Anti-Cancer Stem-Cell-Targeted Therapies in Prostate Cancer
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Epidemiology
1.2. Screening and Diagnosis
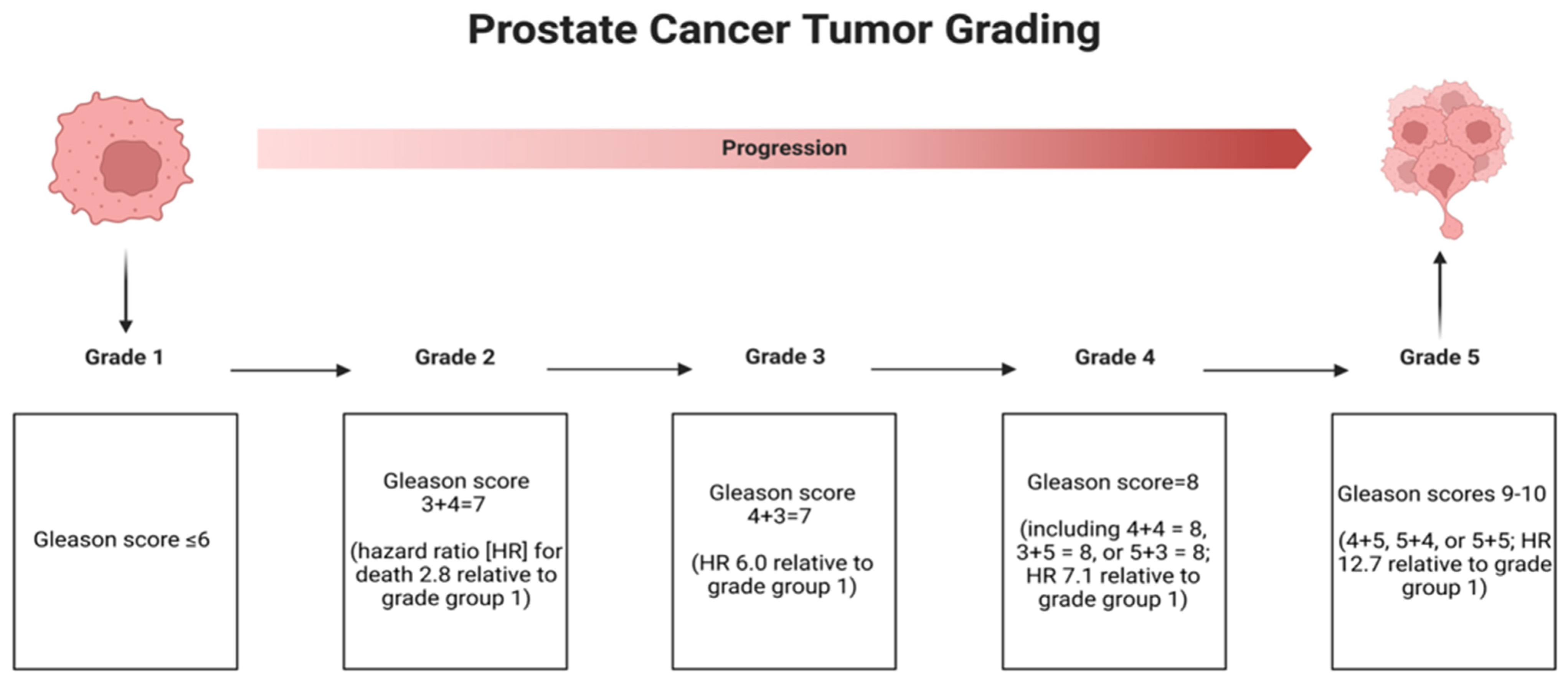
1.3. Grading of Prostate Cancer
1.4. Treatment of Prostate Cancer
1.5. Mechanisms of Progression into Castration-Resistant Prostate Cancer
1.6. Need for Targeted Therapies
2. Cancer Stem Cells in Prostate Cancer
3. Targeting Prostate CSC-Related Signaling Pathways
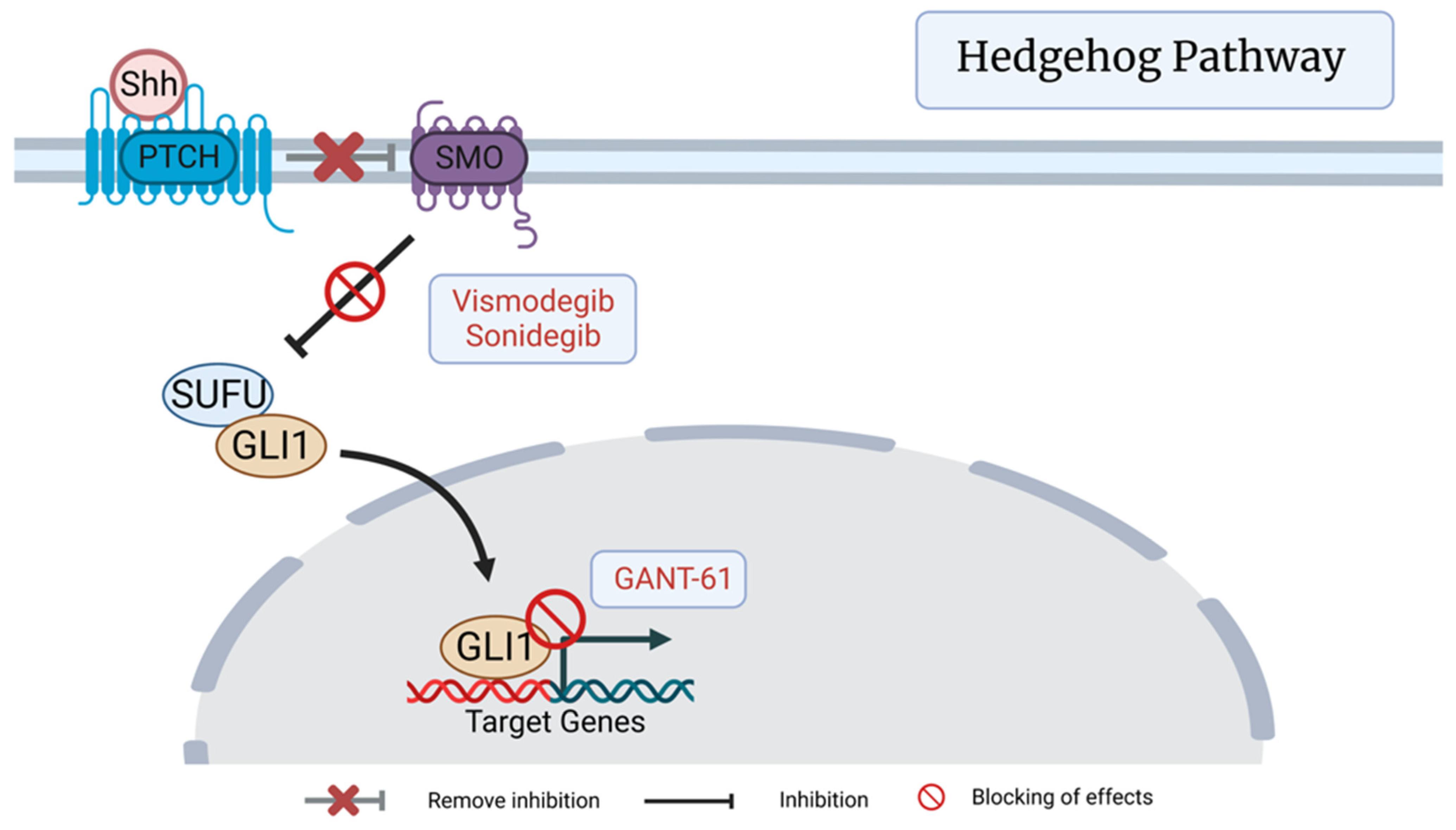
3.1. Hedgehog (Hh) Pathway
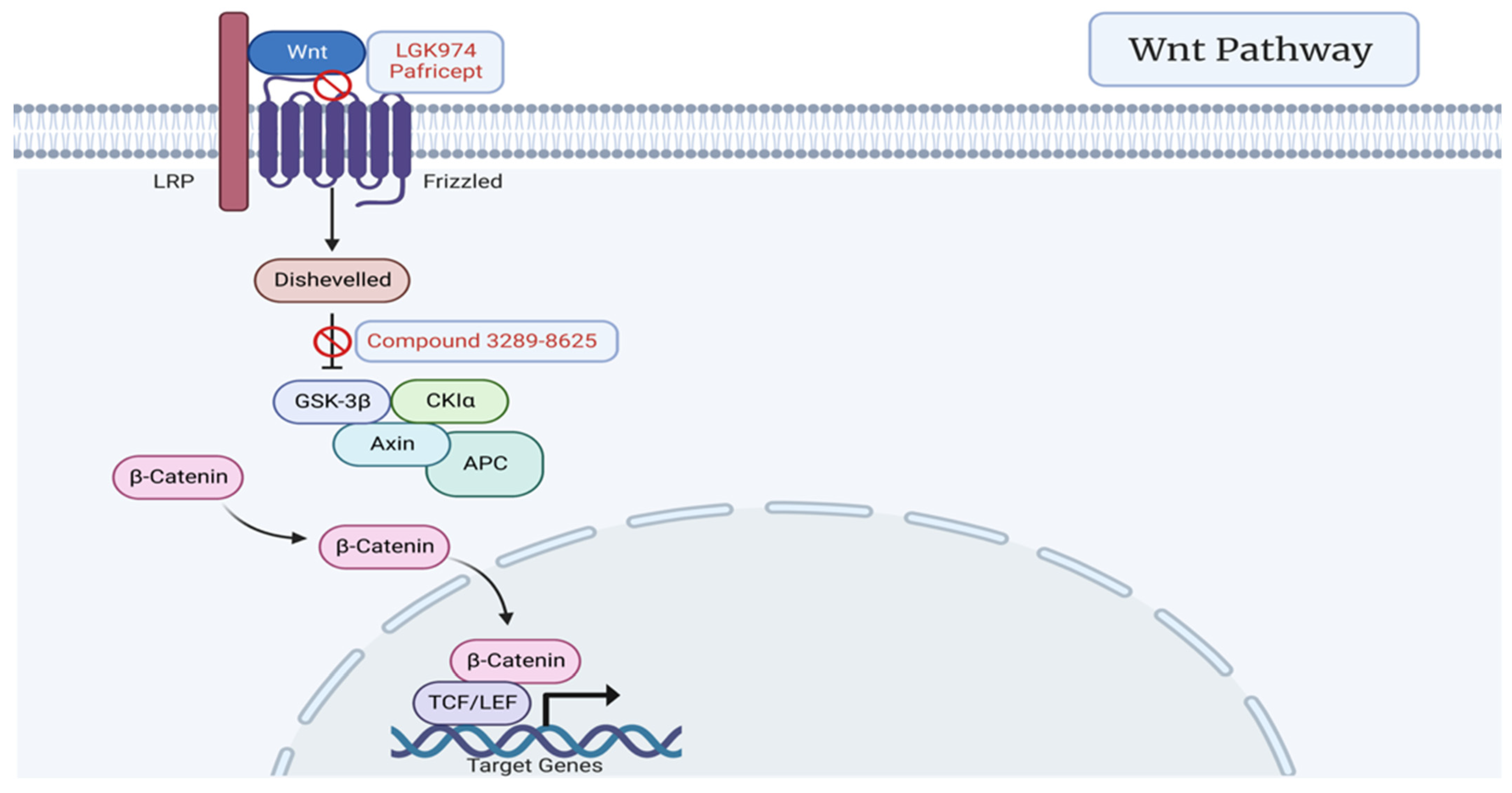
3.2. Wnt Signaling Pathway
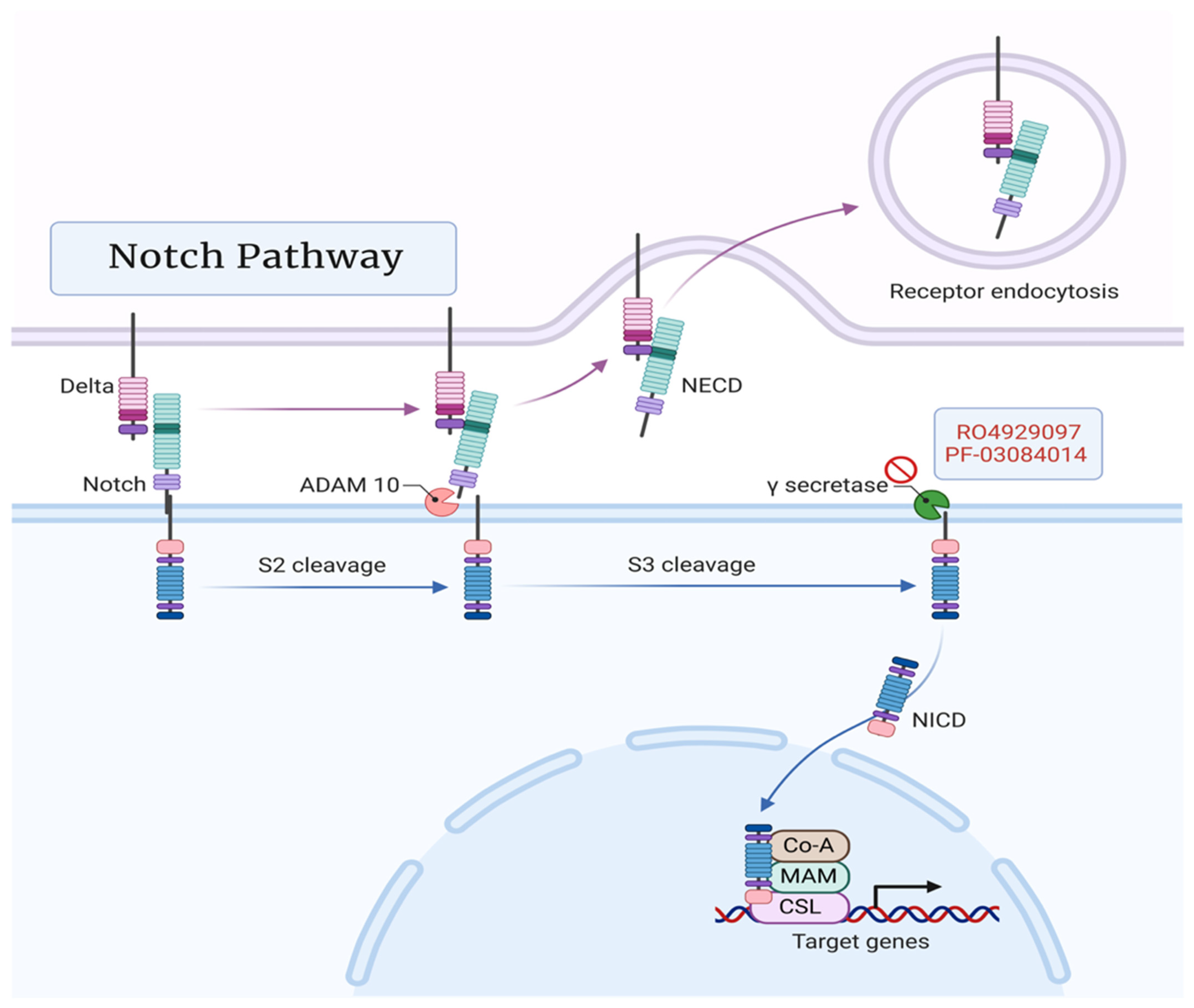
3.3. Notch Pathway
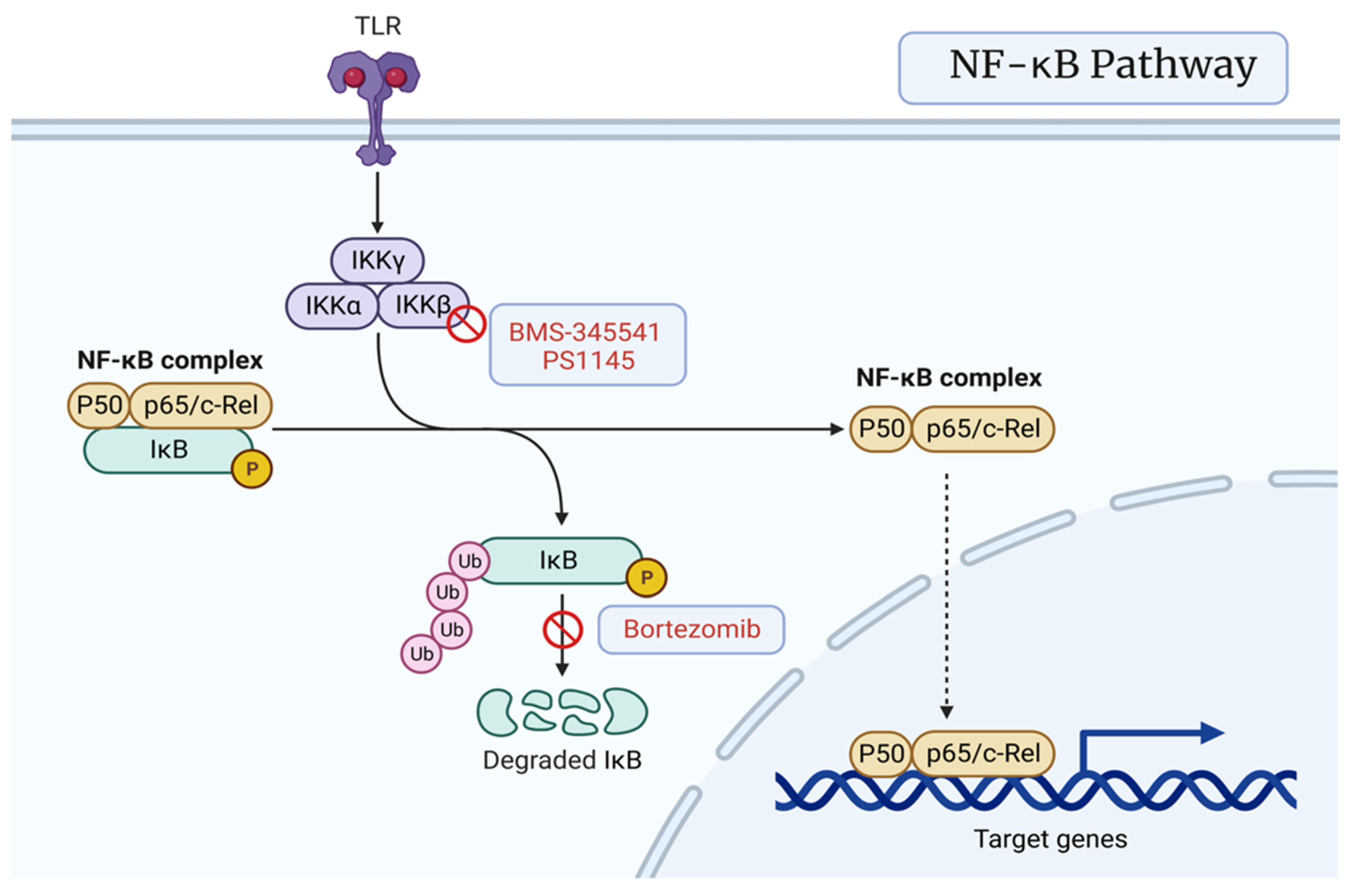
3.4. NF-κB Signaling Pathway
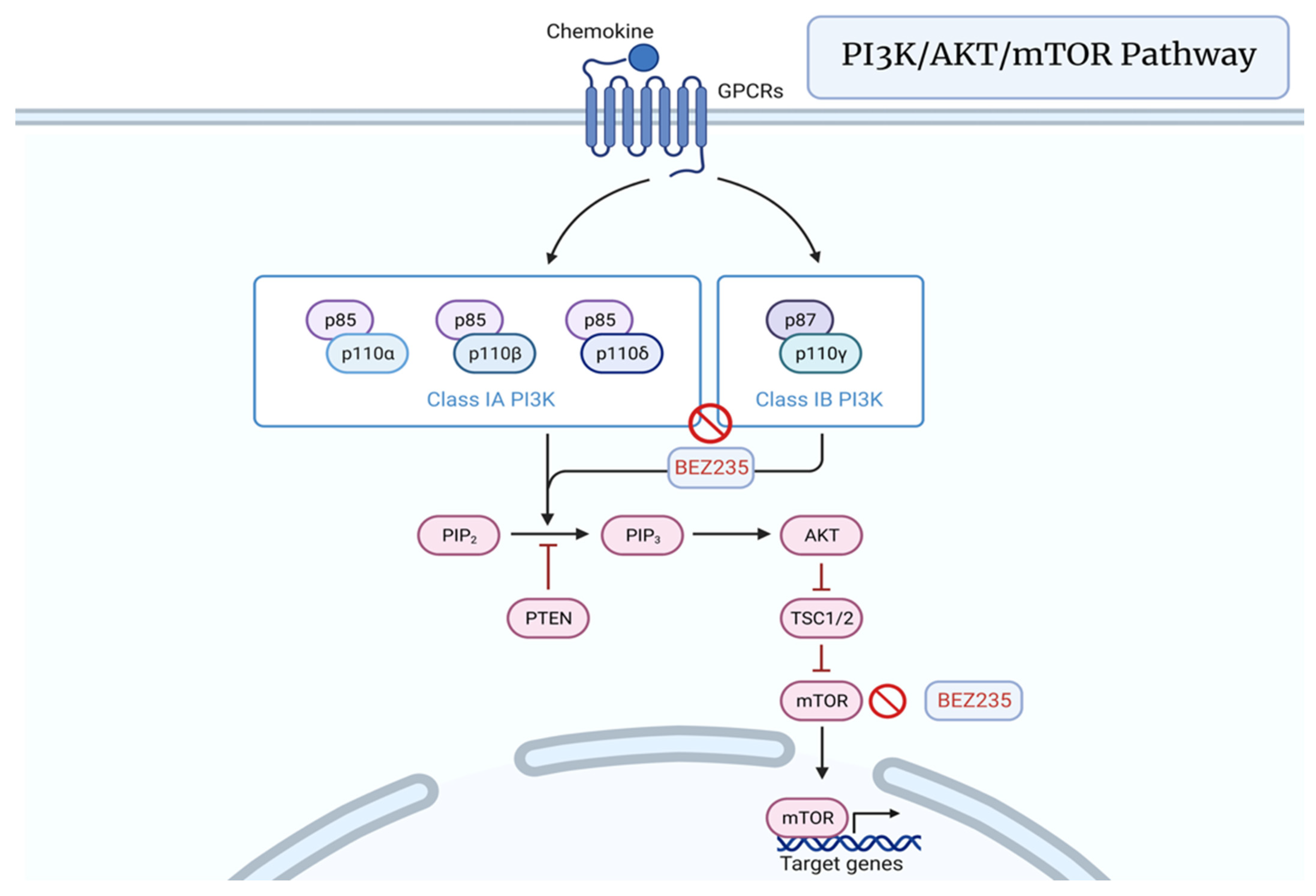
3.5. PI3K/AKT/mTOR Pathway
4. Targeting Prostate CSC Microenvironment
5. Immunotherapies Targeting Prostate CSCs
5.1. (CAR)-Modified T-Cell Therapy Targeting CSC-Associated Tumor Antigens
