Adjuvant Docetaxel in Node-Negative Breast Cancer Patients: A Randomized Trial of AGO-Breast Study Group, German Breast Group, and EORTC-Pathobiology Group
Abstract
Simple Summary
Abstract
1. Introduction
- To investigate whether substituting the last three courses of a standard adjuvant FE100C (six courses) by three courses of docetaxel improves the disease-free survival of high-risk node-negative breast cancer patients.
- To quantify the effects of the tumor-biological risk assessment (uPA/PAI-1) and the clinico-pathological risk assessment with regard to disease-free survival and the proportion of low-risk patients in node-negative breast cancer patients.
- With current follow-up, data are only mature to report on the first question.
2. Materials and Methods
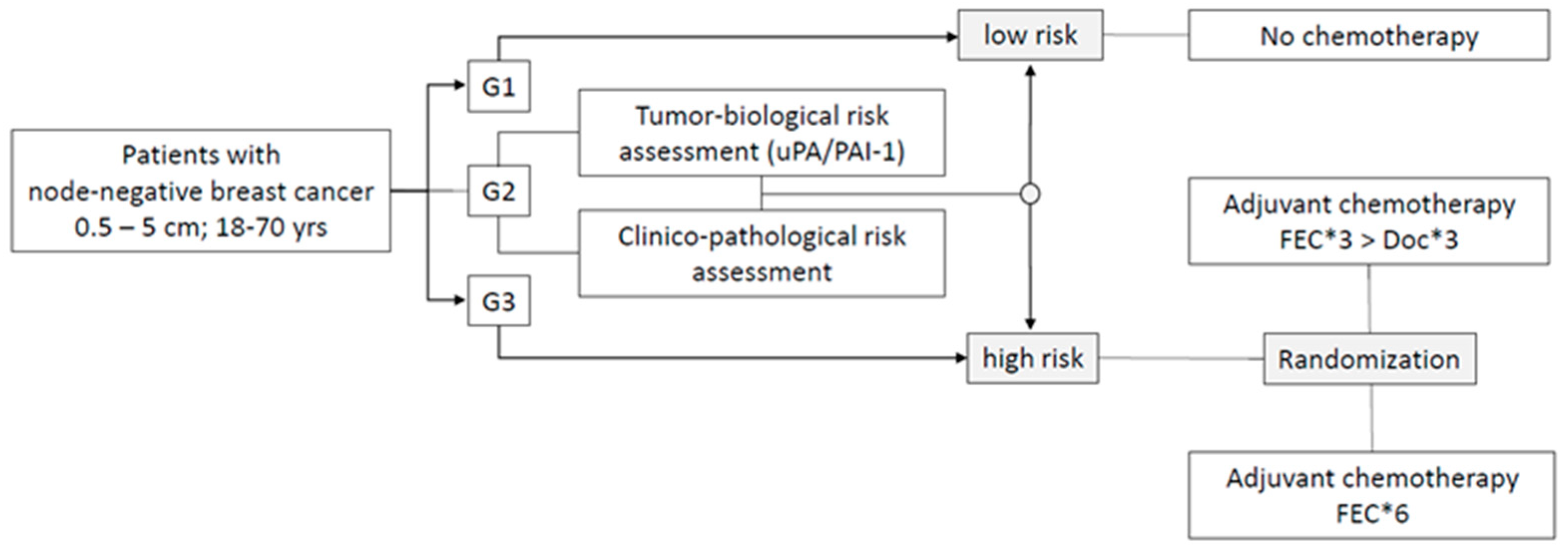
2.1. The Objectives
2.2. Patient Population
2.3. Study Design and Procedures
2.4. Mode of Risk Assessment
2.5. uPA and PAI-1 Assessment
2.6. Quality Assurance of uPA and PAI-1 Testing
2.7. Data Collection and Statistical Analysis
3. Results
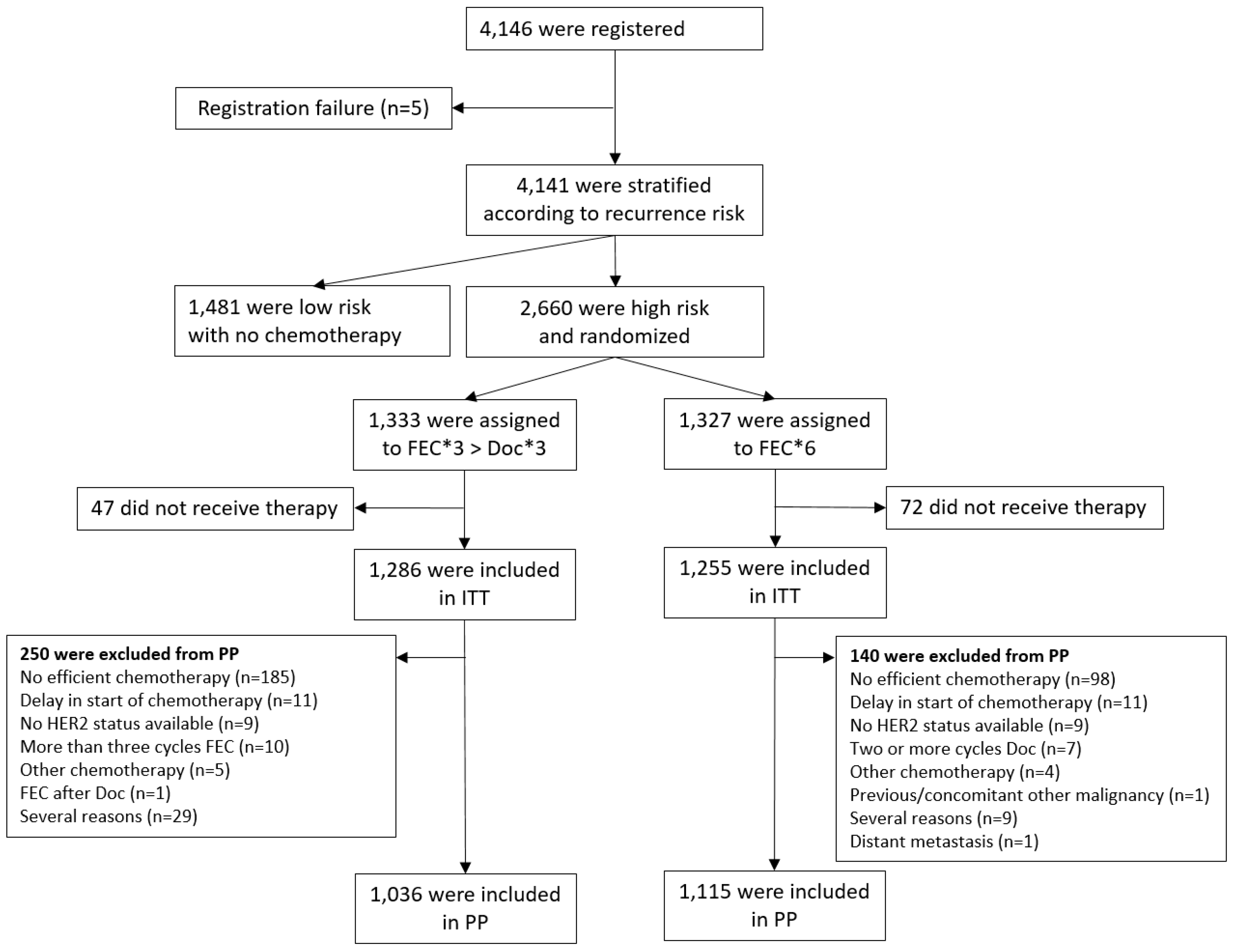
3.1. Patient Population
3.2. Patient Characteristics
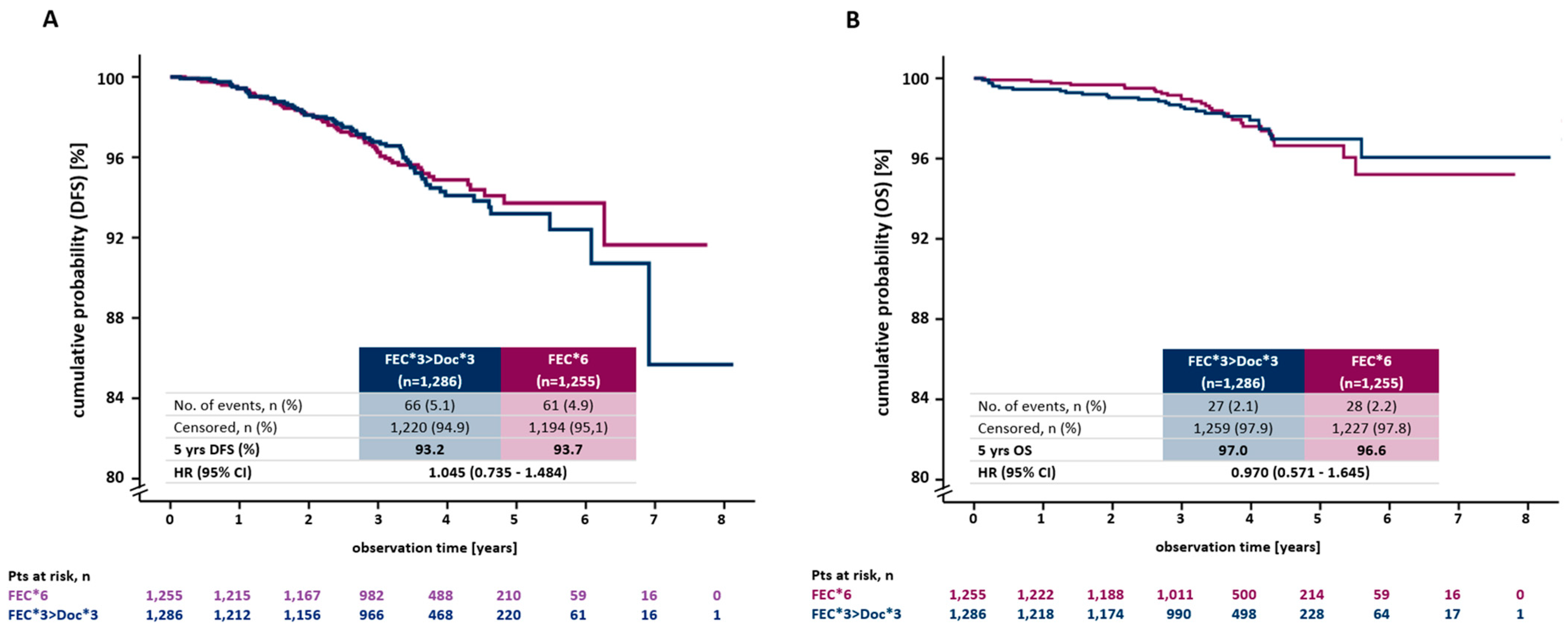
3.3. Disease-Free Survival
3.4. Overall Survival
3.5. Prognostic Factors
3.6. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martín, M.; Ruiz, A.; Ruiz Borrego, M.; Barnadas, A.; González, S.; Calvo, L.; Margelí Vila, M.; Antón, A.; Rodríguez-Lescure, A.; Seguí-Palmer, M.A.; et al. Fluorouracil, doxorubicin, and cyclophosphamide (FAC) versus FAC followed by weekly paclitaxel as adjuvant therapy for high-risk, node-negative breast cancer: Results from the GEICAM/2003-02 study. J. Clin. Oncol. 2013, 31, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Seguí, M.A.; Antón, A.; Ruiz, A.; Ramos, M.; Adrover, E.; Aranda, I.; Rodríguez-Lescure, A.; Grosse, R.; Calvo, L.; et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N. Engl. J. Med. 2010, 363, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Victor, A.; Bratzel, D.; Boehm, D.; Cotarelo, C.; Lebrecht, A.; Siggelkow, W.; Hengstler, J.G.; Elsässer, A.; Gehrmann, M.; et al. Long-term outcome prediction by clinicopathological risk classification algorithms in node-negative breast cancer--comparison between Adjuvant!, St Gallen, and a novel risk algorithm used in the prospective randomized Node-Negative-Breast Cancer-3 (NNBC-3) trial. Ann. Oncol. 2009, 20, 258–264. [Google Scholar] [CrossRef]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef] [PubMed]
