Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
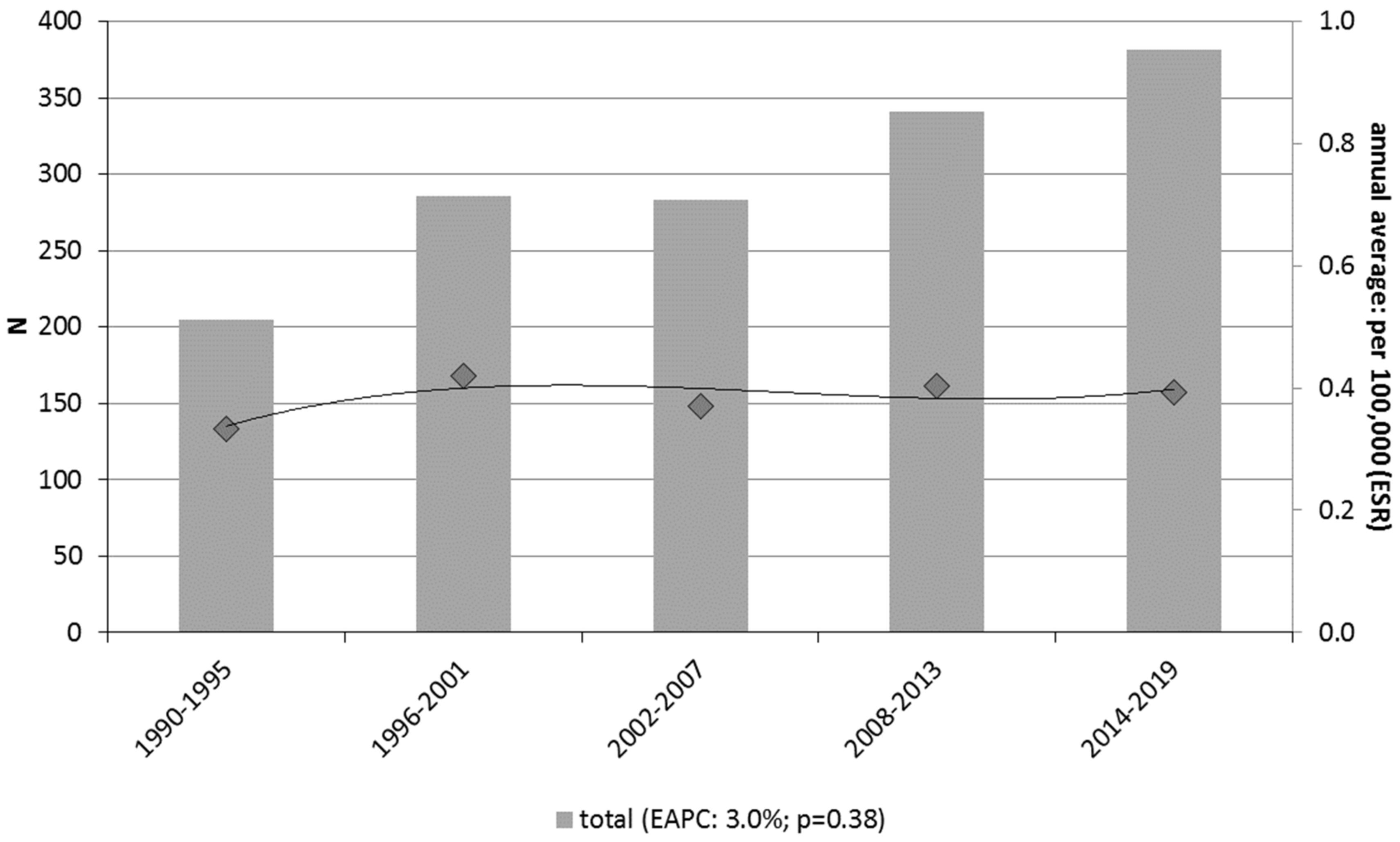
3.1. Incidence
3.2. Survival
3.3. Predictors for Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elder, D.E.; Bastian, B.C.; Cree, I.A.; Massi, D.; Scolyer, R.A. The 2018 World Health Organization Classification of Cutaneous, Mucosal, and Uveal Melanoma: Detailed Analysis of 9 Distinct Subtypes Defined by Their Evolutionary Pathway. Arch. Pathol. Lab. Med. 2020, 144, 500–522. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Li, S.; Sheng, X.; Si, L.; Cui, C.; Han, M.; Guo, J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: A study of 522 consecutive cases. BMC Cancer 2011, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, C.C.; Wu, X.-C.; Jemal, A.; Martin, H.J.; Roche, L.M.; Chen, V.W. Incidence of noncutaneous melanomas in the U.S. Cancer 2005, 103, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: A summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Vlajkovic, S.; Jovanovic, P.; Stefanovic, V. Primary mucosal melanomas: A comprehensive review. Int. J. Clin. Exp. Pathol. 2012, 5, 739–753. [Google Scholar]
- Koomen, E.R.; de Vries, E.; van Kempen, L.C.; van Akkooi, A.C.J.; Guchelaar, H.J.; Louwman, M.W.J.; Nijsten, T.; Coebergh, J.-W.W. Epidemiology of Extracutaneous Melanoma in the Netherlands. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1453–1459. [Google Scholar] [CrossRef]
- Lian, B.; Cui, C.L.; Zhou, L.; Song, X.; Zhang, X.S.; Wu, D.; Si, L.; Chi, Z.H.; Sheng, X.N.; Mao, L.L.; et al. The natural history and patterns of metastases from mucosal melanoma: An analysis of 706 prospectively-followed patients. Ann Oncol. 2017, 28, 868–873. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I. Vulvar Melanoma: Molecular Characteristics, Diagnosis, Surgical Management, and Medical Treatment. Am. J. Clin. Dermatol. 2021, 22, 639–651. [Google Scholar] [CrossRef]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nisticò, S.P. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef]
- Teterycz, P.; Czarnecka, A.M.; Indini, A.; Spałek, M.J.; Labianca, A.; Rogala, P.; Cybulska-Stopa, B.; Quaglino, P.; Ricardi, U.; Badellino, S.; et al. Multimodal Treatment of Advanced Mucosal Melanoma in the Era of Modern Immunotherapy. Cancers 2020, 12, 3131. [Google Scholar] [CrossRef]
- Che, G.; Huang, B.; Xie, Z.; Zhao, J.; Yan, Y.; Wu, J.; Sun, H.; Ma, H. Trends in incidence and survival in patients with melanoma, 1974–2013. Am. J. Cancer Res. 2019, 9, 1396–1414. [Google Scholar] [PubMed]
