Behavioral Voluntary and Social Bioassays Enabling Identification of Complex and Sex-Dependent Pain-(-Related) Phenotypes in Rats with Bone Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
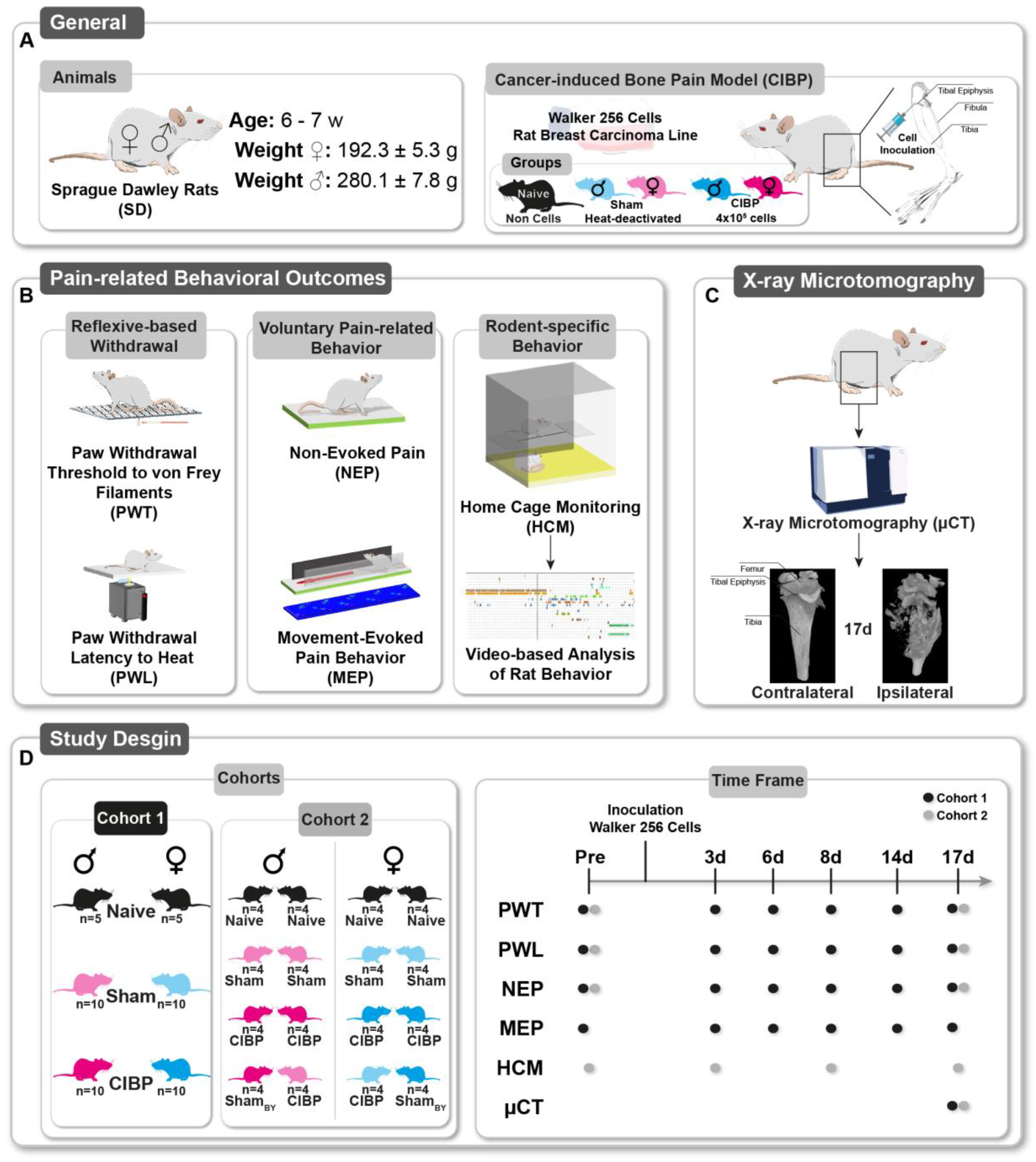
2.1. General
2.2. Culture of Walker 256 Cells
2.3. Pain Model: Cancer-Induced Bone Pain (CIBP)
2.4. Multidimensional Assessment of Pain-Related Behaviors
2.4.1. Paw Withdrawal to Von Frey Filaments (PWT)
2.4.2. Paw Withdrawal Latency to Heat (PWL)
2.5. Voluntary Pain-Related Behaviors
2.5.1. Non-Evoked Pain Assessment (NEP)
2.5.2. Movement-Evoked Pain Assessment (MEP)
- Print area: area of the whole paw
- Stand duration (s): duration of ground contact for a single paw
- Swing duration (s): duration of any swing cycle of a single paw
- Swing speed (cm/s): rate at which a paw is not in contact with the glass plate
2.5.3. Home-Cage Monitoring (HCM)
2.6. microCT Visualization
2.7. Data Analyses
2.8. Study Design
2.8.1. Cohort 1
2.8.2. Cohort 2: Home-Cage Monitoring
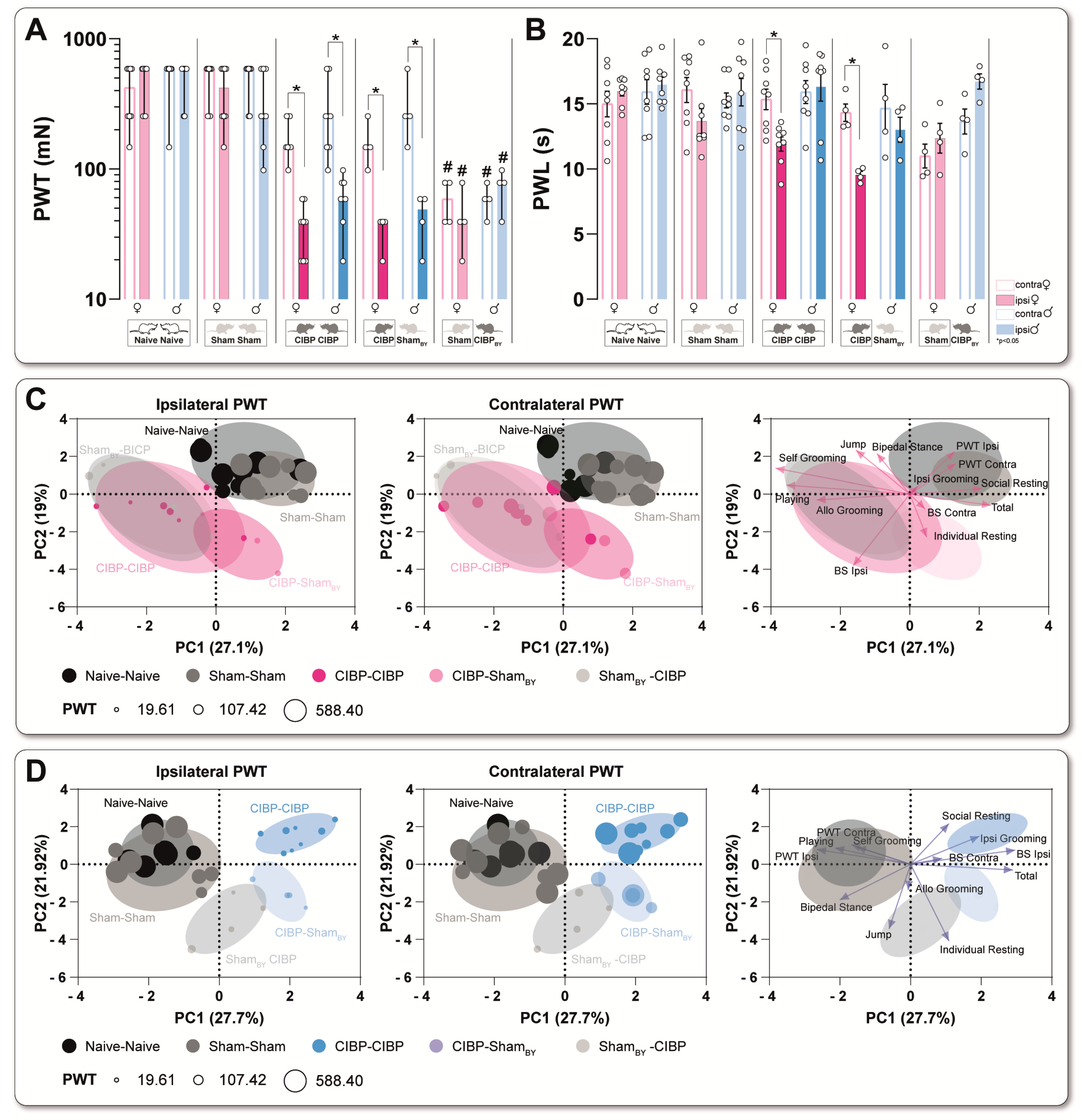
3. Results
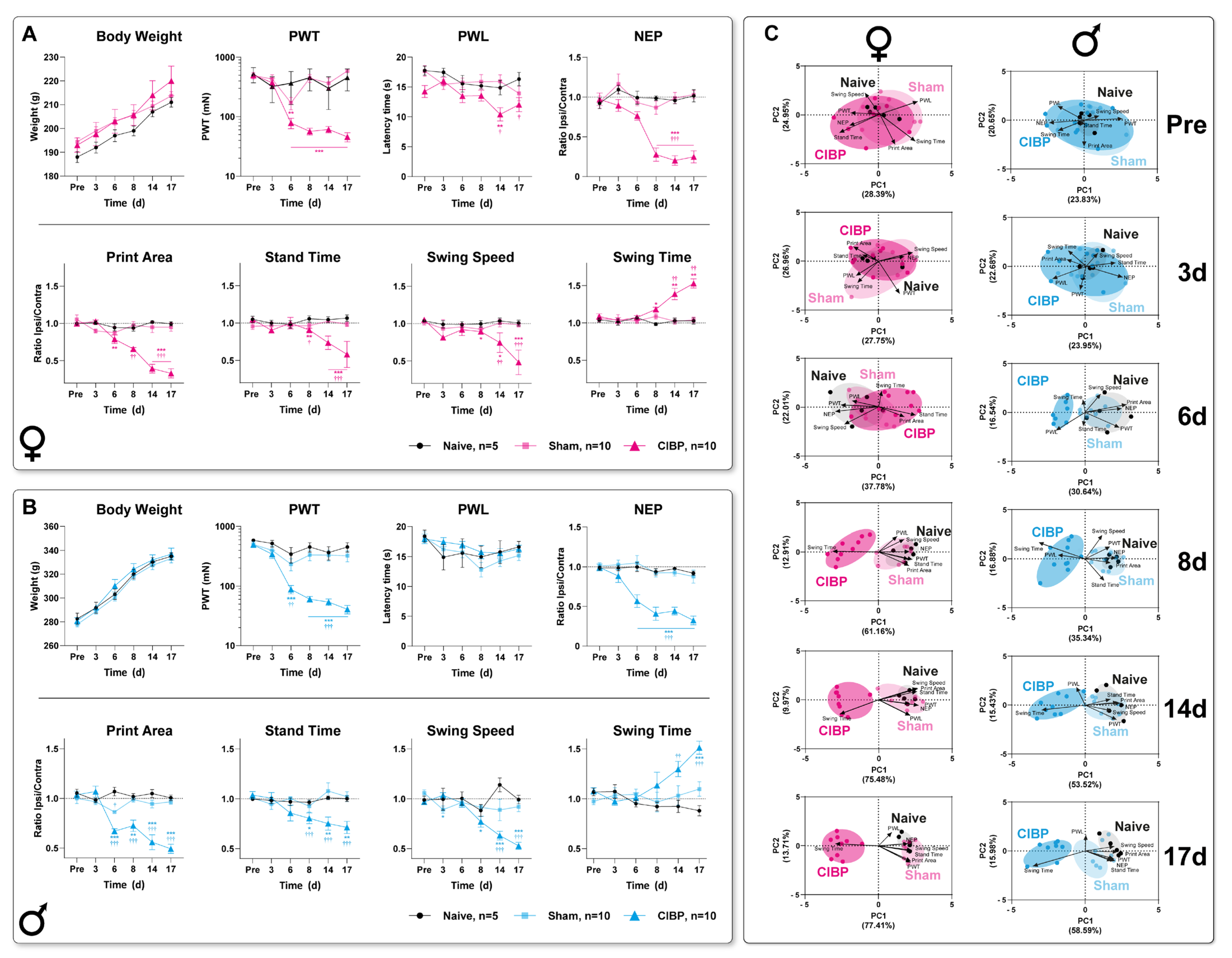
3.1. Bone Cancer Causes Distinct Pain-Related Behavioral Trajectories in Rats of Both Sexes
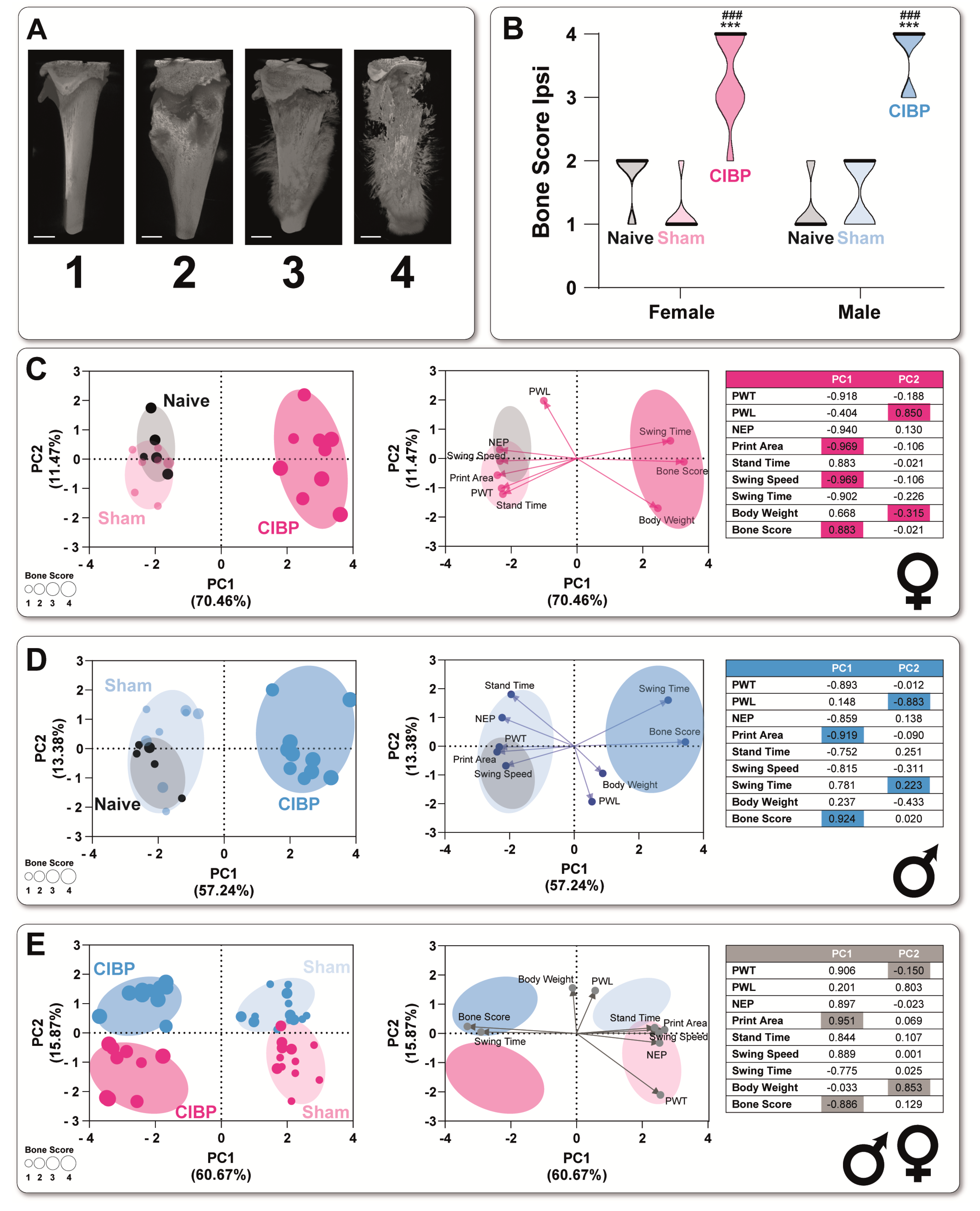
3.2. Cancer-Induced Bone Destruction Is Associated with Pain-Related Behavior
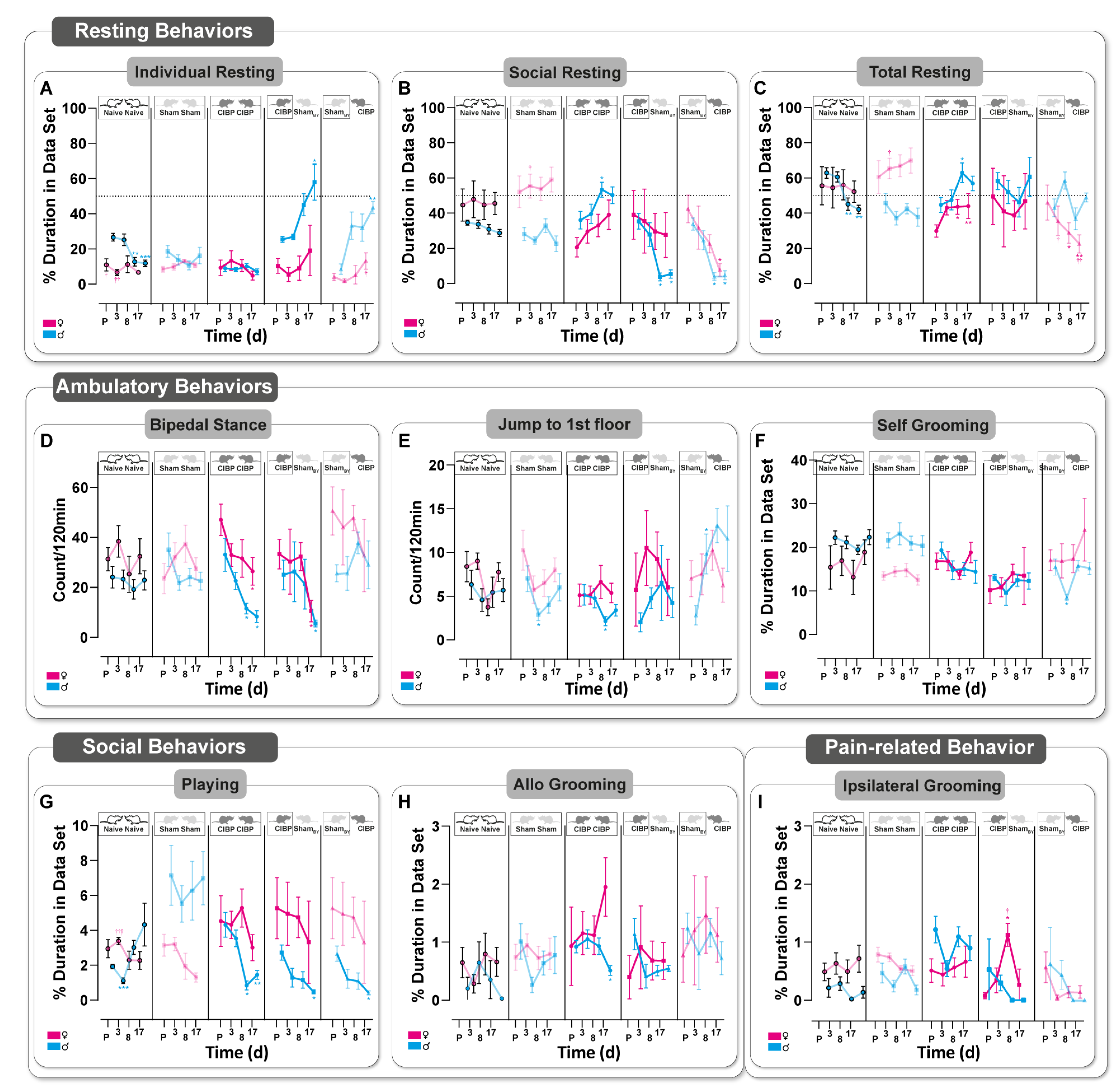
3.3. Bone Cancer Alters Rodent-Specific Complex Behavior in Rats of Both Sexes in a Home-Cage Setting
3.4. Social Transfer of Pain-Related Behavior from CIBP Rats to Sham Bystanders and Alterations in Complex Behavior Caused in Both Sexes
4. Discussion
4.1. Bone Cancer Causes a Sex-Specific Pain Phenotype Associated with Bone Destruction
4.2. Social Interaction with a Cage Mate Suffering from CIBP Alters Rodent-Specific Behavior
4.3. Limitations of the Study
4.4. Implications for Study Planning and Severity Assessment in CIBP
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Saxena, A.K.; Vasudev, P.; Sundriyal, D.; Kumar, A. Cancer induced bone pain: Current management and future perspectives. Med. Oncol. 2021, 38, 134. [Google Scholar] [CrossRef]