- Ditsch, N.; Wöcke, A.; Untch, M.; Jackisch, C.; Albert, U.-S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J.-U.; Budach, W.; Dall, P.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2022. Breast Care 2022, 17, 403–420. [Google Scholar] [CrossRef]
- Harris, L.; Fritsche, H.; Mennel, R.; Norton, L.; Ravdin, P.; Taube, S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J. Clin. Oncol. 2007, 25, 5287–5312. [Google Scholar] [CrossRef]
- Jänicke, F.; Schmitt, M.; Ulm, K.; Gössner, W.; Graeff, H. Urokinase-type plasminogen activator antigen and early relapse in breast cancer. Lancet 1989, 334, 1049. [Google Scholar] [CrossRef]
- Jänicke, F.; Schmitt, M.; Graeff, H. Clinical Relevance of the Urokinase-Type and Tissue-Type Plasminogen Activators and of Their Type 1 Inhibitor in Breast Cancer. Semin. Thromb. Hemost. 1991, 17, 303–312. [Google Scholar] [CrossRef]
- Jänicke, F.; Schmitt, M.; Pache, L.; Ulm, K.; Harbeck, N.; Höfler, H.; Gössner, W. Urokinase (uPA) and its inhibitorPAI-1 are strong and independent prognostic factors in node-negative breast cancer. Breast Cancer Res. Treat. 1993, 24, 195–208. [Google Scholar] [CrossRef]
- Jänicke, F.; Prechtl, A.; Thomssen, C.; Harbeck, N.; Meisner, C.; Untch, M.; Sweep, C.G.; Selbmann, H.K.; Graeff, H.; Schmitt, M. Randomized adjuvant chemotherapy trial in high-risk, lymph node-negative breast cancer patients identified by urokinase-type plasminogen activator and plasminogen activator inhibitor type 1. J. Natl. Cancer Inst. 2001, 93, 913–920. [Google Scholar] [CrossRef]
- Schmitt, M.; Harbeck, N.; Brünner, N.; Jänicke, F.; Meisner, C.; Mühlenweg, B.; Jansen, H.; Dorn, J.; Nitz, U.; Kantelhardt, E.J.; et al. Cancer therapy trials employing level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev. Mol. Diagn. 