- Kuk, D.; Shoushtari, A.N.; Barker, C.A.; Panageas, K.S.; Munhoz, R.R.; Momtaz, P.; Ariyan, C.E.; Brady, M.S.; Coit, D.G.; Bogatch, K.; et al. Prognosis of Mucosal, Uveal, Acral, Nonacral Cutaneous, and Unknown Primary Melanoma from the Time of First Metastasis. Oncologist 2016, 21, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbe, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination with Ipilimumab in Patients with Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Leeneman, B.; Schreuder, K.; Uyl-de Groot, C.A.; van Akkooi, A.C.J.; Haanen, J.B.A.G.; Wakkee, M.; Franken, M.G.; Louwman, M.W.J. Stage-specific trends in incidence and survival of cutaneous melanoma in the Netherlands (2003–2018): A nationwide population-based study. Eur. J. Cancer 2021, 154, 111–119. [Google Scholar] [CrossRef]
- Van Zeijl, M.C.T.; Boer, F.L.; van Poelgeest, M.I.E.; van den Eertwegh, A.J.M.; Wouters, M.; de Wreede, L.C.; Aarts, M.J.B.; van den Berkmortel, F.; de Groot, J.W.B.; Hospers, G.A.P.; et al. Survival outcomes of patients with advanced mucosal melanoma diagnosed from 2013 to 2017 in the Netherlands—A nationwide population-based study. Eur. J. Cancer 2020, 137, 127–135. [Google Scholar] [CrossRef]
- Menzies, A.M.; Amaria, R.N.; Rozeman, E.A.; Huang, A.C.; Tetzlaff, M.T.; van de Wiel, B.A.; Lo, S.; Tarhini, A.A.; Burton, E.M.; Pennington, T.E.; et al. Pathological response and survival with neoadjuvant therapy in melanoma: A pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 2021, 27, 301–309. [Google Scholar] [CrossRef]
- Boer, F.L.; ten Eikelder, M.L.G.; van Geloven, N.; Kapiteijn, E.H.; Gaarenstroom, K.N.; Hughes, G.; Nooij, L.S.; Jozwiak, M.; Tjiong, M.Y.; de Hullu, J.M.A.; et al. Evaluation of treatment, prognostic factors, and survival in 198 vulvar melanoma patients: Implications for clinical practice. Gynecol. Oncol. 2021, 161, 202–210. [Google Scholar] [CrossRef]
- Salari, B.; Foreman, R.K.; Emerick, K.S.; Lawrence, D.P.; Duncan, L.M. Sinonasal Mucosal Melanoma: An Update and Review of the Literature. Am. J. Dermatopathol. 2022, 44, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Lian, B.; Zhou, L.; Song, X.; Zhang, X.; Wu, D.; Chi, Z.; Si, L.; Sheng, X.; Kong, Y.; et al. Multifactorial Analysis of Prognostic Factors and Survival Rates Among 706 Mucosal Melanoma Patients. Ann. Surg. Oncol. 2018, 25, 2184–2192. [Google Scholar] [CrossRef]