- Currie, G.L.; Delaney, A.; Bennett, M.I.; Dickenson, A.H.; Egan, K.J.; Vesterinen, H.M.; Sena, E.S.; Macleod, M.R.; Colvin, L.A.; Fallon, M.T. Animal models of bone cancer pain: Systematic review and meta-analyses. Pain 2013, 154, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Doré-Savard, L.; Otis, V.; Belleville, K.; Lemire, M.; Archambault, M.; Tremblay, L.; Beaudoin, J.-F.; Beaudet, N.; Lecomte, R.; Lepage, M.; et al. Behavioral, Medical Imaging and Histopathological Features of a New Rat Model of Bone Cancer Pain. PLoS ONE 2010, 5, e13774. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Rogers, S.D.; Schwei, M.J.; Salak-Johnson, J.L.; Luger, N.M.; Sabino, M.C.; Clohisy, D.R.; Mantyh, P.W. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 2000, 98, 585–598. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain-United States, 2016. MMWR Recomm. Rep. 2016, 65, 1–49. [Google Scholar] [CrossRef]
- Mouraux, A.; Bannister, K.; Becker, S.; Finn, D.P.; Pickering, G.; Pogatzki-Zahn, E.; Graven-Nielsen, T. Challenges and opportunities in translational pain research—An opinion paper of the working group on translational pain research of the European pain federation (EFIC). Eur. J. Pain 2021, 25, 731–756. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wordliczek, J. Bone Pain in Cancer Patients: Mechanisms and Current Treatment. Int. J. Mol. Sci. 2019, 20, 6047. [Google Scholar] [CrossRef]
- Dale, R.; Stacey, B. Multimodal Treatment of Chronic Pain. Med. Clin. North Am. 2016, 100, 55–64. [Google Scholar] [CrossRef]
- Sadler, K.E.; Mogil, J.S.; Stucky, C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2021, 23, 70–85. [Google Scholar] [CrossRef]
- González-Cano, R.; Montilla-García, Á.; Ruiz-Cantero, M.C.; Bravo-Caparrós, I.; Tejada, M.Á.; Nieto, F.R.; Cobos, E.J. The search for translational pain outcomes to refine analgesic development: Where did we come from and where are we going? Neurosci. Biobehav. Rev. 2020, 113, 238–261. [Google Scholar] [CrossRef]
- Turk, D.C.; Fillingim, R.B.; Ohrbach, R.; Patel, K.V. Assessment of Psychosocial and Functional Impact of Chronic Pain. J. Pain 2016, 17, T21–T49. [Google Scholar] [CrossRef]
