HIPK2 in Angiogenesis: A Promising Biomarker in Cancer Progression and in Angiogenic Diseases
Abstract
Simple Summary
Abstract
1. Introduction
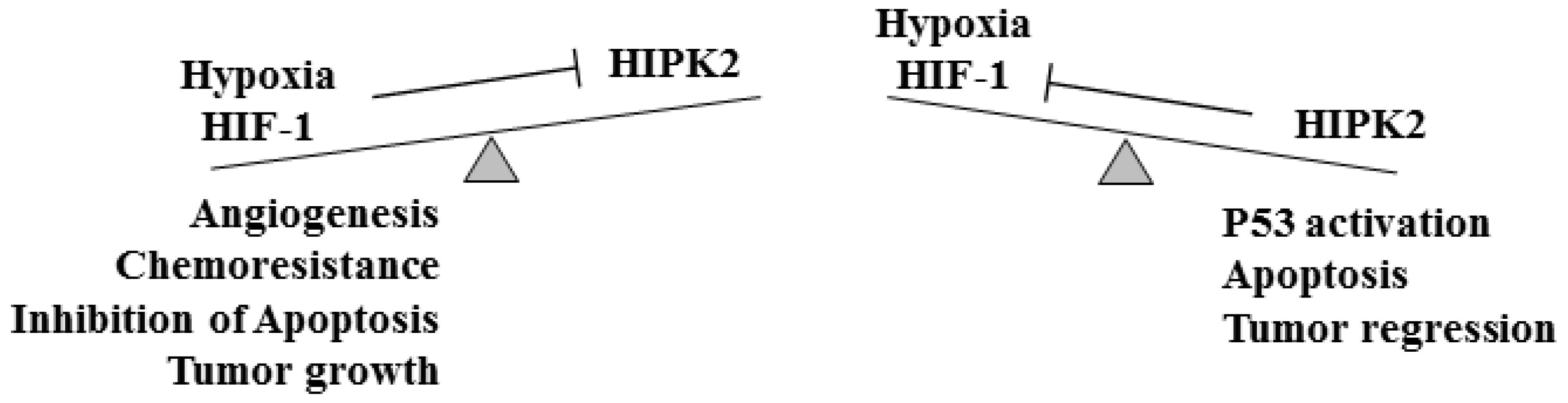
2. HIPK2 and Tumor Angiogenesis
2.1. HIPK2 and HIF-1/VEGF in Tumor Angiogenesis
2.2. HIPK2 and microRNA in Tumor Angiogenesis
2.3. HIPK2 and Circular RNA in Tumor Angiogenesis
3. HIPK2 and Other Angiogenic Diseases
3.1. HIPK2 and Angiogenesis in Gestational Complications and in Myocardial Infarction (MI)
3.2. HIPK2 in Diabetic Retinopathy (DR) and in Diabetic Wound Healing
| miRNA | Cell Type | Disease Model | Target | Reference |
|---|---|---|---|---|
| miR-100-5p | HPMEC 1 | Gestational hypertension | ↓ HIPK2 | [68] |
| miR-126-5p | HUVEC 2 | Myocardial infarction (MI) | ↑ HIPK2 | [74] |
| miR-423-5p | REC 3 | Diabetic retinopathy | ↓ HIPK2 | [79] |
| miR-221-3p | HUVEC 2 | Diabetic foot ulcer | ↓ HIPK2 | [85] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- La Mendola, D.; Trincavelli, M.L.; Martini, C. Angiogenesis in disease. Int. J. Mol. Sci. 2022, 23, 10962. [Google Scholar] [CrossRef]
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019, 110, 775–785. [Google Scholar] [CrossRef]
- Hanahan, D.; Folkman, J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowaska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar] [CrossRef]
- Pepper, M.S. Positive and negative regulation of angiogenesis: From cell biology to the clinic. Vasc. Med. 1996, 1, 259–266. [Google Scholar] [CrossRef]
- Carmeliet, P.; Rakesh, K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix–loop–helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef]
- Jiang, B.H.; Semenza, G.L.; Bauer, H.; Marti, H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. Cell Physiol. 1996, 271, C1172–C1180. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.-M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Kershert, Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- D’Orazi, G.; Rinaldo, C.; Soddu, S. Updates on HIPK2: A resourceful oncosuppressor for clearing cancer. J. Exp. Clin. Cancer Res. 2012, 31, 63. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, L.; Sun, X.; Li, Q. Homeodomain-interacting protein kinase 2 (HIPK2): A promising target for anti-cancer therapies. Oncotarget 2017, 8, 20452–20461. [Google Scholar] [CrossRef]
- Conte, A.; Valente, V.; Paladino, S.; Pierantoni, G.M. HIPK2 in cancer biology and therapy: Recent findings and future perspectives. Cell. Signal. 2023, 101, 110491. [Google Scholar] [CrossRef]
- Calzado, M.A.; Renner, F.; Roscic, A.; Schitz, M.L. HIPK2: A versatile switchboard regulating the transcription machinery and cell death. Cell Cycle 2014, 6, 139–143. [Google Scholar] [CrossRef]
