Prognostic Value of Simple Non-Invasive Tests for the Risk Stratification of Incident Hepatocellular Carcinoma in Cirrhotic Individuals with Non-Alcoholic Fatty Liver Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
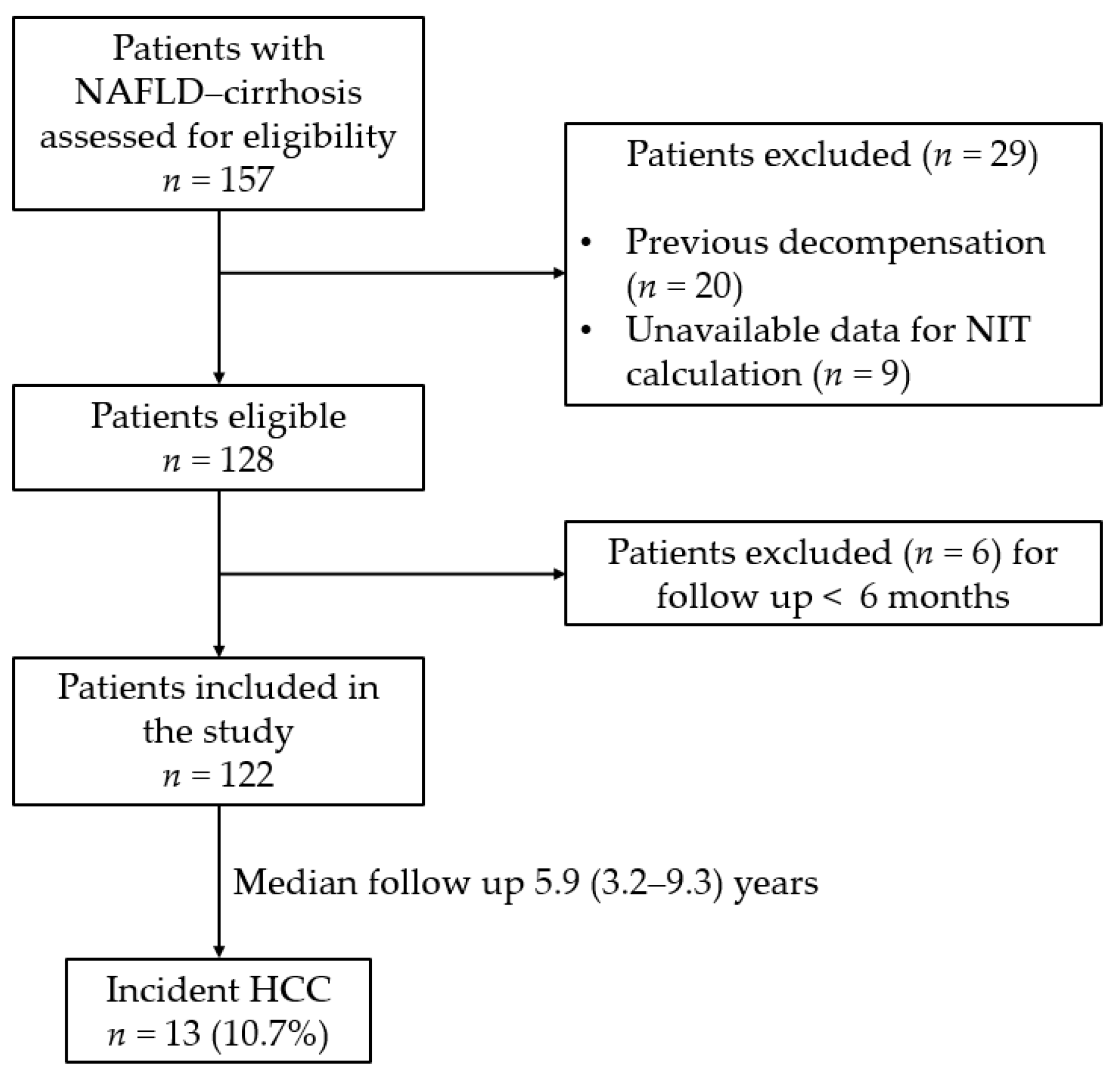
2.1. Study Design and Study Population
2.2. Non-Invasive Tests
2.3. Statistical Analysis
3. Results
3.1. Baseline Charcateristics of the Study Cohort
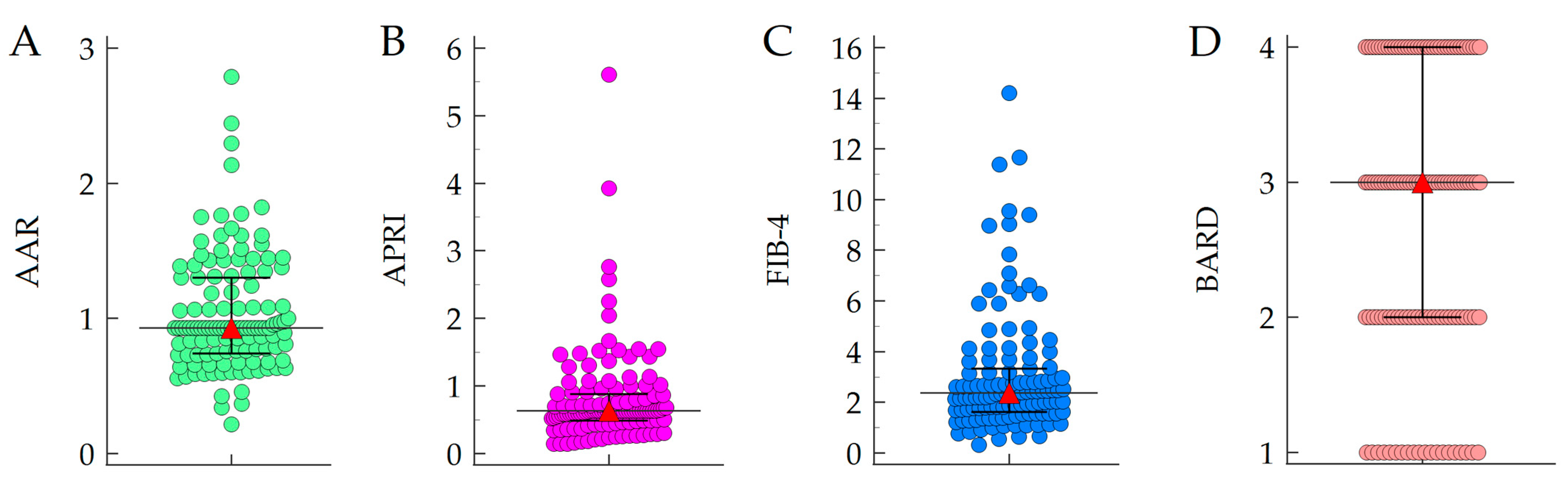
3.2. Baseline NITs Values and Association with HCC Developemnt
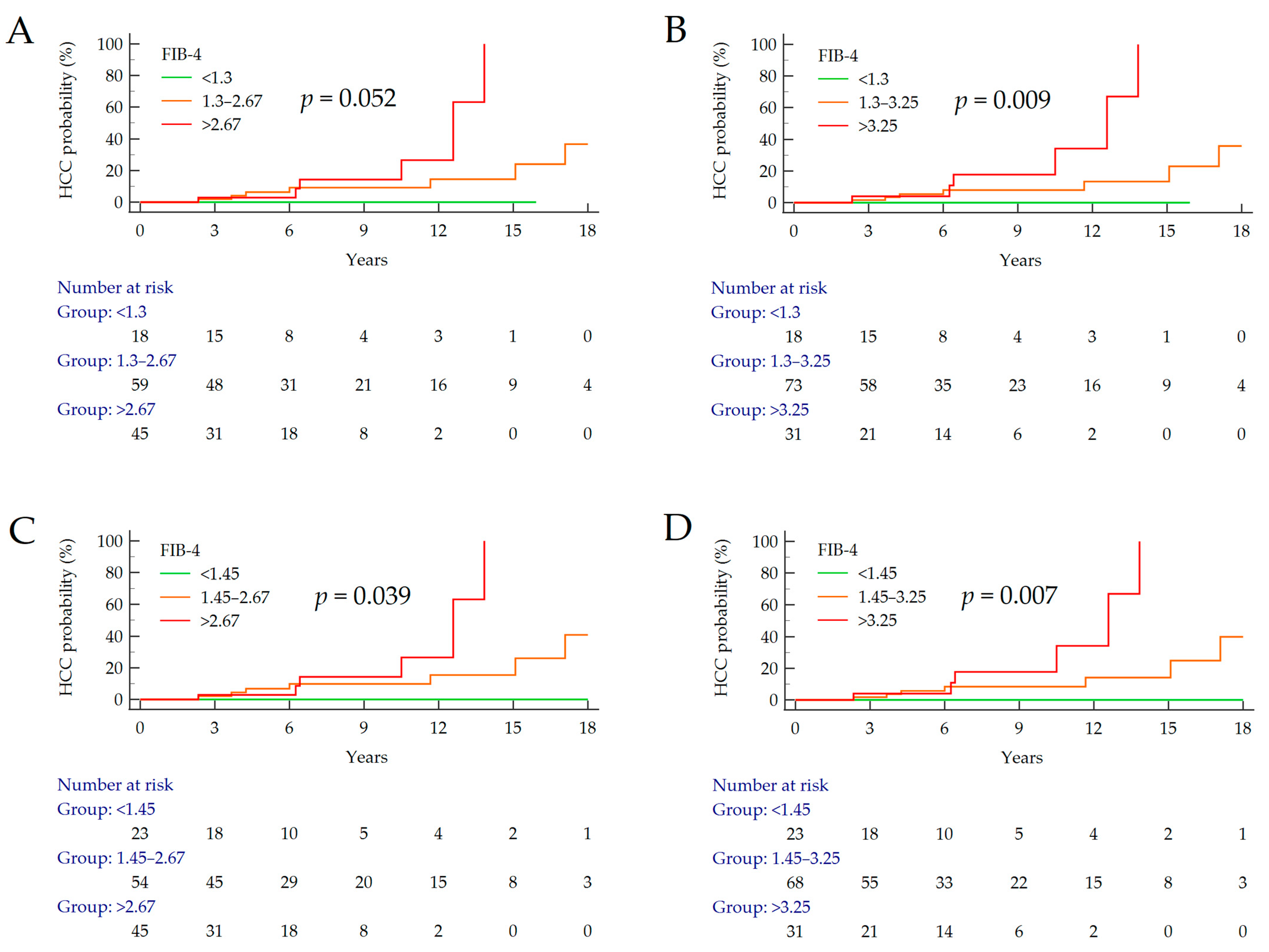
3.3. Stratifcation of the Risk of HCC According to FIB-4
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Leone, N.; Vanni, E.; Marchesini, G.; Brunello, F.; Carucci, P.; Musso, A.; Paolis, P.D.; Capussotti, L.; Salizzoni, M.; et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef]
- Armandi, A.; Bugianesi, E. Natural history of NASH. Liver Int. 2021, 41 (Suppl. S1), 78–82. [Google Scholar] [CrossRef]
- Reddy, S.K.; Steel, J.L.; Chen, H.W.; DeMateo, D.J.; Cardinal, J.; Behari, J.; Humar, A.; Marsh, J.W.; Geller, D.A.; Tsung, A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012, 55, 1809–1819. [Google Scholar] [CrossRef]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Ciancio, A.; Ribaldone, D.G.; Spertino, M.; Risso, A.; Ferrarotti, D.; Caviglia, G.P.; Carucci, P.; Gaia, S.; Rolle, E.; Sacco, M.; et al. Who Should Not Be Surveilled for HCC Development after Successful Therapy with DAAS in Advanced Chronic Hepatitis C? Results of a Long-Term Prospective Study. Biomedicines 2023, 11, 166. [Google Scholar] [CrossRef]