5.2. Anti-CD133 CAR-T Therapy
5.3. CAR T Cells Targeting the CSC Marker EpCAM
6. Targeted Nanoparticles
6.1. CD44-Targeting Therapies
6.2. CD133-Targeted Therapy
7. Anti-CSC Targeted Therapies: Clinical Trials
7.1. Hedgehog (Hh) Pathway
7.2. WNT Signaling Pathway
7.3. Notch Pathway
7.4. NF-kB Pathway
7.5. PI3K/AKT/mTOR Pathway
7.6. Microenvironment (VEGF)
7.7. CAR-T
7.8. Nanoparticles
8. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Butler, S.S.; Muralidhar, V.; Zhao, S.G.; Sanford, N.N.; Franco, I.; Fullerton, Z.H.; Chavez, J.; D’Amico, A.V.; Feng, F.Y.; Rebbeck, T.R.; et al. Prostate cancer incidence across stage, NCCN risk groups, and age before and after USPSTF Grade D recommendations against prostate-specific antigen screening in 2012. Cancer 2020, 126, 717–724. [Google Scholar] [CrossRef]
- Jemal, A.; Fedewa, S.A.; Ma, J.; Siegel, R.; Lin, C.C.; Brawley, O.; Ward, E.M. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA 2015, 314, 2054–2061. [Google Scholar] [CrossRef]
- Force, U.P.S.T. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef]
- Bell, K.J.L.; Del Mar, C.; Wright, G.; Dickinson, J.; Glasziou, P. Prevalence of incidental prostate cancer: A systematic review of autopsy studies. Int. J. Cancer 2015, 137, 1749–1757. [Google Scholar] [CrossRef]
- Sakr, W.A.; Grignon, D.J.; Haas, G.P.; Heilbrun, L.K.; Pontes, J.E.; Crissman, J.D. Age and racial distribution of prostatic intraepithelial neoplasia. Eur. Urol. 1996, 30, 138–144. [Google Scholar] [CrossRef]
- Haas, G.P.; Sakr, W.A. Epidemiology of prostate cancer. CA A Cancer J. Clin. 1997, 47, 273–287. [Google Scholar] [CrossRef]
- Noone, A.; Howlader, N.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D. SEER Cancer Statistics Review (CSR) 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020.