2011, 11, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Schmitt, M.; Meisner, C.; Friedel, C.; Untch, M.; Schmidt, M.; Sweep, C.G.J.; Lisboa, B.W.; Lux, M.P.; Beck, T.; et al. Ten-year analysis of the prospective multicentre Chemo-N0 trial validates American Society of Clinical Oncology (ASCO)-recommended biomarkers uPA and PAI-1 for therapy decision making in node-negative breast cancer patients. Eur. J. Cancer 2013, 49, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Kates, R.E.; Look, M.P.; Meijer-Van Gelder, M.E.; Klijn, J.G.M.; Krüger, A.; Kiechle, M.; Jänicke, F.; Schmitt, M.; Foekens, J.A. Enhanced benefit from adjuvant chemotherapy in breast cancer patients classified high-risk according to urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (n = 3424). Cancer Res. 2002, 62, 4617–4622. [Google Scholar]
- Look, M.P.; van Putten, W.L.J.; Duffy, M.J.; Harbeck, N.; Christensen, I.J.; Thomssen, C.; Kates, R.; Spyratos, F.; Fernö, M.; Eppenberger-Castori, S.; et al. Pooled analysis of prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in 8377 breast cancer patients. J. Natl. Cancer Inst. 2002, 94, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Look, M.; van Putten, W.; Duffy, M.; Harbeck, N.; Christensen, I.J.; Thomssen, C.; Kates, R.; Spyratos, F.; Fernö, M.; Eppenberger-Castori, S.; et al. Pooled analysis of prognostic impact of uPA and PAI-1 in breast cancer patients. Thromb. Haemost. 2003, 90, 538–548. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Gelber, R.D.; Coates, A.S.; Thürlimann, B.; Senn, H.-J. Meeting highlights: Updated international expert consensus on the primary therapy of early breast cancer. J. Clin. Oncol. 2003, 21, 3357–3365. [Google Scholar] [CrossRef]
- Kantelhardt, E.J.; Vetter, M.; Schmidt, M.; Veyret, C.; Augustin, D.; Hanf, V.; Meisner, C.; Paepke, D.; Schmitt, M.; Sweep, F.; et al. Prospective evaluation of prognostic factors uPA/PAI-1 in node-negative breast cancer: Phase III NNBC3-Europe trial (AGO, GBG, EORTC-PBG) comparing 6×FEC versus 3×FEC/3×Docetaxel. BMC Cancer 2011, 11, 140. [Google Scholar] [CrossRef]
- Bonneterre, J.; Roché, H.; Kerbrat, P.; Brémond, A.; Fumoleau, P.; Namer, M.; Goudier, M.-J.; Schraub, S.; Fargeot, P.; Chapelle-Marcillac, I. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J. Clin. Oncol. 2005, 23, 2686–2693. [Google Scholar] [CrossRef]