- Cui, C.; Lian, B.; Zhang, X.; Wu, D.; Li, K.; Si, L.; Yang, Y.; Tian, H.; Zhou, L.; Chi, Z.; et al. An Evidence-Based Staging System for Mucosal Melanoma: A Proposal. Ann. Surg. Oncol. 2022, 29, 5221–5234. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Roesch, A.; Weide, B.; Gutzmer, R.; Meier, F.; Loquai, C.; Kahler, K.C.; Gesierich, A.; Meissner, M.; von Bubnoff, D.; et al. Prognostic factors and treatment outcomes in 444 patients with mucosal melanoma. Eur. J. Cancer. 2017, 81, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.M.; Lee, K.G.; Choi, W.; Cheong, S.H.; Myung, K.B.; Hahn, H.J. An updated review of mucosal melanoma: Survival meta-analysis. Mol. Clin. Oncol. 2019, 11, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Altieri, L.; Eguchi, M.; Peng, D.H.; Cockburn, M. Predictors of mucosal melanoma survival in a population-based setting. J. Am. Acad. Dermatol. 2019, 81, 136–142.e2. [Google Scholar] [CrossRef] [PubMed]
- Carbo-Bague, A.; Rubio-Casadevall, J.; Puigdemont, M.; Sanvisens, A.; Oliveras, G.; Coll, M.; Del Olmo, B.; Perez-Bueno, F.; Marcos-Gragera, R. Epidemiology and Molecular Profile of Mucosal Melanoma: A Population-Based Study in Southern Europe. Cancers 2022, 14, 780. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, H.; Tan, L.; Lai, Y.; Li, Z. Different clinico-pathological and prognostic features of vulvar, vaginal, and cervical melanomas. Hum. Pathol. 2023, 131, 87–97. [Google Scholar] [CrossRef]
- Joste, M.; Dion, L.; Brousse, S.; Nyangoh Timoh, K.; Rousseau, C.; Reilhac, A.; Laviolle, B.; Lesimple, T.; Lavoue, V.; Leveque, J. Vulvar and vaginal melanomas: A retrospective study spanning 19 years from a tertiary center. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102091. [Google Scholar] [CrossRef]
- Michielin, O.; van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U.; clinicalguidelines@esmo.org EGCEa. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef]
- Dimitriou, F.; Namikawa, K.; Reijers, I.L.M.; Buchbinder, E.I.; Soon, J.A.; Zaremba, A.; Teterycz, P.; Mooradian, M.J.; Armstrong, E.; Nakamura, Y.; et al. Single-agent anti-PD-1 or combined with ipilimumab in patients with mucosal melanoma: An international, retrospective, cohort study. Ann. Oncol. 2022, 33, 968–980. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Yoshikawa, S.; Kiniwa, Y.; Maekawa, T.; Yamasaki, O.; Isei, T.; Matsushita, S.; Nomura, M.; Nakai, Y.; et al. Anti-PD-1 antibody monotherapy versus anti-PD-1 plus anti-CTLA-4 combination therapy as first-line immunotherapy in unresectable or metastatic mucosal melanoma: A retrospective, multicenter study of 329 Japanese cases (JMAC study). ESMO Open 2021, 6, 100325. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Wagstaff, J.; Ascierto, P.A.; Butler, M.O.; Lao, C.D.; Márquez-Rodas, I.; Chiarion-Sileni, V.; Dummer, R.; Ferrucci, P.F.; Lorigan, P.C.; et al. CheckMate 067: Long-term outcomes in patients with mucosal melanoma. J. Clin. Oncol. 