- Rinaldo, C.; Prodosmo, A.; Siepi, F.; Soddu, S. HIPK2: A multitalented partner for transcription factors in DNA damage response and development. Biochem. Cell Biol. 2007, 85, 411–418. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; Givol, D.; D’Orazi, G. Regulation of p53 activity by HIPK2: Molecular mechanisms and therapeutical implications. Oncogene 2010, 29, 4378–4387. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; Gal, H.; Rechavi, G.; Amariglio, N.; Domany, E.; Notterman, D.A.; Scarsella, M.; Leonetti, C.; Sacchi, A.; et al. Reversible dysfunction of wild-type p53 following homeodomain-interacting protein kinase-2 knockdown. Cancer Res. 2008, 68, 3707–3714. [Google Scholar] [CrossRef]
- Bon, G.; Di Carlo, S.E.; Folgiero, V.; Avetrani, P.; Lazzari, C.; D’Orazi, G.; Brizzi, M.F.; Sacchi, A.; Soddu, S.; Blandino, G.; et al. Negative regulation of beta(β) integrin transcription by homeodomain-interacting protein kinase e and p53 impairs tumor progression. Cancer Res. 2009, 69, 5978–5986. [Google Scholar] [CrossRef]
- Garufi, A.; Pistritto, G.; D’Orazi, G. HIPK2 as a novel regulator of fibrosis. Cancers 2023, 15, 1059. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; Guidolin, D.; Belloni, A.S.; Bossi, G.; Michiels, C.; Sacchi, A.; Onisto, M.; D’Orazi, G. Transcriptional regulation of hypoxia-inducible factor 1alpha by HIPK2 suggests a novel mechanism to restrain tumor growth. Biochim. et Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 368–377. [Google Scholar] [CrossRef]
- Calzado, M.A.; de la Vega, L.; Moller, A.; Bowtell, D.D.; Schmitz, M.L. An inducible autoregulatory loop between HIPK2 and Siah2 at the apex of the hypoxic response. Nat. Cell Biol. 2009, 11, 85–91. [Google Scholar] [CrossRef]
- Calzado, M.A.; De La Vega, L.; Munoz, E.; Schmitz, M.L. From top to bottom: The two faces of HIPK2 for regulation of the hypoxic response. Cell Cycle 2009, 8, 1659–1664. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; Givol, D.; D’Orazi, G. HIPK2-a therapeutical target to be (re)activated for tumor suppression: Role in p53 activation and HIF-1α inhibition. Cell Cycle 2010, 9, 1270–1275. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; D’Orazi, G. HIF-1α antagonizes p53-mediated apoptosis by triggering HIPK2 degradation. Aging (Albany NY) 2011, 3, 33–43. [Google Scholar] [CrossRef]
- D’Orazi, G.; Sciulli, M.G.; Di Stefano, V.; Riccioni, S.; Frattini, M.; Falcioni, R.; Bertario, L.; Sacchi, A.; Patrignani, P. Homeodomain-interacting protein kinase-2 restrains cytosolic phospholipase A2-dependent prostaglandin E2 generation in human colorectal cancer cells. Clin. Cancer Res. 2006, 12, 735–741. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Pantisano, V.; Puca, R.; Porru, M.; Aiello, A.; Grasselli, A.; Leonetti, C.; Safran, M.; Rechavi, G.; Givol, D.; et al. Zinc downregulates HIF-1α and inhibits its activity in tumor cells in vitro and in vivo. PLoS ONE 2010, 5, e15048. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; Sacchi, A.; Rechavi, G.; Givol, D.; D’Orazi, G. Targeting hypoxia in cancer cells by restoring homeodomain-interacting protein kinase 2 and p53 activity and suppressing HIF-1alpha. PLoS ONE 2009, 4, e6819. [Google Scholar] [CrossRef]
- Nardinocchi, L.; Puca, R.; Sacchim, A.; D’Orazi, G. Inhibition of HIF-1alpha activity hy homeodomain-interacting protein kinase-2 correlates with ssensitization of chemoresistant cells to undergo apoptosis. Mol. Cancer 2009, 8, 1. [Google Scholar] [CrossRef]