- Du Percie Sert, N.; Rice, A.S.C. Improving the translation of analgesic drugs to the clinic: Animal models of neuropathic pain. Br. J. Pharmacol. 2014, 171, 2951–2963. [Google Scholar] [CrossRef]
- Segelcke, D.; Fischer, H.K.; Hütte, M.; Dennerlein, S.; Benseler, F.; Brose, N.; Pogatzki-Zahn, E.M.; Schmidt, M. Tmem160 contributes to the establishment of discrete nerve injury-induced pain behaviors in male mice. Cell Rep. 2021, 37, 110152. [Google Scholar] [CrossRef]
- Pitzer, C.; Kuner, R.; Tappe-Theodor, A. EXPRESS: Voluntary and evoked behavioral correlates in neuropathic pain states under different housing conditions. Mol. Pain 2016, 12, 174480691665663. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Gomez-Varela, D.; Erdmann, G.; Kaschube, K.; Segelcke, D.; Schmidt, M. A proteome signature for acute incisional pain in dorsal root ganglia of mice. Pain 2021, 162, 2070–2086. [Google Scholar] [CrossRef]
- Djouhri, L.; Koutsikou, S.; Fang, X.; McMullan, S.; Lawson, S.N. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J. Neurosci. 2006, 26, 1281–1292. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; Kuner, R. Studying ongoing and spontaneous pain in rodents--challenges and opportunities. Eur. J. Neurosci. 2014, 39, 1881–1890. [Google Scholar] [CrossRef]
- Tappe-Theodor, A.; King, T.; Morgan, M.M. Pros and Cons of Clinically Relevant Methods to Assess Pain in Rodents. Neurosci. Biobehav. Rev. 2019, 100, 335–343. [Google Scholar] [CrossRef]
- Sorge, R.E.; Martin, L.J.; Isbester, K.A.; Sotocinal, S.G.; Rosen, S.; Tuttle, A.H.; Wieskopf, J.S.; Acland, E.L.; Dokova, A.; Kadoura, B.; et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat. Methods 2014, 11, 629–632. [Google Scholar] [CrossRef]
- Von Kortzfleisch, V.T.; Ambrée, O.; Karp, N.A.; Meyer, N.; Novak, J.; Palme, R.; Rosso, M.; Touma, C.; Würbel, H.; Kaiser, S.; et al. Do multiple experimenters improve the reproducibility of animal studies? PLoS Biol. 2022, 20, e3001564. [Google Scholar] [CrossRef] [PubMed]