- Sweep, C.G.; Geurts-Moespot, J.; Grebenschikov, N.; de Witte, J.H.; Heuvel, J.J.; Schmitt, M.; Duffy, M.J.; Jänicke, F.; Kramer, M.D.; Foekens, J.A.; et al. External quality assessment of trans-European multicentre antigen determinations (enzyme-linked immunosorbent assay) of urokinase-type plasminogen activator (uPA) and its type 1 inhibitor (PAI-1) in human breast cancer tissue extracts. Br. J. Cancer 1998, 78, 1434–1441. [Google Scholar] [CrossRef]
- Harbeck, N.; Kates, R.E.; Schmitt, M. Clinical relevance of invasion factors urokinase-type plasminogen activator and plasminogen activator inhibitor type 1 for individualized therapy decisions in primary breast cancer is greatest when used in combination. J. Clin. Oncol. 2002, 20, 1000–1007. [Google Scholar] [CrossRef]
- Schmitt, M.; Sturmheit, A.S.; Welk, A.; Schnelldorfer, C.; Harbeck, N. Procedures for the quantitative protein determination of urokinase and its inhibitor, PAI-1, in human breast cancer tissue extracts by ELISA. Methods Mol. Med. 2006, 120, 245–265. [Google Scholar] [CrossRef] [PubMed]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.-A.W.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Roché, H.; Fumoleau, P.; Spielmann, M.; Canon, J.-L.; Delozier, T.; Serin, D.; Symann, M.; Kerbrat, P.; Soulié, P.; Eichler, F.; et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J. Clin. Oncol. 2006, 24, 5664–5671. [Google Scholar] [CrossRef]
- Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Taylor, C.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar] [CrossRef]
- Sakr, H.; Hamed, R.H.; Anter, A.H.; Yossef, T. Sequential docetaxel as adjuvant chemotherapy for node-positive or/and T3 or T4 breast cancer: Clinical outcome (Mansoura University). Med. Oncol. 2013, 30, 457. [Google Scholar] [CrossRef]
- Coudert, B.; Asselain, B.; Campone, M.; Spielmann, M.; Machiels, J.-P.; Pénault-Llorca, F.; Serin, D.; Lévy, C.; Romieu, G.; Canon, J.-L.; et al. Extended Benefit from Sequential Administration of Docetaxel after Standard Fluorouracil, Epirubicin, and Cyclophosphamide Regimen for Node-Positive Breast Cancer: The 8-Year Follow-Up Results of the UNICANCER-PACS01 Trial. Oncologist 2012, 17, 900–909. [Google Scholar] [CrossRef]
- Kerbrat, P.; Desmoulins, I.; Roca, L.; Levy, C.; Lortholary, A.; Marre, A.; Delva, R.; Rios, M.; Viens, P.; Brain, É.; et al. Optimal duration of adjuvant chemotherapy for high-risk node-negative (N-) breast cancer patients: 6-year results of the prospective randomised multicentre phase III UNICANCER-PACS 05 trial (UCBG-0106). Eur. J. Cancer 2017, 79, 166–175. [Google Scholar] [CrossRef]