- Chen, P.; Duan, X.; Li, X.; Li, J.; Ba, Q.; Wang, H. HIPK2 suppresses tumor growth and progression of hepatocellular carcinoma through promoting the degradation of HIF-1α. Oncogene 2020, 39, 2863–2876. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The impact of VEGF on cancer metastasis and systemic disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef]
- Morse, M.A.; Sun, W.; Kim, R.; He, A.R.; Abada, P.B.; Mynderse, M.; Finn, R.S. The role of angiogenesis in hepatocellular carcinoma. Clin. Cancer Res. 2019, 25, 912–920. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Conte, A.; Pierantoni, G.M. Update on the regulation of HIPK1, HIPK2 and HIPK3 protein kinases by microRNAs. Microrna 2018, 7, 178–186. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef]
- Maia, J.; Caja, S.; Strano Moraes, M.C.; Couto, N.; Costa-Silva, B. Exosome-based cell-cell communication in the tumor microenvironment. Front. Cell Dev. Biol. 2018, 6, 18. [Google Scholar] [CrossRef]
- Hu, H.Y.; Yu, C.H.; Zhang, H.H.; Zhang, S.Z.; Yu, W.Y.; Yang, Y.; Chen, Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int. J. Biol. Macromol. 2019, 132, 470–477. [Google Scholar] [CrossRef]
- Sacillotto, N.; Chouliaras, K.M.; Nikitenki, L.L.; Lu, Y.W.; Fritzsche, M.; Wallace, M.D.; Nornes, S.; Garcia-Moreno, F.; Payne, S.; Bridges, E.; et al. MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev. 2016, 30, 2297–2309. [Google Scholar] [CrossRef]
- Shang, Y.; Doan, C.N.; Arnold, T.D.; Lee, S.; Tang, A.A.; Reichardt, L.F.; Huang, E.J. Transcriptional corepressors HIPK1 and HIPK2 control angiogenesis via TGF-b-TAK1-dpendent mechanism. PLOS Biol. 2013, 11, e1001527. [Google Scholar] [CrossRef]
- Tan, Z.; Zheng, H.; Liu, X.; Zhang, W.; Zhu, J.; Wu, G.; Cao, L.; Song, J.; Wu, S.; Song, L.; et al. MicroRNA-1229 overexpression promotes cell proliferation and tumorigenicity and activates Wnt/β-catenin signaling in breast cancer. Oncotarget 2016, 17, 24076–24087. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Lecarpentier, Y.; Schussler, O.; Henert, J.L.; Vallée, A. Multiple targets of the canonical WNT/β-catenin signaling in cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, J.E.; Sung, K.S.; Choi, D.W.; Lee, B.J.; Choi, C.Y. Homeodomain-interacting protein kinase 2 (HIPK2) targets beta-catenin for phosphorylation and proteasomal degradation. Biochem. Biophys. Res. Commun. 2010, 394, 966–971. [Google Scholar] [CrossRef]
- Wei, G.; Ku, S.; Ma, G.K.; Saito, S.; Tang, A.A.; Zhang, J.; Mail, J.H.; Appella, E.; Balmain, A.; HIang, E.J. HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 13040–13045. [Google Scholar] [CrossRef]