- Deuis, J.R.; Dvorakova, L.S.; Vetter, I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Social modulation of and by pain in humans and rodents. Pain 2015, 156 (Suppl. 1), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Du Percie Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Shenoy, P.A.; Kuo, A.; Vetter, I.; Smith, M.T. The Walker 256 Breast Cancer Cell- Induced Bone Pain Model in Rats. Front. Pharmacol. 2016, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Harford-Wright, E.; Vink, R.; Ghabriel, M.N. Characterisation of Walker 256 breast carcinoma cells from two tumour cell banks as assessed using two models of secondary brain tumours. Cancer Cell Int. 2013, 13, 5. [Google Scholar] [CrossRef]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Gabriel, A.F.; Marcus, M.A.E.; Honig, W.M.M.; Walenkamp, G.H.I.M.; Joosten, E.A.J. The CatWalk method: A detailed analysis of behavioral changes after acute inflammatory pain in the rat. J. Neurosci. Methods 2007, 163, 9–16. [Google Scholar] [CrossRef]
- Ferreira-Gomes, J.; Adães, S.; Castro-Lopes, J.M. Assessment of movement-evoked pain in osteoarthritis by the knee-bend and CatWalk tests: A clinically relevant study. J. Pain 2008, 9, 945–954. [Google Scholar] [CrossRef]
- Segelcke, D.; Pradier, B.; Reichl, S.; Schäfer, L.C.; Pogatzki-Zahn, E.M. Investigating the Role of Ly6G+ Neutrophils in Incisional and Inflammatory Pain by Multidimensional Pain-Related Behavioral Assessments: Bridging the Translational Gap. Front. Pain Res. 2021, 2, 735838. [Google Scholar] [CrossRef]
- Budaev, S.V. Using Principal Components and Factor Analysis in Animal Behaviour Research: Caveats and Guidelines. Ethology 2010, 116, 472–480. [Google Scholar] [CrossRef]
- Rosenholm, M.; Paro, E.; Antila, H.; Võikar, V.; Rantamäki, T. Repeated brief isoflurane anesthesia during early postnatal development produces negligible changes on adult behavior in male mice. PLoS ONE 2017, 12, e0175258. [Google Scholar] [CrossRef]
- Diana, P.; Joksimovic, S.M.; Faisant, A.; Jevtovic-Todorovic, V. Early exposure to general anesthesia impairs social and emotional development in rats. Mol. Neurobiol. 2020, 57, 41–50. [Google Scholar] [CrossRef]