- Singal, A.G.; Lok, A.S.; Feng, Z.; Kanwal, F.; Parikh, N.D. Conceptual Model for the Hepatocellular Carcinoma Screening Continuum: Current Status and Research Agenda. Clin. Gastroenterol. Hepatol. 2022, 20, 9–18. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Troshina, G.; Santaniello, U.; Rosati, G.; Bombaci, F.; Birolo, G.; Nicolosi, A.; Saracco, G.M.; Ciancio, A. Long-Term Hepatocellular Carcinoma Development and Predictive Ability of Non-Invasive Scoring Systems in Patients with HCV-Related Cirrhosis Treated with Direct-Acting Antivirals. Cancers 2022, 14, 828. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis-2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef] [PubMed]
- Younes, R.; Caviglia, G.P.; Govaere, O.; Rosso, C.; Armandi, A.; Sanavia, T.; Pennisi, G.; Liguori, A.; Francione, P.; Gallego-Duran, R.; et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Nasr, P.; Ekstedt, M.; Stål, P.; Hultcrantz, R.; Kechagias, S. Accuracy of Noninvasive Scoring Systems in Assessing Risk of Death and Liver-Related Endpoints in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 1148–1156. [Google Scholar] [CrossRef]
- Cholankeril, G.; Kramer, J.R.; Chu, J.; Yu, X.; Balakrishnan, M.; Li, L.; El-Serag, H.B.; Kanwal, F. Longitudinal changes in fibrosis markers are associated with risk of cirrhosis and hepatocellular carcinoma in non-alcoholic fatty liver disease. J. Hepatol. 2022, 78, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Masson, S.; Anstee, Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: Cofactors for progressive fatty liver disease. J. Hepatol. 2018, 68, 251–267. [Google Scholar] [CrossRef]
- Gaia, S.; Campion, D.; Evangelista, A.; Spandre, M.; Cosso, L.; Brunello, F.; Ciccone, G.; Bugianesi, E.; Rizzeto, M. Non-invasive score system for fibrosis in chronic hepatitis: Proposal for a model based on biochemical, FibroScan and ultrasound data. Liver Int. 2015, 35, 2027–2035. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Armandi, A.; Rosso, C.; Gaia, S.; Aneli, S.; Rolle, E.; Abate, M.L.; Olivero, A.; Nicolosi, A.; Guariglia, M.; et al. Biomarkers of Oncogenesis, Adipose Tissue Dysfunction and Systemic Inflammation for the Detection of Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancers 2021, 13, 2305. [Google Scholar] [CrossRef]
- De Ritis, F.; Coltorti, M.; Giusti, G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin. Chim. Acta. 1957, 2, 70–74. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Bugianesi, E.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Barrera, F.; Haflidadottir, S.; Day, C.P.; George, J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 782–789. [Google Scholar] [CrossRef]
- Ioannou, G.N. HCC surveillance after SVR in patients with F3/F4 fibrosis. J. Hepatol. 2021, 74, 458–465. [Google Scholar] [CrossRef]
- Sebastiani, G.; Alshaalan, R.; Wong, P.; Rubino, M.; Salman, A.; Metrakos, P.; Deschenes, M.; Ghali, P. Prognostic Value of Non-Invasive Fibrosis and Steatosis Tools, Hepatic Venous Pressure Gradient (HVPG) and Histology in Nonalcoholic Steatohepatitis. PLoS ONE 2015, 10, e0128774. [Google Scholar] [CrossRef]
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef]
- Vieira Barbosa, J.; Milligan, S.; Frick, A.; Broestl, J.; Younossi, Z.; Afdhal, N.H.; Lai, M. Fibrosis-4 Index as an Independent Predictor of Mortality and Liver-Related Outcomes in NAFLD. Hepatol. Commun. 2022, 6, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Önnerhag, K.; Hartman, H.; Nilsson, P.M.; Lindgren, S. Non-invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non-alcoholic fatty liver disease (NAFLD). Scand. J. Gastroenterol. 2019, 54, 328–334. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J.; Network, N.C.R. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef]
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
