute Myeloid Leukemia (AML) Patients Undergoing Allogeneic Hematopoietic Stem-Cell Transplantation with Intermediate or Adverse Risk at Diagnosis Compared to At-Risk Group According to European Leukemia Net 2017 Risk Stratification. Cancers 2022, 14, 3199. [Google Scholar] [CrossRef]
- Park, S.; Min, G.J.; Park, S.S.; Yahng, S.A.; Jeon, Y.W.; Shin, S.H.; Yoon, J.H.; Lee, S.E.; Cho, B.S.; Eom, K.S.; et al. Comparison of Myeloablative (CyTBI, BuCy) versus Reduced-Intensity (FluBu2TBI400) Peripheral Blood Stem Cell Transplantation in Acute Myeloid Leukemia Patients with Pretransplant Low WT1 Expression. Biol. Blood Marrow Transplant. 2020, 26, 2018–2026. [Google Scholar] [CrossRef]
- Martino, M.; Pitino, A.; Gori, M.; Bruno, B.; Crescimanno, A.; Federico, V.; Picardi, A.; Tringali, S.; Ingrosso, C.; Carluccio, P.; et al. Letermovir Prophylaxis for Cytomegalovirus Infection in Allogeneic Stem Cell Transplantation: A Real-World Experience. Front. Oncol. 2021, 11, 740079. [Google Scholar] [CrossRef]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e381. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, H.J.; Park, S.S.; Jeon, Y.W.; Lee, S.E.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; Lee, S.; Min, C.K.; et al. Long-term clinical outcomes of hematopoietic cell transplantation for intermediate-to-poor-risk acute myeloid leukemia during first remission according to available donor types. Oncotarget 2017, 8, 41590–41604. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, G.D.; Park, J.; Yoon, J.H.; Kim, H.J.; Min, W.S.; Kim, M. Quantitative fragment analysis of FLT3-ITD efficiently identifying poor prognostic group with high mutant allele burden or long ITD length. Blood Cancer J. 2015, 5, e336. [Google Scholar] [CrossRef]
- Zwaan, C.M.; Meshinchi, S.; Radich, J.P.; Veerman, A.J.; Huismans, D.R.; Munske, L.; Podleschny, M.; Hahlen, K.; Pieters, R.; Zimmermann, M.; et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: Prognostic significance and relation to cellular drug resistance. Blood 2003, 102, 2387–2394. [Google Scholar] [CrossRef]
- Lee, G.; Kim, J.; Lee, S.; Jang, W.; Park, J.; Chae, H.; Kim, M.; Kim, Y. Fragment Analysis for Detection of theFLT3-Internal Tandem Duplication: Comparison with Conventional PCR and Sanger Sequencing. Lab. Med. Online 2017, 7, 13–19. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Derkach, A.; Gowda, L.; Menghrajani, K.; DeWolf, S.; Ruiz, J.D.; Ponce, D.M.; Shaffer, B.C.; Tamari, R.; Young, J.W.; et al. Venetoclax-based combinations in AML and high-risk MDS prior to and following allogeneic hematopoietic cell transplant. Leuk. Lymphoma 2021, 62, 3394–3401. [Google Scholar] [CrossRef]
- Aoudjhane, M.; Labopin, M.; Gorin, N.C.; Shimoni, A.; Ruutu, T.; Kolb, H.J.; Frassoni, F.; Boiron, J.M.; Yin, J.L.; Finke, J.; et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: A retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 2005, 19, 2304–2312. [Google Scholar] [CrossRef]