- Jackisch, C.; Cortazar, P.; Geyer, C.E.; Gianni, L.; Gligorov, J.; Machackova, Z.; Perez, E.A.; Schneeweiss, A.; Tolaney, S.M.; Untch, M.; et al. Risk-based decision-making in the treatment of HER2-positive early breast cancer: Recommendations based on the current state of knowledge. Cancer Treat. Rev. 2021, 99, 102229. [Google Scholar] [CrossRef]
- Petrelli, F.; Bertaglia, V.; Parati, M.C.; Borgonovo, K.; de Silva, P.; Luciani, A.; Novello, S.; Scartozzi, M.; Emens, L.A.; Solinas, C. Adjuvant chemotherapy for resected triple negative breast cancer patients: A network meta-analysis. Breast 2023, 67, 8–13. [Google Scholar] [CrossRef]
- Del Mastro, L.; Poggio, F.; Blondeaux, E.; De Placido, S.; Giuliano, M.; Forestieri, V.; De Laurentiis, M.; Gravina, A.; Bisagni, G.; Rimanti, A.; et al. Gruppo Italiano Mammella Investigators. Fluorouracil and dose-dense adjuvant chemotherapy in patients with early-stage breast cancer (GIM2): End-of-study results from a randomised, phase 3 trial. Lancet Oncol. 2022, 23, 1571–1582. [Google Scholar] [CrossRef] [PubMed]

| Total (ITT) n = 2541 | FEC*3 > Doc*3 n = 1286 | FEC*6 n = 1255 | ||||
|---|---|---|---|---|---|---|
| Variable | n | (%) | n | (%) | n | (%) |
| Age (mean) | 52.6 y | 52.7 y | 52.5 y | |||
| Peri-/premenopausal | 1051 | (41.4%) | 524 | (40.7%) | 527 | (42.0%) |
| Postmenopausal | 1442 | (56.7%) | 737 | (57.3%) | 705 | (56.2%) |
| Missing | 48 | (1.9%) | 25 | (1.9%) | 23 | (1.8%) |
| Tumor size (median/mean) | 1.9 cm/2.98 cm | 1.9 cm/3.08 cm | 2.0 cm/2.89 cm | |||
| pT1b | 222 | (8.7%) | 130 | (10.1%) | 92 | (7.3%) |
| pT1c | 1216 | (47.9%) | 610 | (47.4%) | 606 | (48.3%) |
| pT2 | 1102 | (43.4%) | 545 | (42.4%) | 557 | (44.4%) |
| Missing | 1 | (0.1%) | 1 | (0.1%) | - | - |
| Invasive ductal cancer | 2097 | (82.5%) | 1073 | (83.4%) | 1024 | (81.6%) |
| Invasive lobular cancer and others | 421 | (16.6%) | 200 | (15.6%) | 221 | (17.6%) |
| Missing | 23 | (0.9%) | 13 | (1.0%) | 10 | (0.8%) |
| Grade 1 | 14 | (0.6%) | 9 | (0.7%) | 5 | (0.4%) |
| Grade 2 | 1151 | (45.3%) | 580 | (45.1%) | 571 | (45.5%) |
| Grade 3 | 1366 | (53.8%) | 691 | (53.7%) | 675 | (53.8%) |