- Cicchetti, D.V.; Sparrow, S.A. Developing criteria for establishing interrater reliability of specific items: Applications to assessment of adaptive behavior. Am. J. Ment. Defic. 1981, 86, 127–137. [Google Scholar]
- Vanderschuren, L. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997, 21, 309–326. [Google Scholar] [CrossRef]
- Leach, M.C.; Klaus, K.; Miller, A.L.; Di Scotto Perrotolo, M.; Sotocinal, S.G.; Flecknell, P.A. The assessment of post-vasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS ONE 2012, 7, e35656. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Omenn, G.S.; Sun, Z.; Maes, M.; Pernemalm, M.; Palaniappan, K.K.; Letunica, N.; Vandenbrouck, Y.; Brun, V.; Tao, S.-C.; et al. Advances and Utility of the Human Plasma Proteome. J. Proteome Res. 2021, 20, 5241–5263. [Google Scholar] [CrossRef]
- Smith, M.L.; Asada, N.; Malenka, R.C. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science 2021, 371, 153–159. [Google Scholar] [CrossRef]
- Chen, J. Empathy for Distress in Humans and Rodents. Neurosci. Bull. 2018, 34, 216–236. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Hostetler, C.M.; Heinricher, M.M.; Ryabinin, A.E. Social transfer of pain in mice. Sci. Adv. 2016, 2, e1600855. [Google Scholar] [CrossRef]
- Colvin, L.; Fallon, M. Challenges in cancer pain management—Bone pain. Eur. J. Cancer 2008, 44, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. The Oxford Handbook of the Neurobiology of Pain: The Measurement of Pain in the Laboratory Rodent; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Do Amaral, L.A.; de Souza, G.H.O.; Santos, M.R.; Said, Y.L.V.; de Souza, B.B.; Oliveira, R.J.; dos Santos, E.F. Walker-256 Tumor: Experimental Model, Implantation Sites and Number of Cells for Ascitic and Solid Tumor Development. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Shenoy, P.; Kuo, A.; Vetter, I.; Smith, M.T. Optimization and In Vivo Profiling of a Refined Rat Model of Walker 256 Breast Cancer Cell-Induced Bone Pain Using Behavioral, Radiological, Histological, Immunohistochemical and Pharmacological Methods. Front. Pharmacol. 2017, 8, 442. [Google Scholar] [CrossRef]