- Ilic, D.; Djulbegovic, M.; Jung, J.H.; Hwang, E.C.; Zhou, Q.; Cleves, A.; Agoritsas, T.; Dahm, P. Prostate cancer screening with prostate-specific antigen (PSA) test: A systematic review and meta-analysis. BMJ 2018, 362, k3519. [Google Scholar] [CrossRef]
- Schröder, F.H.; Hugosson, J.; Carlsson, S.; Tammela, T.; Määttänen, L.; Auvinen, A.; Kwiatkowski, M.; Recker, F.; Roobol, M.J. Screening for prostate cancer decreases the risk of developing metastatic disease: Findings from the European Randomized Study of Screening for Prostate Cancer (ERSPC). Eur. Urol. 2012, 62, 745–752. [Google Scholar] [CrossRef]
- Etzioni, R.; Penson, D.F.; Legler, J.M.; di Tommaso, D.; Boer, R.; Gann, P.H.; Feuer, E.J. Overdiagnosis due to prostate-specific antigen screening: Lessons from U.S. prostate cancer incidence trends. J. Natl. Cancer Inst. 2002, 94, 981–990. [Google Scholar] [CrossRef]
- Draisma, G.; Etzioni, R.; Tsodikov, A.; Mariotto, A.; Wever, E.; Gulati, R.; Feuer, E.; de Koning, H. Lead time and overdiagnosis in prostate-specific antigen screening: Importance of methods and context. J. Natl. Cancer Inst. 2009, 101, 374–383. [Google Scholar] [CrossRef]
- Draisma, G.; Boer, R.; Otto, S.J.; van der Cruijsen, I.W.; Damhuis, R.A.; Schröder, F.H.; de Koning, H.J. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J. Natl. Cancer Inst. 2003, 95, 868–878. [Google Scholar] [CrossRef]
- McNaughton-Collins, M.; Fowler, F.J., Jr.; Caubet, J.F.; Bates, D.W.; Lee, J.M.; Hauser, A.; Barry, M.J. Psychological effects of a suspicious prostate cancer screening test followed by a benign biopsy result. Am. J. Med. 2004, 117, 719–725. [Google Scholar] [CrossRef]
- Fowler, F.J., Jr.; Barry, M.J.; Walker-Corkery, B.; Caubet, J.F.; Bates, D.W.; Lee, J.M.; Hauser, A.; McNaughton-Collins, M. The impact of a suspicious prostate biopsy on patients’ psychological, socio-behavioral, and medical care outcomes. J. Gen. Intern. Med. 2006, 21, 715–721. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of Prognosis for Prostatic Adenocarcinoma by Combined Histological Grading and Clinical Staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Bostwick, D.G. Gleason grading of prostatic needle biopsies. Correlation with grade in 316 matched prostatectomies. Am. J. Surg. Pathol. 1994, 18, 796–803. [Google Scholar] [CrossRef]
- Epstein, J.I. An update of the Gleason grading system. J. Urol. 2010, 183, 433–440. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- van Leenders, G.; van der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, e87–e99. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer; National Comprehensive Cancer Network: Fort Washington, PA, USA, 2022. [Google Scholar]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef]
- Loblaw, D.A.; Virgo, K.S.; Nam, R.; Somerfield, M.R.; Ben-Josef, E.; Mendelson, D.S.; Middleton, R.; Sharp, S.A.; Smith, T.J.; Talcott, J.; et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J. Clin. Oncol. 2007, 25, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.J.; Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 2001, 1, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.G.; De Marzo, A.M.; Isaacs, W.B. Prostate cancer. N. Engl. J. Med. 2003, 349, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Debes, J.D.; Tindall, D.J. Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef]
- Shah, R.B.; Mehra, R.; Chinnaiyan, A.M.; Shen, R.; Ghosh, D.; Zhou, M.; Macvicar, G.R.; Varambally, S.; Harwood, J.; Bismar, T.A.; et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: Lessons from a rapid autopsy program. Cancer Res. 2004, 64, 9209–9216. [Google Scholar] [CrossRef]
- Pienta, K.J.; Smith, D.C. Advances in prostate cancer chemotherapy: A new era begins. CA A Cancer J. Clin. 2005, 55, 300–318; quiz 323–325. [Google Scholar] [CrossRef]
- Loberg, R.D.; Logothetis, C.J.; Keller, E.T.; Pienta, K.J. Pathogenesis and treatment of prostate cancer bone metastases: Targeting the lethal phenotype. J. Clin. Oncol. 2005, 23, 8232–8241. [Google Scholar] [CrossRef]
- Pienta, K.J.; Robertson, B.A.; Coffey, D.S.; Taichman, R.S. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clin. Cancer Res. 2013, 19, 5849–5855. [Google Scholar] [CrossRef]
- Richardson, G.D.; Robson, C.N.; Lang, S.H.; Neal, D.E.; Maitland, N.J.; Collins, A.T. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 2004, 117, 3539–3545. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef]
- Hamburger, A.W.; Salmon, S.E. Primary bioassay of human tumor stem cells. Science 1977, 197, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Heppner, G.H. Tumor heterogeneity. Cancer Res. 1984, 44, 2259–2265. [Google Scholar] [PubMed]
- Karhadkar, S.S.; Bova, G.S.; Abdallah, N.; Dhara, S.; Gardner, D.; Maitra, A.; Isaacs, J.T.; Berman, D.M.; Beachy, P.A. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004, 431, 707–712. [Google Scholar] [CrossRef]
- Gonnissen, A.; Isebaert, S.; Haustermans, K. Hedgehog signaling in prostate cancer and its therapeutic implication. Int. J. Mol. Sci. 2013, 14, 13979–14007. [Google Scholar] [CrossRef]
- Datta, S.; Datta, M.W. Sonic Hedgehog signaling in advanced prostate cancer. Cell. Mol. Life Sci. 2006, 63, 435–448. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Ross, A.E.; Hughes, R.M.; Glavaris, S.; Ghabili, K.; He, P.; Anders, N.M.; Harb, R.; Tosoian, J.J.; Marchionni, L.; Schaeffer, E.M.; et al. Pharmacodynamic and pharmacokinetic neoadjuvant study of hedgehog pathway inhibitor Sonidegib (LDE-225) in men with high-risk localized prostate cancer undergoing prostatectomy. Oncotarget 2017, 8, 104182–104192. [Google Scholar] [CrossRef]
- LoRusso, P.M.; Rudin, C.M.; Reddy, J.C.; Tibes, R.; Weiss, G.J.; Borad, M.J.; Hann, C.L.; Brahmer, J.R.; Chang, I.; Darbonne, W.C.; et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin. Cancer Res. 2011, 17, 2502–2511. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Qiu, L.; Qi, M.; Liu, J.; Hu, K.; Lin, W.; Huang, Y.; Fu, J. GANT-61 and GDC-0449 induce apoptosis of prostate cancer stem cells through a GLI-dependent mechanism. J. Cell. Biochem. 2018, 119, 3641–3652. [Google Scholar] [CrossRef] [PubMed]
- Gonnissen, A.; Isebaert, S.; McKee, C.M.; Dok, R.; Haustermans, K.; Muschel, R.J. The hedgehog inhibitor GANT61 sensitizes prostate cancer cells to ionizing radiation both in vitro and in vivo. Oncotarget 2016, 7, 84286–84298. [Google Scholar] [CrossRef]
- Sun, W.; Li, L.; Du, Z.; Quan, Z.; Yuan, M.; Cheng, H.; Gao, Y.; Luo, C.; Wu, X. Combination of phospholipase Cε knockdown with GANT61 sensitizes castration-resistant prostate cancer cells to enzalutamide by suppressing the androgen receptor signaling pathway. Oncol. Rep. 2019, 41, 2689–2702. [Google Scholar] [CrossRef]