2020, 38, 10019. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kan, H.; Zhao, L.; Sun, Z.; Bai, C. Immune checkpoint inhibitors in advanced or metastatic mucosal melanoma: A systematic review. Ther. Adv. Med. Oncol. 2020, 12, 1758835920922028. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Mattei, J.; Tetzlaff, M.; Williams, M.D.; Davies, M.A.; Diab, A.; Oliva, I.C.G.; McQuade, J.; Patel, S.P.; Tawbi, H.; et al. Neoadjuvant checkpoint inhibitor immunotherapy for resectable mucosal melanoma. Front. Oncol. 2022, 12, 1001150. [Google Scholar] [CrossRef]



| Total | 1990–2013 | 2014–2019 | |||||
|---|---|---|---|---|---|---|---|
| N = 1496 | N = 1115 | N = 381 | |||||
| n | % | n | % | n | % | p | |
| Sex | 0.43 | ||||||
| Male | 401 | 26.8% | 293 | 26.3% | 108 | 28.3% | |
| Female | 1095 | 73.2% | 822 | 73.7% | 273 | 71.7% | |
| Age at diagnosis (years) | 0.32 | ||||||
| 0–59 | 323 | 21.6% | 242 | 21.7% | 81 | 21.3% | |
| 60–69 | 297 | 19.9% | 209 | 18.7% | 88 | 23.1% | |
| 70–79 | 429 | 28.7% | 324 | 29.1% | 105 | 27.6% | |
| ≥80 | 447 | 29.9% | 340 | 30.5% | 107 | 28.1% | |
| Median (interquartile range) | 72 (62–81) | 73 (62–81) | 71 (62–80) | ||||
| Tumour site | 0.04 | ||||||
| Head and neck | 505 | 33.8% | 380 | 34.1% | 125 | 32.8% | |
| Gastrointestinal tract | 76 | 5.1% | 51 | 4.6% | 25 | 6.6% | |
| Anorectal tract | 248 | 16.6% | 176 | 15.8% | 72 | 18.9% | |
| Female genital tract | 640 | 42.8% | 488 | 43.8% | 152 | 39.9% | |
| Urinary tract | 16 | 1.1% | 9 | 0.8% | 7 | 1.8% | |
| Respiratory tract | 11 | 0.7% | 11 | 1.0% | 0 | 0.0% | |
| Tumour stage | 0.15 | ||||||
| Local/locally advanced disease | 983 | 65.7% | 741 | 66.5% | 242 | 63.5% | |
| Locoregional spread disease | 254 | 17.0% | 184 | 16.5% | 70 | 18.4% | |
| Distant spread disease | 226 | 15.1% | 161 | 14.4% | 65 | 17.1% | |
| Unknown | 33 | 2.2% | 29 | 2.6% | 4 | 1.0% | |
| Surgery | <0.01 | ||||||
| No | 344 | 23.0% | 233 | 20.9% | 111 | 29.1% | |
| Yes | 1152 | 77.0% | 882 | 79.1% | 270 | 70.9% | |
| Hospital of first surgery | 0.04 ** | ||||||
| Academic centre | 504 | 43.8% | 351 | 39.8% | 153 | 56.7% | |
| General hospital | 459 | 39.8% | 347 | 39.3% | 112 | 41.5% | |
| Unknown | 189 | 16.4% | 184 | 20.9% | 5 | 1.9% | |
| Radiotherapy | 0.38 | ||||||
| No | 1036 | 69.3% | 779 | 69.9% | 257 | 67.5% | |
| Yes | 460 | 30.7% | 336 | 30.1% | 124 | 32.5% | |
| Systemic therapy * | <0.01 | ||||||
| No | 1409 | 94.2% | 1079 | 96.8% | 330 | 86.6% | |
| Yes | 87 | 5.8% | 36 | 3.2% | 51 | 13.4% | |
| Chemotherapy | <0.01 | ||||||
| No | 1462 | 97.7% | 1081 | 97.0% | 381 | 100.0% | |
| Yes | 34 | 2.3% | 34 | 3.0% | 0 | 0.0% | |