- Martin, L.J.; Tuttle, A.H.; Mogil, J.S. The interaction between pain and social behavior in humans and rodents. Curr. Top. Behav. Neurosci. 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Aubert, A. Sickness and behaviour in animals: A motivational perspective. Neurosci. Biobehav. Rev. 1999, 23, 1029–1036. [Google Scholar] [CrossRef]
- Hart, B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988, 12, 123–137. [Google Scholar] [CrossRef]
- Viana, L.R.; Chiocchetti, G.D.M.E.; Oroy, L.; Vieira, W.F.; Busanello, E.N.B.; Marques, A.C.; Salgado, C.D.M.; de Oliveira, A.L.R.; Vieira, A.S.; Suarez, P.S.; et al. Leucine-Rich Diet Improved Muscle Function in Cachectic Walker 256 Tumour-Bearing Wistar Rats. Cells 2021, 10, 3272. [Google Scholar] [CrossRef]
- Bao, Y.; Hua, B.; Hou, W.; Shi, Z.; Li, W.; Li, C.; Chen, C.; Liu, R.; Qin, Y. Involvement of protease-activated receptor 2 in nociceptive behavior in a rat model of bone cancer. J. Mol. Neurosci. 2014, 52, 566–576. [Google Scholar] [CrossRef]
- Xu, H.; Peng, C.; Chen, X.-T.; Yao, Y.-Y.; Chen, L.-P.; Yin, Q.; Shen, W. Chemokine receptor CXCR4 activates the RhoA/ROCK2 pathway in spinal neurons that induces bone cancer pain. Mol. Pain 2020, 16, 1744806920919568. [Google Scholar] [CrossRef]
- Bloom, A.P.; Jimenez-Andrade, J.M.; Taylor, R.N.; Castañeda-Corral, G.; Kaczmarska, M.J.; Freeman, K.T.; Coughlin, K.A.; Ghilardi, J.R.; Kuskowski, M.A.; Mantyh, P.W. Breast cancer-induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J. Pain 2011, 12, 698–711. [Google Scholar] [CrossRef]
- Hu, S.; Mao-Ying, Q.-L.; Wang, J.; Wang, Z.-F.; Mi, W.-L.; Wang, X.-W.; Jiang, J.-W.; Huang, Y.-L.; Wu, G.-C.; Wang, Y.-Q. Lipoxins and aspirin-triggered lipoxin alleviate bone cancer pain in association with suppressing expression of spinal proinflammatory cytokines. J. Neuroinflamm. 2012, 9, 278. [Google Scholar] [CrossRef]
- Dance, A. Why the sexes don’t feel pain the same way. Nature 2019, 567, 448–450. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.S.; Dalgarno, R.; Tu, Y.; Moriarty, O.; Beggs, S.; Kwok, C.H.T.; Halievski, K.; Assi, S.; Mogil, J.S.; Trang, T.; et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain 2018, 159, 1752–1763. [Google Scholar] [CrossRef]