- Youssef, M.; Moussa, N.; Helmy, M.; Haroun, M. Unraveling the therapeutic potential of GANT61/Dactolisib combination as a novel prostate cancer modality. Med. Oncol. 2022, 39, 143. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 2008, 8, 387–398. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Yokoyama, N.N.; Shao, S.; Hoang, B.H.; Mercola, D.; Zi, X. Wnt signaling in castration-resistant prostate cancer: Implications for therapy. Am. J. Clin. Exp. Urol. 2014, 2, 27–44. [Google Scholar]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef]
- de la Taille, A.; Rubin, M.A.; Chen, M.W.; Vacherot, F.; de Medina, S.G.; Burchardt, M.; Buttyan, R.; Chopin, D. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin. Cancer Res. 2003, 9, 1801–1807. [Google Scholar]
- Chen, G.; Shukeir, N.; Potti, A.; Sircar, K.; Aprikian, A.; Goltzman, D.; Rabbani, S.A. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: Potential pathogenetic and prognostic implications. Cancer 2004, 101, 1345–1356. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Xu, H. Wnt/β-catenin signal transduction pathway in prostate cancer and associated drug resistance. Discov. Oncol. 2021, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.; Guo, Y.; Simoneau, A.R.; Hope, C.; Xie, J.; Holcombe, R.F.; Hoang, B.H. Expression of Frzb/secreted Frizzled-related protein 3, a secreted Wnt antagonist, in human androgen-independent prostate cancer PC-3 cells suppresses tumor growth and cellular invasiveness. Cancer Res. 2005, 65, 9762–9770. [Google Scholar] [CrossRef]
- Yee, D.S.; Tang, Y.; Li, X.; Liu, Z.; Guo, Y.; Ghaffar, S.; McQueen, P.; Atreya, D.; Xie, J.; Simoneau, A.R.; et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol. Cancer 2010, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, L.; Li, J.; Farah, E.; Atallah, N.M.; Pascuzzi, P.E.; Gupta, S.; Liu, X. Inhibition of the Wnt/β-Catenin Pathway Overcomes Resistance to Enzalutamide in Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 3147–3162. [Google Scholar] [CrossRef] [PubMed]
- Grandy, D.; Shan, J.; Zhang, X.; Rao, S.; Akunuru, S.; Li, H.; Zhang, Y.; Alpatov, I.; Zhang, X.A.; Lang, R.A.; et al. Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J. Biol. Chem. 2009, 284, 16256–16263. [Google Scholar] [CrossRef]
- Ma, F.; Ye, H.; He, H.H.; Gerrin, S.J.; Chen, S.; Tanenbaum, B.A.; Cai, C.; Sowalsky, A.G.; He, L.; Wang, H.; et al. SOX9 drives WNT pathway activation in prostate cancer. J. Clin. Investig. 2016, 126, 1745–1758. [Google Scholar] [CrossRef]
- Canesin, G.; Evans-Axelsson, S.; Hellsten, R.; Krzyzanowska, A.; Prasad, C.P.; Bjartell, A.; Andersson, T. Treatment with the WNT5A-mimicking peptide Foxy-5 effectively reduces the metastatic spread of WNT5A-low prostate cancer cells in an orthotopic mouse model. PLoS ONE 2017, 12, e0184418. [Google Scholar] [CrossRef]
- Jimeno, A.; Gordon, M.; Chugh, R.; Messersmith, W.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Anusewicz, D.; Orzechowska, M.; Bednarek, A.K. Notch Signaling Pathway in Cancer-Review with Bioinformatic Analysis. Cancers 2021, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, T.; Riedinger, M.; Lin, S.; Faltermeier, C.M.; Smith, B.A.; Zhang, K.X.; Going, C.C.; Goldstein, A.S.; Lee, J.K.; Drake, J.M.; et al. Activation of Notch1 synergizes with multiple pathways in promoting castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6457–E6466. [Google Scholar] [CrossRef]
- Kwon, O.J.; Zhang, L.; Wang, J.; Su, Q.; Feng, Q.; Zhang, X.H.; Mani, S.A.; Paulter, R.; Creighton, C.J.; Ittmann, M.M.; et al. Notch promotes tumor metastasis in a prostate-specific Pten-null mouse model. J. Clin. Investig. 2016, 126, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef]
- Li, J.L.; Sainson, R.C.; Shi, W.; Leek, R.; Harrington, L.S.; Preusser, M.; Biswas, S.; Turley, H.; Heikamp, E.; Hainfellner, J.A.; et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function, and promotes tumor growth in vivo. Cancer Res. 2007, 67, 11244–11253. [Google Scholar] [CrossRef]
- Rice, M.A.; Hsu, E.C.; Aslan, M.; Ghoochani, A.; Su, A.; Stoyanova, T. Loss of Notch1 Activity Inhibits Prostate Cancer Growth and Metastasis and Sensitizes Prostate Cancer Cells to Antiandrogen Therapies. Mol. Cancer Ther. 2019, 18, 1230–1242. [Google Scholar] [CrossRef]
- Wang, L.; Zi, H.; Luo, Y.; Liu, T.; Zheng, H.; Xie, C.; Wang, X.; Huang, X. Inhibition of Notch pathway enhances the anti-tumor effect of docetaxel in prostate cancer stem-like cells. Stem Cell Res. Ther. 2020, 11, 258. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Tan, S.H.; Xavier, C.P.; Katta, S.; Huang, W.; Ravindranath, L.; Jamal, M.; Li, H.; Srivastava, M.; Srivatsan, E.S.; et al. Synergistic Activity with NOTCH Inhibition and Androgen Ablation in ERG-Positive Prostate Cancer Cells. Mol. Cancer Res. 2017, 15, 1308–1317. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Ghanbari-Movahed, Z.; Momtaz, S.; Kilpatrick, K.L.; Farzaei, M.H.; Bishayee, A. Unlocking the Secrets of Cancer Stem Cells with γ-Secretase Inhibitors: A Novel Anticancer Strategy. Molecules 2021, 26, 972. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Messersmith, W.A.; Mikulski, S.M.; Papadopoulos, K.P.; Kwak, E.L.; Gibbon, D.G.; Patnaik, A.; Falchook, G.S.; Dasari, A.; Shapiro, G.I.; et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J. Clin. Oncol. 2012, 30, 2348–2353. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; DiPaola, R.S.; Mayer, T.M.; Jeyamohan, C.; Metzger, D.; Anand, M.; Ivy, S.P.; Consortium, P.C.C.T. A randomized phase II study of bicalutamide (BIC) followed by placebo or gamma secretase inhibitor RO4929097 (RO492) in men with rising PSA. J. Clin. Oncol. 2012, 30, 219. [Google Scholar] [CrossRef]
- Cui, D.; Dai, J.; Keller, J.M.; Mizokami, A.; Xia, S.; Keller, E.T. Notch Pathway Inhibition Using PF-03084014, a γ-Secretase Inhibitor (GSI), Enhances the Antitumor Effect of Docetaxel in Prostate Cancer. Clin. Cancer Res. 2015, 21, 4619–4629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ning, J.; Wakimoto, H.; Wu, S.; Wu, C.-l.; Humphrey, M.R.; Rabkin, S.D.; Martuza, R.L. Oncolytic Herpes Simplex Virus and PI3K Inhibitor BKM120 Synergize to Promote Killing of Prostate Cancer Stem-like Cells. Mol. Ther. Oncolytics 2019, 13, 58–66. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Guttridge, D.C.; Mayo, M.W.; Baldwin, A.S. NF-κB Induces Expression of the Bcl-2 Homologue A1/Bfl-1 To Preferentially Suppress Chemotherapy-Induced Apoptosis. Mol. Cell. Biol. 1999, 19, 5923–5929. [Google Scholar] [CrossRef]
- Peng, Y.-M.; Zheng, J.-B.; Zhou, Y.-B.; Li, J. Characterization of a novel curcumin analog P1 as potent inhibitor of the NF-κB signaling pathway with distinct mechanisms. Acta Pharmacol. Sin. 2013, 34, 939–950. [Google Scholar] [CrossRef]