| Immune and targeted therapy | <0.01 | ||||||
| No | 1443 | 96.5% | 1113 | 99.8% | 330 | 86.6% | |
| Yes | 53 | 3.5% | 2 | 0.2% | 51 | 13.4% | |
| Hospital of first contact | 0.43 ** | ||||||
| Academic centre | 202 | 13.5% | 155 | 13.9% | 47 | 12.3% | |
| General hospital | 1291 | 86.3% | 957 | 85.8% | 334 | 87.7% | |
| Unknown | 3 | 0.2% | 3 | 0.3% | 0 | 0.0% | |
| Tumour Site | Total | Local/Locally Advanced Disease | Locoregional Spread Disease | Distant Spread Disease | Unknown | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1496 | 65.7% | 17.0% | 15.1% | 2.2% | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Head and neck | 505 | 33.8% | 403 | 79.8% | 50 | 9.9% | 51 | 10.1% | 1 | 0.2% |
| Oral | 83 | 5.5% | 58 | 69.9% | 15 | 18.1% | 10 | 12.0% | 0 | 0.0% |
| Sinonasal | 412 | 27.5% | 342 | 83.0% | 32 | 7.8% | 37 | 9.0% | 1 | 0.2% |
| Pharynx/glottis | 10 | 0.7% | 3 | 30.0% | 3 | 30.0% | 4 | 40.0% | 0 | 0.0% |
| Gastrointestinal tract | 76 | 5.1% | 27 | 35.5% | 10 | 13.2% | 39 | 51.3% | 0 | 0.0% |
| Anorectal tract | 248 | 16.6% | 104 | 41.9% | 70 | 28.2% | 73 | 29.4% | 1 | 0.4% |
| Rectum | 136 | 9.1% | 45 | 33.1% | 40 | 29.4% | 51 | 37.5% | 0 | 0.0% |
| Anus | 112 | 7.5% | 59 | 52.7% | 30 | 26.8% | 22 | 19.6% | 1 | 0.9% |
| Female genital tract | 640 | 42.8% | 433 | 67.7% | 122 | 19.1% | 54 | 8.4% | 31 | 4.8% |
| Vulva | 458 | 30.6% | 301 | 65.7% | 101 | 22.1% | 26 | 5.7% | 30 | 6.6% |
| Vagina | 157 | 10.5% | 111 | 70.7% | 20 | 12.7% | 25 | 15.9% | 1 | 0.6% |
| Other | 25 | 1.7% | 21 | 84.0% | 1 | 4.0% | 3 | 12.0% | 0 | 0.0% |
| Urinary tract | 16 | 1.1% | 11 | 68.8% | 0 | 0.0% | 5 | 31.3% | 0 | 0.0% |
| Respiratory tract | 11 | 0.7% | 5 | 45.5% | 2 | 18.2% | 4 | 36.4% | 0 | 0.0% |
| 1-Year OS | 2-Year OS | 5-Year OS | Median OS | 5-Year RS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | 95%CI | % | 95%CI | % | 95%CI | Years | 95%CI | % | 95%CI | |
| All | 67.2 | (64.7–69.5) | 44.4 | (41.9–46.9) | 23.8 | (21.6–26.0) | 1.7 | (1.6–1.8) | 29.0 | (26.2–31.8) |
| Tumour stage | ||||||||||
| Local/locally advanced | 77.2 | (74.5–79.7) | 55.2 | (52.1–58.3) | 30.8 | (27.9–33.7) | 2.4 | (2.1–2.7) | 37.5 | (33.7–41.2) |
| Locoregional spread disease | 62.2 | (55.9–67.8) | 33.1 | (27.4–38.9) | 14.0 | (10.0–18.8) | 1.3 | (1.1–1.6) | 17.4 | (12.1–23.4) |
| Distant spread disease | 31.4 | (25.5–37.5) | 12.8 | (8.9–17.6) | 5.2 | (2.8–8.8) | 0.6 | (0.4–0.7) | 6.7 | (3.4–11.5) |
| Tumour site | ||||||||||
| Head and neck | 68.7 | (64.5–72.6) | 47.7 | (43.3–52) | 24.7 | (20.9–28.6) | 1.9 | (1.7–2.1) | 30.8 | (26.0–35.7) |