- Puca, R.; Nardinocchi, L.; D’Orazi, G. Regulation of vascular endothelial growth factor expression by homeodomain-interacting protein kinase-2. J. Exp. Clin. Cancer Res. 2008, 27, 22. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, H.; Choi, Y.J.; Kang, M.H. Exosomal miR-1260b derived from non-small cell lung cancer promotes tumor metastasis through the inhibition of HIPK2. Cell Death Dis. 2021, 12, 747. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, K.; Hu, L.Q.; Zhou, C.R.; Lu, Z.B.; Zhan, G.; Pan, X.L.; Pan, C.F.; Wang, J.; Wen, W.; et al. Exosome- mediated transfer of miR-1 260b promotes cell invasion through Wnt/beta-catenin signaling pathway in lung adenocarcinoma. J. Cell. Physiol. 2020, 235, 843–853. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Q.; Shen, J.; Yang, B.B.; Ding, X. Circbank: A comprehensive database for circRNA with standard nomenclature. RNA Biol. 2019, 16, 899–905. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- Verduci, L.; Tarcitano, E.; Strano, S.; Yarden, Y.; Blandino, G. CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 2021, 12, 468. [Google Scholar] [CrossRef]
- Ren, M.; Song, X.; Niu, J.; Tang, G.; Sun, Z.; Li, Y.; Kong, F. The malignant property of circHIPK2 for angiogenesis and chemoresistance in non-small cell lung cancer. Exp. Cell Res. 2022, 419, 113276. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Y.; Han, B.; Bai, Y.; Zhou, R.; Gan, G.; Chao, J.; Hu, G.; Yao, H. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy 2017, 13, 1722–1741. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, X.; Li, M.; Gao, Y. CircHIPK2 contributes to DDP resistance and malignant behaviors of DDP-resistant ovarian cancer cells both in vitro and in vivo through circHIPK2/miR-338-3p/CHTOP ceRNA pathway. OncoTargets Ther. 2021, 14, 3151–3165. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Tan, Y.; Ma, X.; Zhao, M.; Chen, B.; Zhang, R.; Chen, Z.; Wang, K. Profiling and functional analysis of circular RNAs in acute promyelocytic leukemia and their dynamic regulation during all-trans retinoic acid treatment. Cell Death Dis. 2018, 9, 651. [Google Scholar] [CrossRef]
- Tong, G.; Chen, B.; Wu, X.; He, L.; Lv, G.; Wang, S. circHIPK2 has a potentially important clinical significance in colorectal cancer progression via HSP90 ubiquitination by miR485-5p. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 33–42. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, H.; Sun, Y.; Cheng, F.; Zhao, S.; Liu, J.; Sun, P. CircHIPK2 promotes proliferation of nasopharyngeal carcinoma by downregulating HIPK2. Transl. Cancer Res. 2022, 11, 2348–2358. [Google Scholar] [CrossRef]
- Birukov, A.; Herse, F.; Nielsen, J.H.; Kyhl, H.B.; Golic, M.; Kraker, K.; Haase, N.; Busjahn, A.; Bruun, S.; Jense, B.L. Blood pressure and angiogenic markers in pregnancy: Contributors to pregnancy-induced hypertension and offspring cardiovascular risk. Hypertension 2020, 76, 901–909. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Agrawal, S.; Staff, A.C.; Redman, C.W.; Vatish, M. Angiogenic factors: Potential to change clinical practice in preeclampsia? BJOG 2018, 125, 1389–1395. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Shu, X.; Gao, Q.; Chen, X. Overexpression of microRNA-100-5p attenuates the endothelial cell dysfunction by targeting HIPK2 under hypoxia and reoxygenation treatment. J. Mol. Histol. 2021, 52, 1115–1125. [Google Scholar] [CrossRef]