- Jin, R.; Yi, Y.; Yull, F.E.; Blackwell, T.S.; Clark, P.E.; Koyama, T.; Smith, J.A., Jr.; Matusik, R.J. NF-κB gene signature predicts prostate cancer progression. Cancer Res. 2014, 74, 2763–2772. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Li, J.; Yadav, S.S.; Tewari, A.K. Recent insights into NF-κB signalling pathways and the link between inflammation and prostate cancer. BJU Int. 2014, 114, 168–176. [Google Scholar] [CrossRef]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, R.; Wei, J.; Zhou, Y.; Ji, W.; Liu, J.; Zhi, X.; Zhang, J. Effects of PI3K inhibitor NVP-BKM120 on overcoming drug resistance and eliminating cancer stem cells in human breast cancer cells. Cell Death Dis. 2015, 6, e2020. [Google Scholar] [CrossRef]
- Heath, E.I.; Hillman, D.W.; Vaishampayan, U.; Sheng, S.; Sarkar, F.; Harper, F.; Gaskins, M.; Pitot, H.C.; Tan, W.; Ivy, S.P.; et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Cancer Res. 2008, 14, 7940–7946. [Google Scholar] [CrossRef]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.W. NF-kappaB in cancer: From innocent bystander to major culprit. Nat. Rev. Cancer 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Lien, E.C.; Lyssiotis, C.A.; Cantley, L.C. Metabolic reprogramming by the PI3K-Akt-mTOR pathway in cancer. In Metabolism in Cancer; Springer: Berlin/Heidelberg, Germany, 2016; pp. 39–72. [Google Scholar] [CrossRef]
- Morgan, T.M.; Koreckij, T.D.; Corey, E. Targeted therapy for advanced prostate cancer: Inhibition of the PI3K/Akt/mTOR pathway. Curr. Cancer Drug Targets 2009, 9, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Febbo, P.G.; Bikoff, R.; Berger, R.; Xue, Q.; McMahon, L.M.; Manola, J.; Brugarolas, J.; McDonnell, T.J.; Golub, T.R.; et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004, 10, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Pitts, T.E.; Gross, T.S.; Poliachik, S.L.; Vessella, R.L.; Corey, E. RAD001 (Everolimus) inhibits growth of prostate cancer in the bone and the inhibitory effects are increased by combination with docetaxel and zoledronic acid. Prostate 2008, 68, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, Q.; Liu, S.; Ning, N.; Zhang, X.; Xu, Y.; Chang, A.E.; Wicha, M.S. Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches. Stem Cells 2015, 33, 2085–2092. [Google Scholar] [CrossRef]
- Lang, S.H.; Frame, F.M.; Collins, A.T. Prostate cancer stem cells. J. Pathol. 2009, 217, 299–306. [Google Scholar] [CrossRef]
- Borovski, T.; De Sousa, E.M.F.; Vermeulen, L.; Medema, J.P. Cancer stem cell niche: The place to be. Cancer Res. 2011, 71, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A perivascular niche for brain tumor stem cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Chen, K.; Huang, Y.H.; Chen, J.L. Understanding and targeting cancer stem cells: Therapeutic implications and challenges. Acta Pharm. Sin. 2013, 34, 732–740. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Boonstra, J. Cancer stem cells and addicted cancer cells. Oncol. Discov. 2013, 1. [Google Scholar] [CrossRef]
- Skvortsov, S.; Skvortsova, I.I.; Tang, D.G.; Dubrovska, A. Concise Review: Prostate Cancer Stem Cells: Current Understanding. Stem Cells 2018, 36, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, E.; Mukhtarova, G.; Alpay, A.; Avci, C.B.; Bagca, B.G.; Oktem, G. Sonic hedgehog signaling is associated with resistance to zoledronic acid in CD133high/CD44high prostate cancer stem cells. Mol. Biol. Rep. 2021, 48, 3567–3578. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.L.; Pursell, B.; Shultz, L.D.; Greiner, D.L.; Brekken, R.A.; Vander Kooi, C.W.; Mercurio, A.M. P-Rex1 Promotes Resistance to VEGF/VEGFR-Targeted Therapy in Prostate Cancer. Cell Rep. 2016, 14, 2193–2208. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.S.; Kerr, B.A. Prostate Cancer Stem Cell Markers Drive Progression, Therapeutic Resistance, and Bone Metastasis. Stem Cells Int. 2017, 2017, 8629234. [Google Scholar] [CrossRef]
- Gill, S.; June, C.H. Going viral: Chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol. Rev. 2015, 263, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Abate-Daga, D.; Lagisetty, K.H.; Tran, E.; Zheng, Z.; Gattinoni, L.; Yu, Z.; Burns, W.R.; Miermont, A.M.; Teper, Y.; Rudloff, U.; et al. A novel chimeric antigen receptor against prostate stem cell antigen mediates tumor destruction in a humanized mouse model of pancreatic cancer. Hum. Gene Ther. 2014, 25, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, J.; Jafarzadeh, A.; Abdolalizadeh, J.; Khan, H.; Philippe, J.; Mirzaei, H.; Mirzaei, H.R. Cancer stem cell-targeted chimeric antigen receptor (CAR)-T cell therapy: Challenges and prospects. Acta Pharm. Sin. B 2021, 11, 1721–1739. [Google Scholar] [CrossRef]
- Yu, H.; Pan, J.; Guo, Z.; Yang, C.; Mao, L. CART cell therapy for prostate cancer: Status and promise. OncoTargets Ther. 2019, 12, 391–395. [Google Scholar] [CrossRef]
- Yang, J.; Aljitawi, O.; Van Veldhuizen, P. Prostate Cancer Stem Cells: The Role of CD133. Cancers 2022, 14, 5448. [Google Scholar] [CrossRef]
- Kanwal, R.; Shukla, S.; Walker, E.; Gupta, S. Acquisition of tumorigenic potential and therapeutic resistance in CD133+ subpopulation of prostate cancer cells exhibiting stem-cell like characteristics. Cancer Lett. 2018, 430, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Prasad, S.; Gaedicke, S.; Hettich, M.; Firat, E.; Niedermann, G. Patient-derived glioblastoma stem cells are killed by CD133-specific CAR T cells but induce the T cell aging marker CD57. Oncotarget 2015, 6, 171–184. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef]
- Imrich, S.; Hachmeister, M.; Gires, O. EpCAM and its potential role in tumor-initiating cells. Cell Adh. Migr. 2012, 6, 30–38. [Google Scholar] [CrossRef]
- Ni, J.; Cozzi, P.J.; Duan, W.; Shigdar, S.; Graham, P.H.; John, K.H.; Li, Y. Role of the EpCAM (CD326) in prostate cancer metastasis and progression. Cancer Metastasis Rev. 2012, 31, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Massoner, P.; Thomm, T.; Mack, B.; Untergasser, G.; Martowicz, A.; Bobowski, K.; Klocker, H.; Gires, O.; Puhr, M. EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br. J. Cancer 2014, 111, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wu, Y.; Ma, W.; Zhang, S.; Zhang, Y.Q. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Li, D.; Zhang, B.; Chen, Y.; Liao, X.; Li, X.; Alexander, P.B.; Wang, Y.; Li, Q.J. Potential lung attack and lethality generated by EpCAM-specific CAR-T cells in immunocompetent mouse models. Oncoimmunology 2020, 9, 1806009. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Moreira, R.B.; Debiasi, M.; Francini, E.; Nuzzo, P.V.; De Velasco, G.; Maluf, F.C.; Fay, A.P.; Bellmunt, J.; Choueiri, T.K.; Schutz, F.A. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: A meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 84572–84578. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Smith, M.R.; Sweeney, C.; ElFiky, A.A.; Logothetis, C.; Corn, P.G.; Vogelzang, N.J.; Small, E.J.; Harzstark, A.L.; Gordon, M.S.; et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J. Clin. Oncol. 2013, 31, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C. Overcoming docetaxel resistance in prostate cancer: A perspective review. Ther. Adv. Med. Oncol. 2012, 4, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef]