| Missing | 10 | (0.4%) | 6 | (0.5%) | 4 | (0.3%) |
| Hormone receptor status positive (ER-pos. and/or PR-pos.) | 1770 | (69.7%) | 893 | (69.4%) | 877 | (69.9%) |
| Hormone receptor status negative (ER-neg. and PR-neg.) | 765 | (30.1%) | 390 | (30.3%) | 375 | (29.9%) |
| Missing | 6 | (0.2%) | 3 | (0.2%) | 3 | (0.2%) |
| HER2-positive | 508 | (20.0%) | 260 | (20.2%) | 248 | (19.8%) |
| HER2-negative | 2012 | (79.2%) | 1015 | (78.9%) | 997 | (79.4%) |
| Missing | 21 | (0.8%) | 11 | (0.9%) | 10 | (0.8%) |
| Vessel invasion (present) | 195 | (7.7%) | 100 | (7.8%) | 95 | (7.6%) |
| Vessel invasion (not present) | 1718 | (67.6%) | 867 | (67.4%) | 851 | (67.8%) |
| Missing | 628 | (24.7%) | 319 | (24.8%) | 309 | (24.6%) |
| Mastectomy | 279 | (11.0%) | 150 | (11.7%) | 129 | (10.3%) |
| Breast conserving therapy (BCT) | 2252 | (88.6%) | 1130 | (87.9%) | 1122 | (89.4%) |
| Missing | 10 | (0.4%) | 6 | (0.5%) | 4 | (0.3%) |
| Biological (uPA/PAI-1) risk assessment | 1452 | (57.1%) | 734 | (57.1%) | 718 | (57.2%) |
| Clinico-pathological risk assessment | 1089 | (42.9%) | 552 | (42.9%) | 537 | (42.8%) |
| Chemotherapy completed * | 2234 | (87.9%) | 1086 | (84.4%) | 1148 | (91.5%) |
| Chemotherapy incomplete <6 courses | 307 | (12.1%) | 200 | (15.6%) | 107 | (8.5%) |
| Chemotherapy incomplete ≤4 courses | 196 | (7.7%) | 124 | (9.6%) | 72 | (5.7%) |
| Follow-up ≥ 2.5 y | 2306 | (90.8%) | 1144 | (89.0%) | 1162 | (92.6%) |
| Distant recurrences | 94 | (3.7%) | 50 | (3.9%) | 44 | (3.5%) |
| Loco-regional recurrences | 51 | (2.0%) | 24 | (1.9%) | 27 | (2.2%) |
| Second cancers | 39 | (1.5%) | 20 | (1.6%) | 19 | (1.5%) |
| Deaths | 55 | (2.2%) | 27 | (2.1%) | 28 | (2.2%) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p-Value | Hazard Ratio | 95% Confidence Interval | p-Value | |
| Disease-free survival | ||||
| Tumor size | 0.03 | 2.94 | 1.08–8.0 | 0.0355 |
| (>1 cm vs. ≤1 cm) | ||||
| Chemotherapy | 0.001 | 2.06 | 1.25–3.38 | 0.005 |
| (<6 courses vs. complete) | ||||
| Estrogen receptor (ER) status | <0.0001 | 1.89 | 1.13–3.17 | 0.015 |
| (negative vs. positive) | ||||
| Progesterone receptor (PR) status | <0.0001 | 1.96 | 1.17–3.29 | 0.01 |
| (negative vs. positive) | ||||
| Vessel invasion | 0.009 | 1.74 | 0.99–3.06 | 0.056 |