- Li, Y.; Lv, Y.; Cheng, C.; Huang, Y.; Yang, L.; He, J.; Tao, X.; Hu, Y.; Ma, Y.; Su, Y.; et al. SPEN induces miR-4652-3p to target HIPK2 in nasopharyngeal carcinoma. Cell Death Dis. 2020, 11, 509. [Google Scholar] [CrossRef]
- Gonzalez-Luis, G.; van Westering-Kroon, E.; Villamor-Martinez, E.; Huizing, M.J.; Kilani, M.A.; Kramer, B.W.; Villamor, E. Tobacco smoking during pregnancy is associated with increased risk of moderate/severe bronchopulmonary dysplasia: A systematic review and meta-analysis. Front. Pediatr. 2020, 8, 160. [Google Scholar] [CrossRef]
- Singh, S.P.; Chand, H.S.; Gundavarapu, S.; Saeed, A.I.; Langley, R.J.; Tesfaigzi, Y.; Mishra, N.C.; Sopori, M.L. HIF-1a plays a critical role in the gestational sidestream smoke-induced bronchopulmonary dysplasia in mice. PLoS ONE 2015, 10, e0137757. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; YU, S.; Yeh, R.F.; Wythe, J.D.; Bruneau, B.G.; Stainer, D.Y.R.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, S.; Munoz-Vega, M.; Contreras, W.E.; Crespo-Avilan, G.E.; Rodriguez-Montesinos, J.; Arias-Carrion, O.; Perez-Mendez, O.; Boisvert, W.A.; Preissner, K.T.; Cabrera-Fuentes, H.A. Responses of endothelial cells towards ischemic conditioning following acute myocardial infarction. Cond. Med. 2018, 1, 247–258. [Google Scholar]
- Liao, Y.; Zou, Y.; Zhang, H. MicroRNA-126-5p facilitates hypoxia-induced vascular endothelial cell injury via HIPK2. Ann. Clin. Lab. Sci. 2022, 52, 918–926. [Google Scholar]
- Schmidt, A.M. Highlighting diabetes mellitus: The epidemic continues. Arter. Thromb. Vasc. Biol. 2018, 38, e1–e8. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.-Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Cataldi, S.; Tramontano, M.; Costa, V.; Aprile, M.; Ciccodicola, A. Diabetic retinopathy: Are lncRNAs new molecular players and targets? Antioxidants 2022, 11, 2021. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, Y.; Sun, H.; Hu, J.; Li, W.; Gao, L. MiR-423-5p activated by E2F1 promotes neovascularization in diabetic retinopathy by targeting HIPK2. Diabetol. Metab. Syndr. 2021, 13, 152. [Google Scholar] [CrossRef]
- Baldari, S.; Garufi, A.; Granato, M.; Cuomo, L.; Pistritto, G.; Cirone, M.; D’Orazi, G. Hyperglycemia triggers HIPK2 protein degradation. Oncotarget 2017, 8, 1190–1203. [Google Scholar] [CrossRef]
- Garufi, A.; Baldari, S.; Toietta, G.; Cirone, M.; D’Orazi, G. p53-dependent PUMA to DRAM antagonistic interplay as a key molecular switch in cell-fate decision in normal/high glucose condition. J. Exp. Clin. Cancer Res. 2017, 36, 126. [Google Scholar] [CrossRef]
- Garufi, A.; Traversi, G.; Gilardini Montani, M.S.; D’Orazi, V.; Pistritto, G.; Cirone, M.; D’Orazi, G. Reduced chemotherapeutic sensitivity in high glucose condition: Implication of antioxidant response. Oncotarget 2019, 10, 4691–4702. [Google Scholar] [CrossRef]