- Huang, W.Y.; Lin, J.N.; Hsieh, J.T.; Chou, S.C.; Lai, C.H.; Yun, E.J.; Lo, U.G.; Pong, R.C.; Lin, J.H.; Lin, Y.H. Nanoparticle Targeting CD44-Positive Cancer Cells for Site-Specific Drug Delivery in Prostate Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 30722–30734. [Google Scholar] [CrossRef]
- Wei, J.; Sun, J.; Liu, Y. Enhanced targeting of prostate cancer-initiating cells by salinomycin-encapsulated lipid-PLGA nanoparticles linked with CD44 antibodies. Oncol. Lett. 2019, 17, 4024–4033. [Google Scholar] [CrossRef]
- Mahira, S.; Kommineni, N.; Husain, G.M.; Khan, W. Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer. Biomed. Pharmacother. 2019, 110, 803–817. [Google Scholar] [CrossRef]
- Sanfilippo, V.; Caruso, V.C.L.; Cucci, L.M.; Inturri, R.; Vaccaro, S.; Satriano, C. Hyaluronan-Metal Gold Nanoparticle Hybrids for Targeted Tumor Cell Therapy. Int. J. Mol. Sci. 2020, 21, 3085. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Ingram, N.; Coletta, P.L.; Millner, P.A.; Tyler, A., II; Hughes, T.A. Hyaluronic-Acid-Tagged Cubosomes Deliver Cytotoxics Specifically to CD44-Positive Cancer Cells. Mol. Pharm. 2022, 19, 4601–4611. [Google Scholar] [CrossRef]
- Kim, W.T.; Ryu, C.J. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017, 50, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Hou, N.; Liu, Y.; Liu, B.; Cao, W.; Zheng, D.; Li, W.; Liu, Y.; Xu, B.; Wang, Z.; et al. CD133 antibody targeted delivery of gold nanostars loading IR820 and docetaxel for multimodal imaging and near-infrared photodynamic/photothermal/chemotherapy against castration resistant prostate cancer. Nanomedicine 2020, 27, 102192. [Google Scholar] [CrossRef] [PubMed]
- Shigdar, S.; Qiao, L.; Zhou, S.F.; Xiang, D.; Wang, T.; Li, Y.; Lim, L.Y.; Kong, L.; Li, L.; Duan, W. RNA aptamers targeting cancer stem cell marker CD133. Cancer Lett. 2013, 330, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Qian, W.; Tao, W.; Zhou, Y.; Xue, B. Delivery of Curcumin Nanoliposomes Using Surface Modified With CD133 Aptamers For Prostate Cancer. Drug Des. Dev. Ther. 2019, 13, 4021–4033. [Google Scholar] [CrossRef]
- Wolf, I.; Gratzke, C.; Wolf, P. Prostate Cancer Stem Cells: Clinical Aspects and Targeted Therapies. Front. Oncol. 2022, 12, 935715. [Google Scholar] [CrossRef] [PubMed]
| Pathway | Target | NCT Number | Phase | Status | Notes |
|---|---|---|---|---|---|
| Hh | Sonidegib | NCT02111187 | 1 | Completed | Primary outcome: change from baseline in tissue GLi1 expression levels in those receiving LDE225, compared to those receiving no treatment prior to prostatectomy. Results: 6/7 participants had at least a 2-fold reduction in GLi1 expression in post treatment vs. pretreatment tumor tissue. |
| NCT02182622 | 1 | Withdrawn | Primary outcome: maximum tolerated dose of LDE225 plus docetaxel/prednisone. Withdrawn: no reason reported. | ||
| GDC-0449 | NCT01163084 | 1,2 | Terminated | Primary outcome: proportion of patients with </=5% tumor involvement in those receiving leuprolide acetate or goserelin acetate with or without vismodegib. Terminated: no reason reported. | |
| NCT 00607724 | 1 | Completed | Primary outcome: percentage of participants with dose-limiting toxicities (DLTs) Results: 0/68 subjects affected. | ||
| NCT02465060 | 2 | Recruiting | Primary outcome: objective response rate in those receiving targeted therapy directed by genetic testing. Recruiting: projected completion 2025. | ||
| Wnt | Foxy-5 | NCT02020291 | 1 | Completed | Primary outcome: safety and tolerability of Foxy-5. No results posted. |
| NCT02655952 | 1 | Completed | Primary outcome: presence of dose limiting toxicities of Foxy-5. No results posted. | ||
| Notch | RO4929097 | NCT01200810 | 2 | Terminated | Primary outcome: time to PSA progression in those receiving bicalutamide and RO4929097. Terminated: lack of study drugs. |
| NF-kB | Bortezomib | NCT00103376 | 2 | Terminated | Primary outcome: PSA response in those receiving bortezomib with or without hormone therapy. Terminated: low accrual. |
| NCT00183937 | 2 | Completed | Primary outcome: number of patients with improved serum PSA response rate in those receiving bortezomib and docetaxel. No results posted. | ||
| NCT00059631 | 1 | Completed | Primary outcome: maximum tolerated dose of mitoxantrone combined with bortezomib. No results posted. | ||
| NCT00193232 | 2 | Completed | Primary outcome: objective response rate. Results: treatment with combination of weekly docetaxel and bortezomib showed no improved efficacy vs. previous results with docetaxel alone, bortezomib has minimal activity in pts with HRPC and is unlikely to make any impact on treatment efficacy. | ||
| NCT00425503 | 2 | Completed | Primary outcome: assess the safety of PS-341 as a pretreatment in patients who are to undergo a radical prostatectomy measured with poor wound healing and excessive bleeding. Results: 0/37 subjects affected. | ||
| NCT00064610 | 1,2 | Completed | Primary outcome: determine the maximum tolerated dose and preliminary activity of PS-341 plus docetaxel. No results posted. | ||
| NCT00667641 | 1 | Completed | Primary outcome: maximum tolerated dose of paclitaxel in combination with bortezomib. No results posted. | ||
| NCT00620295 | 1 | Completed | Primary outcome: maximum tolerated dose of bortezomib and gemcitabine. No results posted. | ||
| Aspirin | NCT03819101 | 3 | Recruiting | Primary outcome: overall survival in those taking aspirin and atorvastatin. Recruiting: projected completion 2034. | |
| NCT02757365 | 4 | Unknown | Primary outcome: aspirin PSA response, digital rectal exam, US of prostate, biopsy, fPSA level. Unknown: last status verification in 2016 | ||
| NCT02420652 | 2 | Terminated | Primary outcome: change in stable PSA rates after 6 months of metformin hydrochloride and aspirin or placebo. Terminated: slow accrual. | ||
| NCT01428869 | Unknown | Completed | Primary outcome: diagnosis of prostate cancer in REDUCE study participants treated with statins, aspirin, and dutasteride. No results posted. | ||
| NCT00234299 | NA | Completed | Primary outcome: assess the effect of oral aspirin on in vivo prostate epithelial cells. No results posted. | ||
| NCT02804815 | 3 | Recruiting | Primary outcome: survival and disease recurrence in those receiving aspirin after primary therapy. Recruiting: projected completion 2026. | ||
| BKM120 | NCT01695473 | 2 | Terminated | Primary outcome: percent of patients with decrease in phosphorylated S6 ICH from baseline, percent with downstream target inhibition of PI3K in prostate tumor tissue measured by ICH when treated with BKM120. Terminated: lack of accrual. | |
| NCT01634061 | 1 | Completed | Primary outcome: incidence of dose limiting toxicities and PSA decline >/= 30% in those getting abiraterone acetate and BEZ235 or BKM120. No results posted. | ||