| Variables | Data |
|---|---|
| Patients, n | 122 |
| Age (years), median (IQR) [122] | 62 (51–67) |
| Male sex, n (%) [122] | 64 (52.5%) |
| BMI (Kg/m2), median (IQR) [122] | 30.5 (27.8–33.3) |
| Lean (BMI < 25.0 Kg/m2), n (%) | 10 (8.2%) |
| Overweight (BMI 25.0–29.9 Kg/m2), n (%) | 43 (35.2%) |
| Obese (BMI ≥ 30.0 Kg/m2), n (%) | 69 (56.6%) |
| T2DM, n (%) [122] | 70 (57.4%) |
| Hb (g/dL), median (IQR) [89] | 13.9 (12.4–15.1) |
| WBC count (×109/L), median (IQR) [61] | 5.90 (4.77–7.38) |
| Platelet count (×109/L), median (IQR) [122] | 155 (108–198) |
| ALT (U/L), median (IQR) [122] | 42 (24–61) |
| AST (U/L), median (IQR) [122] | 39 (26–47) |
| γGT (U/L), median (IQR) [122] | 97 (45–173) |
| ALP (U/L), median (IQR) [57] | 91 (71–123) |
| INR, median (IQR) [61] | 1.09 (1.01–1.22) |
| Albumin (g/dL), median (IQR) [54] | 4.2 (3.9–4.4) |
| Total bilirubin (mg/dL), median (IQR) [78] | 0.7 (0.5–1.2) |
| Total cholesterol (mg/dL), median (IQR) [47] | 179 (148–205) |
| HDL cholesterol (mg/dL), median (IQR) [46] | 45 (36–59) |
| Triglycerides (mg/dL), median (IQR) [57] | 136 (84–206) |
| Liver stiffness (kPa), median (IQR) [60] | 20.5 (14.3–27.3) |
| FIB-4 Cut-Offs | HCC (n) | Cumulative (%) | p Value 1 | HR (95% CI) | p Value 2 |
|---|---|---|---|---|---|
| <1.3 1.3–2.67 >2.67 | 0/18 7/59 6/45 | 0% 11.9% 13.3% | 0.052 | 3.79 (1.21–1.90) | 0.023 |
| <1.3 1.3–3.25 >3.25 | 0/18 7/73 6/31 | 0% 9.6% 19.4% | 0.009 | 4.95 (1.57–15.59) | 0.006 |
| <1.45 1.45–2.67 >2.67 | 0/23 7/54 6/45 | 0% 13.0% 13.3% | 0.039 | 3.86 (1.28–11.66) | 0.017 |
| <1.45 1.45–3.25 >3.25 | 0/23 7/68 6/31 | 0% 10.3% 19.4% | 0.007 | 4.97 (1.63–15.13) | 0.005 |
| Baseline Variables | HR (95% CI) | p Value |
|---|---|---|
| FIB-4 (<1.45, 1.45–3.25, >3.25) | 6.40 (1.71–24.00) | 0.006 |
| Male sex | 0.75 (0.32–4.86) | 0.748 |