- Sorge, R.E.; Lacroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.-S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef]
- Lewejohann, L.; Schwabe, K.; Häger, C.; Jirkof, P. Impulse for animal welfare outside the experiment. Lab. Anim. 2020, 54, 150–158. [Google Scholar] [CrossRef]
- Tawfik, V.L.; Huck, N.A.; Baca, Q.J.; Ganio, E.A.; Haight, E.S.; Culos, A.; Ghaemi, S.; Phongpreecha, T.; Angst, M.S.; Clark, J.D.; et al. Systematic Immunophenotyping Reveals Sex-Specific Responses After Painful Injury in Mice. Front. Immun. 2020, 11, 1652. [Google Scholar] [CrossRef]
- Dawes, J.M.; Bennett, D.L. Addressing the gender pain gap. Neuron 2021, 109, 2641–2642. [Google Scholar] [CrossRef]
- Gregus, A.M.; Levine, I.S.; Eddinger, K.A.; Yaksh, T.L.; Buczynski, M.W. Sex differences in neuroimmune and glial mechanisms of pain. Pain 2021, 162, 2186–2200. [Google Scholar] [CrossRef]
- Grossman, N.; Binyamin, L.-A.; Bodner, L. Effect of rat salivary glands extracts on the proliferation of cultured skin cells--a wound healing model. Cell Tissue Bank. 2004, 5, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.D.C. What can evolutionary theory tell us about chronic pain? Pain 2016, 157, 788–790. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.-F.; Li, C.-L.; Wang, Y.; Sun, W.; He, T.; Chen, X.-F.; Wang, X.-L.; Chen, J. Social interaction with a cagemate in pain facilitates subsequent spinal nociception via activation of the medial prefrontal cortex in rats. Pain 2014, 155, 1253–1261. [Google Scholar] [CrossRef]
- Li, C.-L.; Yu, Y.; He, T.; Wang, R.-R.; Geng, K.-W.; Du, R.; Luo, W.-J.; Wei, N.; Wang, X.-L.; Wang, Y.; et al. Validating Rat Model of Empathy for Pain: Effects of Pain Expressions in Social Partners. Front. Behav. 2018, 12, 242. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Lahvis, G.P. Rodent empathy and affective neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K.; Walker, P.; McLean, L.; Carrive, P. Fear and the Defense Cascade: Clinical Implications and Management. Harv. Rev. Psychiatry 2015, 23, 263–287. [Google Scholar] [CrossRef] [PubMed]