- Garufi, A.; Ricci, A.; Trisciuoglio, D.; Iorio, E.; Carpinelli, G.; Pistritto, G.; Cirone, M.; D’Orazi, G. Glucose restriction induces cell death in parental but not in homeodomain-interacting protein kinase 2 depleted RKO colon cancer cells: Molecular mechanisms and implications for tumor therapy. Cell Death Dis. 2013, 4, e639. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Panunzi, A.; Madotto, F.; Sangalli, E.; Riccio, F.; Sganzaroli, A.B.; Galenda, P.; Bertulessi, A.; Barmina, M.F.; Ludovico, O.; Fortunato, O.; et al. Results of a prospective observational study of autologous peripheral blood mononuclear cell therapy for no-option critical limb-threatening ischemia and severe diabetic foot ulcers. Cardiovasc. Diabetol. 2022, 21, 196. [Google Scholar] [CrossRef]
- Fui, L.W.; Lok, M.P.W.; Govindasamy, V.; Yong, T.K.; Lek, T.K.; Das, A.K. Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic applications of stem cells conditioned medium in the healing process. J. Tissue Eng. Regen. Med. 2019, 13, 2218–2233. [Google Scholar] [CrossRef]
- Xu, J.; Bai, S.; Cao, Y.; Liu, L.; Fang, Y.; Du, J.; Luo, L.; Chen, M.; Shen, B.; Zhang, Q. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1259–1270. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, L.; Zhang, X.; Chang, H.; Ma, S.; Xie, Z.; Tang, S.; Ju, X.; Zhu, H.; Shen, B.; et al. MiR-221-3p targets HIPK2 to promote diabetic wound healing. Microvasc. Res. 2022, 140, 104306. [Google Scholar] [CrossRef]
- Oh, H.; Kato, M.; Deshpande, S.; Zhang, E.; Das, S.; Lanting, L.; Wang, M.; Natarajan, R. Inhibition of the processing of miR-25 by HIPK2-Phosphorylated-MeCP2 induces NOX4 in early diabetic nephropathy. Sci. Rep. 2016, 6, 38789. [Google Scholar] [CrossRef]
| miRNA/circHIPK2 | Tumor Type | Biological Effect | Target | Tissues | Cell Lines | Reference |
|---|---|---|---|---|---|---|
| miR-1229 | CRC 1 | angiogenesis, metastasis | ↓ HIPK2 | + | + | [45] |
| miR-1260b | NSCLC 2 | angiogenesis, metastasis | ↓ HIPK2 | + | + | [55] |
| circHIPK2 | DDP-resistant NSCLC 3 | angiogenesis, drug resistance | ↓ miR-1249-3p, ↑ VEGF | + | + | [60] |
| circHIPK2 | CRC 1 | reduced overall survival | ? | + | + | [64] |
| circHIPK2 | NPC 4 | tumor progression | ? | + | + | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garufi, A.; D’Orazi, V.; Pistritto, G.; Cirone, M.; D’Orazi, G. HIPK2 in Angiogenesis: A Promising Biomarker in Cancer Progression and in Angiogenic Diseases. Cancers 2023, 15, 1566. https://doi.org/10.3390/cancers15051566
Garufi A, D’Orazi V, Pistritto G, Cirone M, D’Orazi G. HIPK2 in Angiogenesis: A Promising Biomarker in Cancer Progression and in Angiogenic Diseases. Cancers. 2023; 15(5):1566. https://doi.org/10.3390/cancers15051566
Chicago/Turabian StyleGarufi, Alessia, Valerio D’Orazi, Giuseppa Pistritto, Mara Cirone, and Gabriella D’Orazi. 2023. "HIPK2 in Angiogenesis: A Promising Biomarker in Cancer Progression and in Angiogenic Diseases" Cancers 15, no. 5: 1566. https://doi.org/10.3390/cancers15051566
APA StyleGarufi, A., D’Orazi, V., Pistritto, G., Cirone, M., & D’Orazi, G. (2023). HIPK2 in Angiogenesis: A Promising Biomarker in Cancer Progression and in Angiogenic Diseases. Cancers, 15(5), 1566. https://doi.org/10.3390/cancers15051566