| NCT01385293 | 2 | Terminated | Primary outcome: progression free survival prostate cancer working group 2 criteria or based on the onset of a skeletal related event in those getting BKM120. Terminated: 1st stage due to futility | ||
| NCT02035124 | 2 | Withdrawn | Primary outcome: number of subjects with serious and non-serious adverse events and progression free survival in those receiving cabazitaxel and BKM120. Withdrawn: slow accrual, no subjects enrolled. | ||
| NCT02487823 | 1 | Terminated | Primary outcome: determine MTD of BKM120 when given orally in combination with daily bicalutamide and LH-RH agonists. Terminated: defect of recruitment. | ||
| NCT01741753 | 1 | Terminated | Primary outcome: safety profile and mTD for BKM120/abiraterone/prednisone. Terminated: slow accrual, supplier of BKM120 asked to cease further enrollment. | ||
| PI3K/AKT/mTOR | BEZ235 | NCT01717898 | 1,2 | Terminated | Primary outcome: number of reported DLT and MTD when combining BEZ235 and abiraterone acetate, decline in PSA > 50%. Terminated: DLT on lowest dose level. |
| NCT01634061 | 1 | Completed | Primary outcome: incidence of DLT and PSA decline >/= 30% in those getting abiraterone acetate and BEZ235 or BKM120. No results posted. | ||
| VEGF | Bevacizumab | NCT00203424 | 2 | Completed | Primary outcome: evaluate efficacy of bevacizumab and erlotinib, time to tumor recurrence. Results: 7/19 subjects had tumor recurrence, average 285 days. |
| NCT00499694 | NA | Completed | Primary outcome: time to progression in those taking satraplatin and bevacizumab in mPCa patients previously treated with docetaxel. Results: average time to progression 7 months. | ||
| NCT00574769 | 1,2 | Completed | Primary outcome: MTD of RAD001 with docetaxel/bevacizumab. No results posted. | ||
| NCT00776594 | 2 | Completed | Primary outcome: relapse-free survival in those treated with ADT vs. ADT plus bevacizumab. Results: average relapse free survival of 13 months vs. 10 months respectively. | ||
| NCT00321646 | 2 | Completed | Primary outcome: endorectal MRI response after completion of 6 cycles of neoadjuvant therapy in subjects receiving bevacizumab plus docetaxel. Results: 22% of participants had a response. | ||
| NCT00027599 | 2,3 | Completed | Primary outcome: efficacy of APC8015 and bevacizumab in terms of decline in PSA and effect on PSA doubling time. No results posted. | ||
| NCT00348998 | 2 | Unknown | Primary outcome: determine the safety and efficacy of bevacizumab with hormonal therapy and radiotherapy. Unknown: last status update in 2008 stated active, not recruiting. | ||
| NCT00089609 | 2 | Completed | Primary outcome: number of participants with PSA response and immune response to docetaxel, thalidomide, prednisone, and bevacizumab. Results: 52/60 subjects had PSA response, immune response not measured. | ||
| NCT01083368 | 1,2 | Completed | Primary outcome: MTD of temsirolimus with fixed dose bevacizumab and objective response via PSA level. Results: MTD of temsirolimus was 25 mg, 5/16 subjects had a change from baseline PSA. | ||
| NCT01656304 | 2 | Completed | Primary outcome: PSA response rate with bevacizumab in non-metastatic CRPC, toxicities of bevacizumab, and time to PSA progression. Results: 5/15 subjects had a PSA decline >/=50%, 14/15 subjects had toxicities, time to PSA progression = average 2.8 months. | ||
| NCT00016107 | 2 | Completed | Primary outcome: time to objective progression, objective and PSA response rates, and toxicity in those receiving chemo plus bevacizumab. No results posted. | ||
| NCT00658697 | 2 | Completed | Primary outcome: PSA progression at 1 year after completing ADT. Results: 98% had PSA progression at 1 year. | ||
| NCT00110214 | 3 | Completed | Primary outcome: overall survival in those receiving docetaxel and prednisone with or without bevacizumab who did not respond to hormone therapy. Results: those taking docetaxel plus placebo survived average 21.5 months, docetaxel plus bevacizumab survived average 22.6 months. | ||
| NCT00942331 | 3 | Completed | Primary outcome: overall survival in those receiving gemcitabine hydrochloride and cisplatin with or without bevacizumab. Results: those taking gemcitabine hydrochloride, cisplatin, and bevacizumab survived average 14.5 months, gemcitabine hydrochloride, cisplatin, and placebo survived average 14.3 months. | ||
| NCT05489211 | 2 | Recruiting | Primary outcome: objective response rate, number of subjects with adverse events in those receiving dato-dxd monotherapy and in combination with anti-cancer drugs including bevacizumab. Recruiting: projected completion 2025. | ||
| CAR-T | EpCAM | NCT03013712 | 1,2 | Unknown | Primary outcome: toxicity profile of EpCAM targeted CAR T cells with CTCAE. Unknown: last status update 2017 stated recruiting. |
| Nanoparticles | Nanoparticle-Based Drug Delivery System | NCT00499291 | NA | Withdrawn | Primary outcome: develop a population pharmacokinetic model in those receiving paclitaxel albumin-stabilized nanoparticle formulation. Withdrawn: no reason reported |
| NCT04221828 | 2 | Terminated | Primary outcome: number of adverse events in those receiving NanoPac (sterile nanoparticle paclitaxel). Terminated: lack of enrollment | ||
| NCT03531827 | 2 | Terminated | Primary outcome: percentage of participants with anti-tumor activity with combined CRLX101 and enzalutamide in those who previously failed enzalutamide therapy. Terminated: closed due to toxicity | ||
| NCT02769962 | 2 | Recruiting | Primary outcome: determine overall response rate of EP005 plus Olaparib in mCRPC. Results: projected completion 2023 | ||
| NCT02646319 | 1 | Completed | Primary outcome: clinical benefit rate, incidence of adverse events, survival time, and time to disease progression in those receiving nanoparticle albumin-bound rapamycin. No results posted. | ||
| NCT01300533 | 1 | Completed | Primary outcome: determine DLT of BIND-014. No results posted. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gogola, S.; Rejzer, M.; Bahmad, H.F.; Alloush, F.; Omarzai, Y.; Poppiti, R. Anti-Cancer Stem-Cell-Targeted Therapies in Prostate Cancer. Cancers 2023, 15, 1621. https://doi.org/10.3390/cancers15051621
Gogola S, Rejzer M, Bahmad HF, Alloush F, Omarzai Y, Poppiti R. Anti-Cancer Stem-Cell-Targeted Therapies in Prostate Cancer. Cancers. 2023; 15(5):1621. https://doi.org/10.3390/cancers15051621
Chicago/Turabian StyleGogola, Samantha, Michael Rejzer, Hisham F. Bahmad, Ferial Alloush, Yumna Omarzai, and Robert Poppiti. 2023. "Anti-Cancer Stem-Cell-Targeted Therapies in Prostate Cancer" Cancers 15, no. 5: 1621. https://doi.org/10.3390/cancers15051621
APA StyleGogola, S., Rejzer, M., Bahmad, H. F., Alloush, F., Omarzai, Y., & Poppiti, R. (2023). Anti-Cancer Stem-Cell-Targeted Therapies in Prostate Cancer. Cancers, 15(5), 1621. https://doi.org/10.3390/cancers15051621