| (present vs. not present) | ||||
| Grading (G3 vs. G2) | 0.005 | 1.06 | 0.69–1.61 | 0.803 |
| Age | 0.095 | 0.76 | 0.53–1.09 | 0.133 |
| (>50 years vs. ≤50 years) | ||||
| Overall Survival | ||||
| Chemotherapy | <0.0001 | 3.92 | 2.10–7.31 | <0.0001 |
| (<6 courses vs. complete) | ||||
| Estrogen receptor status | 0.038 | 1.3 | 0.62–2.72 | 0.484 |
| (negative vs. positive) | ||||
| Progesterone receptor status (negative vs. positive) | 0.008 | 1.89 | 0.91–3.92 | 0.086 |
| Age | 0.045 | 1.88 | 1.03–3.46 | 0.041 |
| (>50 years vs. ≤50 years) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomssen, C.; Vetter, M.; Kantelhardt, E.J.; Meisner, C.; Schmidt, M.; Martin, P.M.; Clatot, F.; Augustin, D.; Hanf, V.; Paepke, D.; et al. Adjuvant Docetaxel in Node-Negative Breast Cancer Patients: A Randomized Trial of AGO-Breast Study Group, German Breast Group, and EORTC-Pathobiology Group. Cancers 2023, 15, 1580. https://doi.org/10.3390/cancers15051580
Thomssen C, Vetter M, Kantelhardt EJ, Meisner C, Schmidt M, Martin PM, Clatot F, Augustin D, Hanf V, Paepke D, et al. Adjuvant Docetaxel in Node-Negative Breast Cancer Patients: A Randomized Trial of AGO-Breast Study Group, German Breast Group, and EORTC-Pathobiology Group. Cancers. 2023; 15(5):1580. https://doi.org/10.3390/cancers15051580
Chicago/Turabian StyleThomssen, Christoph, Martina Vetter, Eva J. Kantelhardt, Christoph Meisner, Marcus Schmidt, Pierre M. Martin, Florian Clatot, Doris Augustin, Volker Hanf, Daniela Paepke, and et al. 2023. "Adjuvant Docetaxel in Node-Negative Breast Cancer Patients: A Randomized Trial of AGO-Breast Study Group, German Breast Group, and EORTC-Pathobiology Group" Cancers 15, no. 5: 1580. https://doi.org/10.3390/cancers15051580
APA StyleThomssen, C., Vetter, M., Kantelhardt, E. J., Meisner, C., Schmidt, M., Martin, P. M., Clatot, F., Augustin, D., Hanf, V., Paepke, D., Meinerz, W., Hoffmann, G., Wiest, W., Sweep, F. C. G. J., Schmitt, M., Jänicke, F., Loibl, S., Minckwitz, G. v., & Harbeck, N., on behalf of the NNBC 3-Europe Study Group. (2023). Adjuvant Docetaxel in Node-Negative Breast Cancer Patients: A Randomized Trial of AGO-Breast Study Group, German Breast Group, and EORTC-Pathobiology Group. Cancers, 15(5), 1580. https://doi.org/10.3390/cancers15051580