- Bodden, C.; Wewer, M.; Kästner, N.; Palme, R.; Kaiser, S.; Sachser, N.; Richter, S.H. Not all mice are alike: Mixed-strain housing alters social behaviour. Physiol. Behav. 2021, 228, 113220. [Google Scholar] [CrossRef]
- Directive 2010/63EU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific purposes, Text with EEA Relevance (europa.eu). 2010.
- Bodden, C.; Siestrup, S.; Palme, R.; Kaiser, S.; Sachser, N.; Richter, S.H. Evidence-based severity assessment: Impact of repeated versus single open-field testing on welfare in C57BL/6J mice. Behav. Brain Res. 2018, 336, 261–268. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segelcke, D.; Linnemann, J.; Pradier, B.; Kronenberg, D.; Stange, R.; Richter, S.H.; Görlich, D.; Baldini, N.; Di Pompo, G.; Verri, W.A., Jr.; et al. Behavioral Voluntary and Social Bioassays Enabling Identification of Complex and Sex-Dependent Pain-(-Related) Phenotypes in Rats with Bone Cancer. Cancers 2023, 15, 1565. https://doi.org/10.3390/cancers15051565
Segelcke D, Linnemann J, Pradier B, Kronenberg D, Stange R, Richter SH, Görlich D, Baldini N, Di Pompo G, Verri WA Jr., et al. Behavioral Voluntary and Social Bioassays Enabling Identification of Complex and Sex-Dependent Pain-(-Related) Phenotypes in Rats with Bone Cancer. Cancers. 2023; 15(5):1565. https://doi.org/10.3390/cancers15051565
Chicago/Turabian StyleSegelcke, Daniel, Jan Linnemann, Bruno Pradier, Daniel Kronenberg, Richard Stange, S. Helene Richter, Dennis Görlich, Nicola Baldini, Gemma Di Pompo, Waldiceu A. Verri, Jr., and et al. 2023. "Behavioral Voluntary and Social Bioassays Enabling Identification of Complex and Sex-Dependent Pain-(-Related) Phenotypes in Rats with Bone Cancer" Cancers 15, no. 5: 1565. https://doi.org/10.3390/cancers15051565
APA StyleSegelcke, D., Linnemann, J., Pradier, B., Kronenberg, D., Stange, R., Richter, S. H., Görlich, D., Baldini, N., Di Pompo, G., Verri, W. A., Jr., Avnet, S., & Pogatzki-Zahn, E. M. (2023). Behavioral Voluntary and Social Bioassays Enabling Identification of Complex and Sex-Dependent Pain-(-Related) Phenotypes in Rats with Bone Cancer. Cancers, 15(5), 1565. https://doi.org/10.3390/cancers15051565