- Couriel, D.R.; Saliba, R.M.; Giralt, S.; Khouri, I.; Andersson, B.; de Lima, M.; Hosing, C.; Anderlini, P.; Donato, M.; Cleary, K.; et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol. Blood Marrow Transplant. 2004, 10, 178–185. [Google Scholar] [CrossRef]
- Cho, B.S.; Min, G.J.; Park, S.; Park, S.S.; Shin, S.H.; Yahng, S.A.; Jeon, Y.W.; Yoon, J.H.; Lee, S.E.; Eom, K.S.; et al. Haploidentical vs matched unrelated donor transplantation for acute myeloid leukemia in remission: A prospective comparative study. Am. J. Hematol. 2021, 96, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, J.H.; Lee, J.H.; Kim, D.Y.; Park, H.S.; Choi, E.J.; Ko, S.H.; Seol, M.; Lee, Y.S.; Kang, Y.A.; et al. Reduced-Intensity Conditioning with Busulfan, Fludarabine, and Antithymocyte Globulin for Hematopoietic Cell Transplantation from Unrelated or Haploidentical Family Donors in Patients with Acute Myeloid Leukemia in Remission. Biol. Blood Marrow Transplant. 2017, 23, 1555–1566. [Google Scholar] [CrossRef]
- Tarantini, F.; Cumbo, C.; Anelli, L.; Zagaria, A.; Specchia, G.; Musto, P.; Albano, F. Can the New and Old Drugs Exert an Immunomodulatory Effect in Acute Myeloid Leukemia? Cancers 2021, 13, 4121. [Google Scholar] [CrossRef]
- Carrington, E.M.; Tarlinton, D.M.; Gray, D.H.; Huntington, N.D.; Zhan, Y.; Lew, A.M. The life and death of immune cell types: The role of BCL-2 anti-apoptotic molecules. Immunol. Cell Biol. 2017, 95, 870–877. [Google Scholar] [CrossRef]
- Chen, K.; Cheng, M.P.; Hammond, S.P.; Einsele, H.; Marty, F.M. Antiviral prophylaxis for cytomegalovirus infection in allogeneic hematopoietic cell transplantation. Blood Adv. 2018, 2, 2159–2175. [Google Scholar] [CrossRef] [PubMed]
- Loke, J.; Malladi, R.; Moss, P.; Craddock, C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: A triumph of hope and experience. Br. J. Haematol. 2020, 188, 129–146. [Google Scholar] [CrossRef]
- Maiti, A.; DiNardo, C.D.; Daver, N.G.; Rausch, C.R.; Ravandi, F.; Kadia, T.M.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Wei, A.H.; Pollyea, D.A.; Fathi, A.T.; Vyas, P.; DiNardo, C.D. New directions for emerging therapies in acute myeloid leukemia: The next chapter. Blood Cancer J. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Shimony, S.; Yeshurun, M.; Wolach, O.; Ram, R.; Rozovski, U.; Shargian, L.; Zukerman, T.; Amit, O.; Bar-On, Y.; Krayem, B.; et al. Post-transplantation maintenance with sorafenib or midostaurin for FLT3 positive AML patients—A multicenter retrospective observational study. Leuk. Lymphoma 2021, 62, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.M.; Li, S.; Fathi, A.T.; Wadleigh, M.; Ho, V.T.; Collier, K.; Connolly, C.; Ballen, K.K.; Cutler, C.S.; Dey, B.R.; et al. Haematopoietic cell transplantation with and without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukaemia in first complete remission. Br. J. Haematol. 2016, 175, 496–504. [Google Scholar] [CrossRef]