| Oral | 77.1 | (66.5–84.7) | 54.1 | (42.8–64.1) | 28.4 | (18.9–38.5) | 2.6 | (1.7–3.5) | 31.0 | (20.2–42.3) |
| Sinonasal | 67.0 | (62.2–71.3) | 46.4 | (41.5–51.1) | 23.8 | (19.7–28.1) | 1.8 | (1.5–2.1) | 30.8 | (25.4–36.3) |
| Gastrointestinal tract | 36.8 | (26.2–47.5) | 19.7 | (11.7–29.3) | 13.2 | (6.7–21.8) | 0.7 | (0.5–0.9) | 17.2 | (9.1–27.4) |
| Anorectal tract | 58.5 | (52.1–64.3) | 30.2 | (24.6–36) | 14.8 | (10.6–19.6) | 1.2 | (1.0–1.4) | 18.3 | (12.9–24.4) |
| Rectum | 52.9 | (44.2–60.9) | 23.5 | (16.8–30.9) | 11.5 | (6.8–17.6) | 1.0 | (0.8–1.3) | 13.9 | (7.9–21.5) |
| Anus | 65.2 | (55.6–73.2) | 38.4 | (29.4–47.3) | 18.9 | (12.2–26.8) | 1.6 | (1.2–1.8) | 23.9 | (15.0–34.0) |
| Female genital tract | 73.8 | (70.2–77.0) | 50.5 | (46.5–54.3) | 27.8 | (24.4–31.4) | 2.0 | (1.8–2.5) | 33.4 | (28.8–38.0) |
| Vulva | 79.0 | (75.0–82.5) | 59.0 | (54.3–63.3) | 34.5 | (30.0–38.9) | 2.9 | (2.5–3.4) | 41.4 | (35.5–47.2) |
| Vagina | 58.0 | (49.8–65.2) | 26.1 | (19.5–33.2) | 9.5 | (5.6–14.7) | 1.1 | (1.0–1.4) | 12.1 | (7.0–18.8) |
| Urinary tract | 68.8 | (40.5–85.6) | 56.3 | (29.5–76.2) | 31.3 | (11.4–53.6) | 2.8 | (0.5–5.1) | 38.0 | (12.4–63.9) |
| Respiratory tract | 18.2 | (2.9–44.2) | 18.2 | (2.9–44.2) | 9.1 | (0.5–33.3) | 0.4 | (0.2–0.9) | 14.1 | (1.2–41.6) |
| Univariable Analyses | Multivariable Analyses: Complete Model | Multivariable Analyses: Definitive Model Stratified for Surgery | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%-CI | p | HR | 95%-CI | p | HR | 95%-CI | p | |
| Period of diagnosis | |||||||||
| 1990–2013 | ref | ref | ref | ||||||
| 2014–2019 | 0.85 | (0.74–0.98) | 0.02 | 0.82 | (0.71–0.95) | <0.01 | 0.82 | (0.71–0.95) | <0.01 |
| Sex | |||||||||
| Male | 1.32 | (1.17–1.49) | <0.01 | 1.11 | (0.96–1.28) | 0.17 | |||
| Female | ref | ref | |||||||
| Age at diagnosis (years) | |||||||||
| 0–59 | ref | ref | ref | ||||||
| 60–69 | 1.23 | (1.03–1.47) | 0.02 | 1.27 | (1.06–1.52) | <0.01 | 1.33 | (1.11–1.59) | 0.01 |
| 70–79 | 1.54 | (1.31–1.82) | <0.01 | 1.51 | (1.28–1.78) | <0.01 | 1.59 | (1.35–1.87) | <0.01 |
| ≥80 | 2.28 | (1.94–2.68) | <0.01 | 2.26 | (1.91–2.67) | <0.01 | 2.34 | (1.98–2.76) | <0.01 |
| Tumour site | |||||||||
| Head and neck | ref | ref | ref | ||||||
| Gastrointestinal tract | 1.87 | (1.45–2.40) | <0.01 | 1.27 | (0.97–1.65) | 0.08 | 1.22 | (0.95–1.60) | 0.12 |
| Anorectal tract | 1.39 | (1.18–1.63) | <0.01 | 1.15 | (0.96–1.38) | 0.13 | 1.12 | (0.95–1.33) | 0.17 |
| Female genital tract | 0.86 | (0.76–0.97) | 0.02 | 0.89 | (0.76–1.05) | 0.17 | 0.82 | (0.72–0.93) | <0.01 |
| Urinary tract | 0.93 | (0.53–1.61) | 0.79 | 0.85 | (0.49–1.50) | 0.58 | 0.83 | (0.47–1.44) | 0.51 |