| BMI (Kg/m2) | 0.94 (0.82–1.08) | 0.398 |
| T2DM | 1.06 (0.27–4.13) | 0.938 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armandi, A.; Caviglia, G.P.; Abdulle, A.; Rosso, C.; Gjini, K.; Castelnuovo, G.; Guariglia, M.; Perez Diaz del Campo, N.; D’Amato, D.; Ribaldone, D.G.; et al. Prognostic Value of Simple Non-Invasive Tests for the Risk Stratification of Incident Hepatocellular Carcinoma in Cirrhotic Individuals with Non-Alcoholic Fatty Liver Disease. Cancers 2023, 15, 1659. https://doi.org/10.3390/cancers15061659
Armandi A, Caviglia GP, Abdulle A, Rosso C, Gjini K, Castelnuovo G, Guariglia M, Perez Diaz del Campo N, D’Amato D, Ribaldone DG, et al. Prognostic Value of Simple Non-Invasive Tests for the Risk Stratification of Incident Hepatocellular Carcinoma in Cirrhotic Individuals with Non-Alcoholic Fatty Liver Disease. Cancers. 2023; 15(6):1659. https://doi.org/10.3390/cancers15061659
Chicago/Turabian StyleArmandi, Angelo, Gian Paolo Caviglia, Amina Abdulle, Chiara Rosso, Kamela Gjini, Gabriele Castelnuovo, Marta Guariglia, Nuria Perez Diaz del Campo, Daphne D’Amato, Davide Giuseppe Ribaldone, and et al. 2023. "Prognostic Value of Simple Non-Invasive Tests for the Risk Stratification of Incident Hepatocellular Carcinoma in Cirrhotic Individuals with Non-Alcoholic Fatty Liver Disease" Cancers 15, no. 6: 1659. https://doi.org/10.3390/cancers15061659
APA StyleArmandi, A., Caviglia, G. P., Abdulle, A., Rosso, C., Gjini, K., Castelnuovo, G., Guariglia, M., Perez Diaz del Campo, N., D’Amato, D., Ribaldone, D. G., Saracco, G. M., & Bugianesi, E. (2023). Prognostic Value of Simple Non-Invasive Tests for the Risk Stratification of Incident Hepatocellular Carcinoma in Cirrhotic Individuals with Non-Alcoholic Fatty Liver Disease. Cancers, 15(6), 1659. https://doi.org/10.3390/cancers15061659