- Wei, Y.; Xiong, X.; Li, X.; Lu, W.; He, X.; Jin, X.; Sun, R.; Lyu, H.; Yuan, T.; Sun, T.; et al. Low-dose decitabine plus venetoclax is safe and effective as post-transplant maintenance therapy for high-risk acute myeloid leukemia and myelodysplastic syndrome. Cancer Sci. 2021, 112, 3636–3644. [Google Scholar] [CrossRef]
- Burchert, A.; Bug, G.; Fritz, L.V.; Finke, J.; Stelljes, M.; Rollig, C.; Wollmer, E.; Wasch, R.; Bornhauser, M.; Berg, T.; et al. Sorafenib Maintenance after Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia with FLT3-Internal Tandem Duplication Mutation (SORMAIN). J. Clin. Oncol. 2020, 38, 2993–3002. [Google Scholar] [CrossRef]
| All | New | R/R | p-Value | |
|---|---|---|---|---|
| Total Number | 50 | 10 | 40 | |
| Sex | 0.308 | |||
| Male | 27 (54.0%) | 7 (70.0%) | 20 (50.0%) | |
| Female | 23 (46.0%) | 3 (30.0%) | 20 (50.0%) | |
| HCT number | 0.022 | |||
| first | 35 (70.0%) | 10 (100%) | 25 (62.5%) | |
| second | 15 (30.0%) | 0 | 15 (37.5%) | |
| AML type | 0.496 | |||
| De novo | 47 (94.0%) | 9 (90.0%) | 38 (95.0%) | |
| Secondary | 3 (6.0%) | 1 (10.0%) | 2 (5.0%) | |
| ELN (2022) risk group | 0.848 | |||
| Favorable | 6 (12.0%) | 1 (10.0%) | 5 (12.5%) | |
| Intermediate | 21 (42.0%) | 5 (50.0%) | 16 (40.0%) | |
| Adverse | 23 (46.0%) | 4 (40.0%) | 19 (47.5%) | |
| Cytogenetic risk group | 0.793 | |||
| Favorable | 1 (2.0%) | 0 | 1 (2.5%) | |
| Intermediate | 37 (74.0%) | 7 (70.0%) | 30 (75.0%) | |
| Adverse | 12 (24.0%) | 3 (30.0%) | 9 (22.5%) | |
| Prior chemotherapy line before VEN-HMA 0 1 2 | 10 (20.0%) 33 (66.0%) 7 (14.0%) | 10 (100%) 0 0 | 0 33 (82.5%) 7 (17.5%) | <0.001 |
| VEN combination | 1.000 | |||
| AZA | 1 (2.0%) | 0 | 1 (2.5%) | |
| DEC | 49 (98.0%) | 10 (100%) | 39 (97.5%) | |
| VEN-HMA cycles | ||||
| Median | 3 | 4 | 2 | <0.001 |
| Range | 1–7 | 3–7 | 1–6 | |
| Age at HCT | ||||
| Median | 54 | 68 | 51 | <0.001 |
| Range | 23–73 | 66–73 | 23–68 | |
| Response before HCT | 0.794 | |||
| Active disease | 4 (8.0%) | 1 (10.0%) | 3 (7.5%) | |
| Response disease | 46 (92.0%) | 9 (90.0%) | 37 (92.5%) | |
| CR/CRi without MRD | 21 (42.0%) | 4 (40.0%) | 17 (42.5%) | |
| CR/CRi with MRD | 11 (22.0%) | 5 (50.0%) | 6 (15.0%) | |
| MLFS without MRD | 7 (14.0%) | 0 (0%) | 7 (17.5%) | |
| MLFS with MRD | 7 (14.0%) | 0 (0%) | 7 (17.5%) | |
| HCT-CI | 0.283 | |||
| 0 | 17 (34.0%) | 3 (30.0%) | 14 (35.0%) | |
| 1–2 | 14 (28.0%) | 1 (10.0%) | 13 (32.5%) | |
| ≥3 | 19 (38.0%) | 6 (60.0%) | 13 (32.5%) | |
| Donor type | 1.000 | |||
| MSD | 9 (18.0%) | 1 (10.0%) | 8 (20.0%) | |
| UD * | 25 (40.0%) | 5 (50.0%) | 20 (50.0%) | |
| HID | 12 (24.0%) | 4 (40.0%) | 8 (20.0%) | |
| CBT | 4 (8.0%) | 0 | 4 (10.0%) | |
| Conditioning regimen | 0.010 | |||
| MAC | 41 (82.0%) | 5 (50.0%) | 36 (90.0%) | |
| RIC | 9 (18.0%) | 5 (50.0%) | 4 (10.0%) | |
| CMV prophylaxis | 0.663 | |||
| Yes | 40 (80.0%) | 9 (90.0%) | 31 (77.5%) | |
| No | 10 (20.0%) | 1 (10.0%) | 9 (22.5%) |
| All | New | R/R | p-Value | |
|---|---|---|---|---|
| Total Number | 50 | 10 | 40 | |
| OS | ||||
| Median | NR | NR | NR | 0.368 |