| Respiratory tract | 2.79 | (1.53–5.08) | <0.01 | 2.42 | (1.30–4.50) | <0.01 | 2.45 | (1.32–4.54) | <0.01 |
| Tumour stage | |||||||||
| Local/locally advanced disease | ref | ref | ref | ||||||
| Locoregional spread disease | 1.67 | (1.44–1.93) | <0.01 | 1.55 | (1.33–1.80) | <0.01 | 1.61 | (1.38–1.87) | <0.01 |
| Distant spread disease | 3.56 | (3.05–4.15) | <0.01 | 2.73 | (2.27–3.30) | <0.01 | 2.56 | (2.13–3.09) | <0.01 |
| Unknown | 1.98 | (1.39–2.82) | <0.01 | 1.75 | (1.22–2.51) | <0.01 | 1.68 | (1.17–2.42) | 0.01 |
| Surgery | |||||||||
| No | ref | ref | |||||||
| Yes | 0.31 | (0.27–0.35) | <0.01 | 0.45 | (0.38–0.52) | <0.01 | |||
| Hospital of first surgery | |||||||||
| Academic centre | ref | ||||||||
| General hospital | 0.97 | (0.84–1.11) | 0.63 | ||||||
| Unknown | 1.03 | (0.86–1.23) | 0.77 | ||||||
| Radiotherapy | |||||||||
| No | ref | ref | |||||||
| Yes | 1.19 | (1.06–1.34) | <0.01 | 1.05 | (0.92–1.21) | 0.48 | |||
| Chemotherapy | |||||||||
| No | ref | ref | |||||||
| Yes | 1.94 | (1.38–2.73) | <0.01 | 0.82 | (0.57–1.18) | 0.29 | |||
| Immune and targeted therapy | |||||||||
| No | ref | ref | ref | ||||||
| Yes | 1.39 | (1.02–1.90) | <0.01 | 0.55 | (0.38–0.79) | <0.01 | 0.60 | (0.42–0.86) | 0.01 |
| Hospital of first contact | |||||||||
| Academic centre | ref | ref | |||||||
| General hospital | 1.08 | (0.92–1.27) | 0.34 | 1.08 | (0.92–1.27) | 0.34 | |||
| Unknown | 0.42 | (0.10–1.69) | 0.22 | 0.42 | (0.10–1.69) | 0.22 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boer, F.L.; Ho, V.K.Y.; Louwman, M.W.J.; Schrader, A.M.R.; Zuur, C.L.; Blank, C.U.; van Poelgeest, M.I.E.; Kapiteijn, E.H.W. Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019). Cancers 2023, 15, 1541. https://doi.org/10.3390/cancers15051541
Boer FL, Ho VKY, Louwman MWJ, Schrader AMR, Zuur CL, Blank CU, van Poelgeest MIE, Kapiteijn EHW. Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019). Cancers. 2023; 15(5):1541. https://doi.org/10.3390/cancers15051541
Chicago/Turabian StyleBoer, Florine L., Vincent K. Y. Ho, Marieke W. J. Louwman, Anne M. R. Schrader, Charlotte L. Zuur, Christian U. Blank, Mariette I. E. van Poelgeest, and Ellen H. W. Kapiteijn. 2023. "Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019)" Cancers 15, no. 5: 1541. https://doi.org/10.3390/cancers15051541
APA StyleBoer, F. L., Ho, V. K. Y., Louwman, M. W. J., Schrader, A. M. R., Zuur, C. L., Blank, C. U., van Poelgeest, M. I. E., & Kapiteijn, E. H. W. (2023). Trends in Incidence and Survival of 1496 Patients with Mucosal Melanoma in The Netherlands (1990–2019). Cancers, 15(5), 1541. https://doi.org/10.3390/cancers15051541