| 1 year [95% CI] | 63.7% [47.3–76.3%] | 77.1% [34.5–93.9%] | 60.5% [42.0–74.7%] | |
| RFS | ||||
| Median | NR | NR | NR | 0.252 |
| 1 year [95% CI] | 59.3% [43.6–72.1%] | 80.0% [40.9–94.6%] | 54.3% [36.7–68.9%] | |
| CIR | ||||
| Median | NR | NR | NR | 0.230 |
| 1 year [95% CI] | 28.5% [16.0–42.4%] | 10.0% [4.0–37.6%] | 33.0% [18.0–48.7%] | |
| NRM | ||||
| Median | NR | NR | NR | 0.827 |
| 1 year [95% CI] | 12.2% [4.9–23.0%] | 10.0% [0.5–37.4%] | 12.7% [4.6–25.3%] | |
| Cumulative incidence of aGVHD | ||||
| II-IV at 100 days | 28.4% | 20.0% | 30.3% | 0.634 |
| III-IV at 100 days | 8.4% | 0.0% | 10.7% | 0.285 |
| Cumulative incidence of cGVHD | ||||
| Mod-Sev at 1 year | 37.4% | 15.6% | 39.3% | 0.495 |
| Severe at 1 year | 20.9% | 0.0% | 23.9% | 0.276 |
| Infectious complications | ||||
| CMV DNAemia | 22 (44.0%) | 7 (70.0%) | 15 (37.5%) | |
| CMV Treatment | 9 (18.0%) | 5 (50.0%) | 4 (10.0%) | |
| Bacteremia | 12 (24.0%) | 5 (50.0%) | 7 (17.5%) | |
| Pneumonia | 8 (16.0%) | 5 (50.0%) | 3 (7.5%) | |
| BK viruria | 12 (24.0%) | 3 (30.0%) | 9 (22.5%) |
| OS | RFS | CIR | NRM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |||||
| p-Value | p-Value | HR [95% CI] | p-Value | p-Value | HR [95% CI] | p-Value | p-Value | HR [95% CI] | p-Value | p-Value | HR [95% CI] | |
| Age at HCT | 0.894 | - | - | 0.887 | - | - | 0.410 | - | - | 0.440 | - | - |
| Sex | 0.472 | - | - | 0.590 | - | - | 0.620 | - | - | 0.850 | - | - |
| Setting (R/R or New) | 0.377 | - | - | 0.265 | - | - | 0.270 | - | - | 0.830 | - | - |
| VEN-HMA cycle (>3 or ≤3) | 0.034 | 0.021 | 0.089 [0.011–0.691] | 0.026 | 0.036 | 0.196 [0.043–0.903] | 0.105 | - | - | 0.996 | - | - |
| Prior HCT (Yes or no) | 0.111 | - | - | 0.099 | 0.045 | 2.704 [1.023–7.142] | 0.420 | - | - | 0.210 | - | - |
| ELN risk group (Poor or others) | 0.086 | 0.041 | 0.264 [0.073–0.947] | 0.154 | - | - | 0.490 | - | - | 0.155 | - | - |
| HCT-CI (≥3 or <3) | 0.128 | 0.087 | 2.724 [0.864–8.587] | 0.401 | 0.517 | 1.468 [0.460–4.687] | 0.700 | 0.427 | 0.588 [0.159–2.179] | 0.120 | 0.185 | 3.267 [0.568–18.810] |
| Response at HCT (No or response) | 0.001 | 0.003 | 9.745 [2.218–42.810] | <0.001 | 0.004 | 8.627 [1.983–37.520] | 0.008 | <0.001 | 19.750 [3.986–97.850] | 0.380 | 0.402 | 2.610 [0.276–24.650] |
| Conditioning (RIC or MAC) | 0.664 | - | - | 0.849 | - | - | 0.910 | - | - | 0.870 | - | - |
| Donor type (Others or matched) | 0.632 | - | - | 0.182 | - | - | 0.180 | - | - | 0.680 | - | - |
| Authors, Year [Reference No.] (Study Design) | Patients N (ND/RR) | Age at HCT (Range) | Cycle Median N (Range) | Prior HCT N (%) | HCT-CI (%) | Response at HCT | Condition -ing | Donor | Median f/u Duration | OS | RFS | CIR | NRM | CMV N (%) | CI of aGVHD (%) | CI of cGVHD (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pasvolsky, 2022 [4] (Retrospective) | 24 (24/0) | 71.7 (43–76) | (1–4) | 0 | ≥3 39% | CR 100% | MAC 12% RIC 88% | MSD 21% MUD 67% HID 13% | 8.0 months | 1 yr 63.2% | 1 yr 58% | - | 1 yr 19.1% | - | 6 months 58% | 1 yr 40% |
| Pollyea, 2022 [15] (Retrospective) | 21 (21/0) | 65 (60–73) | 3 (1–19) | 0 | ≥2 57% | CR/CRi 81% MLFS 19% | MAC 9.5% RIC 57.1% NMA 33.4% | MSD 33% CBT 67% | 22.9 months | NR | - | 1 yr 20% | 1 yr 11% | - | II–IV; 48% III–IV; 10% | Any; 43% Mod-Sev; 10% |
| Winters, 2022 [16] (Retrospective) | 29 (29/0) | 65 (22–73) (at Dx.) | 3 (1–19) | 0 | - | CR 86% MLFS 14% | MAC 31.0% RIC 41.4% NMA 27.6% | MSD 28% HID 3% CBT 69% | 14.3 months | 1 yr 76.3% | 1 yr 73.2% | - | - | - | - | - |
| Current study (Retrospective) | 50 (10/40) | 54 | 3 (1–7) | 15 (30%) | ≥3 38% | CR/CRi 64% MLFS 28% R/R 8% | MAC 82% RIC 18% | MSD 18% UD 40% HID 24% CBT 8% | 13.7 months | 1 yr 63.7% | 1 yr 59.3% | 1 yr 28.5% | 1 yr 12.2% | 9 (18.0%) treatment | 100 days II-IV; 28.4% III-IV; 8.4% | 1 yr Any; 55.2% Mod-Sev; 37.4% |
| Sandhu, 2020 [13] (Retrospective) | 32 (19/13) | 62 (18–73) | 2 (1–12) | 0 | ≥2 31.3% | CR/CRi 68.8% Refractory 31.3% | MAC 6.2% RIC 62.5% NMA 31.3% | MSD 25% MUD 68.8% HID 6.3% | 14.4 months | 1 yr 62.5% | 1 yr 43.8% | 1 yr 37.5% | 1 yr 18.8% | 5 (15.6%) reactivation | 100 days II-IV; 43.8% III-IV; 21.9% | 1 yr Any; 31.3% Ext.; 28.1% |
| Kennedy, 2022 [14] (Retrospective) | 88 (46/42) | 67 (24–77) | 3 (1–13) | 5 (6%) | ≥3 35% | CR 70% CRi 15% MLFS 15% | MAC 28% RIC 55% NMA 17% | MSD 25% MUD 55% HID 19% CBT 1% | 10.9 months | 1 yr 73% | - | 1 yr 36% | 1 yr 17% | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, S.-Y.; Park, S.; Kwag, D.; Lee, J.H.; Min, G.-J.; Park, S.-S.; Yoon, J.-H.; Lee, S.-E.; Cho, B.-S.; Eom, K.-S.; et al. A Successful Bridge Therapy Combining Hypomethylating Agents with Venetoclax for Adult Patients with Newly Diagnosed or Relapsed/Refractory Acute Myeloid Leukemia. Cancers 2023, 15, 1666. https://doi.org/10.3390/cancers15061666
Bang S-Y, Park S, Kwag D, Lee JH, Min G-J, Park S-S, Yoon J-H, Lee S-E, Cho B-S, Eom K-S, et al. A Successful Bridge Therapy Combining Hypomethylating Agents with Venetoclax for Adult Patients with Newly Diagnosed or Relapsed/Refractory Acute Myeloid Leukemia. Cancers. 2023; 15(6):1666. https://doi.org/10.3390/cancers15061666
Chicago/Turabian StyleBang, Su-Yeon, Silvia Park, Daehun Kwag, Jong Hyuk Lee, Gi-June Min, Sung-Soo Park, Jae-Ho Yoon, Sung-Eun Lee, Byung-Sik Cho, Ki-Seong Eom, and et al. 2023. "A Successful Bridge Therapy Combining Hypomethylating Agents with Venetoclax for Adult Patients with Newly Diagnosed or Relapsed/Refractory Acute Myeloid Leukemia" Cancers 15, no. 6: 1666. https://doi.org/10.3390/cancers15061666
APA StyleBang, S.-Y., Park, S., Kwag, D., Lee, J. H., Min, G.-J., Park, S.-S., Yoon, J.-H., Lee, S.-E., Cho, B.-S., Eom, K.-S., Kim, Y.-J., Lee, S., Min, C.-K., Cho, S.-G., Lee, J. W., & Kim, H.-J. (2023). A Successful Bridge Therapy Combining Hypomethylating Agents with Venetoclax for Adult Patients with Newly Diagnosed or Relapsed/Refractory Acute Myeloid Leukemia. Cancers, 15(6), 1666. https://doi.org/10.3390/cancers15061666






