Simple Summary
For early-stage hepatocellular carcinoma (HCC) (size ≤ 5 cm), the prediction of microvascular invasion (MVI) before operation is important for the therapeutic strategy. This study aimed to construct deep learning (DL) models based only on the venous phase (VP) of contrast-enhanced computed tomography (CECT), and to evaluate the performance of these models for preoperative prediction of MVI. A novel transformer-based end-to-end DL model is proposed for the first time, named MVI-TR, to capture features automatically from radiomics and to perform MVI preoperative assessments. For patient cohorts, it achieved superior outcomes in six performance measures of MVI predication status: accuracy, precision, receiver operating characteristic (ROC), area under the curve (AUC), recalling rate, and F1-score.
Abstract
In this study, we considered preoperative prediction of microvascular invasion (MVI) status with deep learning (DL) models for patients with early-stage hepatocellular carcinoma (HCC) (tumor size ≤ 5 cm). Two types of DL models based only on venous phase (VP) of contrast-enhanced computed tomography (CECT) were constructed and validated. From our hospital (First Affiliated Hospital of Zhejiang University, Zhejiang, P.R. China), 559 patients, who had histopathological confirmed MVI status, participated in this study. All preoperative CECT were collected, and the patients were randomly divided into training and validation cohorts at a ratio of 4:1. We proposed a novel transformer-based end-to-end DL model, named MVI-TR, which is a supervised learning method. MVI-TR can capture features automatically from radiomics and perform MVI preoperative assessments. In addition, a popular self-supervised learning method, the contrastive learning model, and the widely used residual networks (ResNets family) were constructed for fair comparisons. With an accuracy of 99.1%, a precision of 99.3%, an area under the curve (AUC) of 0.98, a recalling rate of 98.8%, and an F1-score of 99.1% in the training cohort, MVI-TR achieved superior outcomes. Additionally, the validation cohort’s MVI status prediction had the best accuracy (97.2%), precision (97.3%), AUC (0.935), recalling rate (93.1%), and F1-score (95.2%). MVI-TR outperformed other models for predicting MVI status, and showed great preoperative predictive value for early-stage HCC patients.
1. Introduction
The most typical primary liver malignant tumor, hepatocellular carcinoma (HCC), poses a major risk to public health [1]. In 2020, it became the second leading cause of death for men and ranks second among all causes of cancer death worldwide [2]. With the development of technology and the change of clinical perception, liver transplantation (LT), surgical resection, targeted therapy, transcatheter arterial chemoembolization (TACE), and immune checkpoint inhibitors are the main therapeutic methods for HCC [3,4]. However, about 60% of patients will have postoperative tumor recurrence [5]. For the systematic treatment of HCC, identifying patients at high risk of recurrence is significant.
Microvascular invasion (MVI) is common in advanced HCC [6]. Different from macrovascular infiltration in advanced HCC, it is a distinct and significant predictor of recurrence after surgical treatment in early-stage HCC patients [7,8,9]. Presently, the pathological evaluation of the surgical specimens obtained after resection or LT can confirm the diagnosis of MVI, and a large tumor diameter is a prognostic factor for MVI [10,11]. An innovative tool with noninvasive and efficient recognition of MVI during early-stage HCC (tumor size ≤ 5 cm) has specific clinical importance for the determination of an individual’s treatment strategy and correspondingly the assessment of recurrence risk.
Medical imageology has been the most important method to diagnose HCC without pathological evidence [12,13]. However, the traditional clinical-radiological model lacks the indicators to predict the clinical prognosis factors, such as MVI and recurrent tumor heterogeneity [14]. With the rapid development of computer-aided diagnosis (CAD), radiomics, which can transform the original image into a great number of statistical features and explain the instinctive pathophysiology of tumors, has become one of the significant means to study the heterogeneity of many different tumors [13,15,16,17,18,19]. On the other hand, DL has achieved great success in many fields. Progressively, there has been some research in applying DL-based models to MVI histopathologic outcome prediction. These include DL-based models for MVI examining based on the images of histopathological sections [20,21]. Meanwhile, Wang and Deng [22,23] propose multimodal deep learning models based on CECT and multi-parameter magnetic resonance imaging (MRI). Based solely on CT data, Wang et al. [24] developed a light-weight transformer model for cancer segmentation and prediction. However, their validation was based on a limited number of patients and showed an unconvincing result. Yang et al. [25] applied six pretrained convolutional neural network (CNN) algorithms (pretrained using ImageNet database) to extract DL features from the sequences of non-contrast (NC), arterial phase (AP), and VP. These sequences provided more valuable information than a single image. Nevertheless, radiomics research addressing MVI diagnosis has shown a number of limitations, especially regarding prediction accuracy and generalizability. Heterogeneity exists in the source of the CNN base pretrained from the MVI.
In this study, we collected a sufficient number of large datasets of annotated medical images. These datasets were able to represent excellent universality and clinical practicality. For early-stage HCC patients (an isolated tumor size ≤ 5 cm), we proposed MVI-TR, a novel transformer-based end-to-end DL model, and developed additional DL models of MVI prediction based only on a single VP of CECT, without any clinical data. To our knowledge, we have established the first transformer-based prediction model and achieved superior accuracy and precision for MVI prediction.
2. Materials and Methods
2.1. Patients
The approval of this work was granted by the review board of our institution (the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China), and the written informed consent was waived. The Declaration of Helsinki was followed in all procedures. We collected data from 559 consecutive HCC patients, from January 2019 to December 2020, who underwent therapeutic resection or LT in our center.
The following were the inclusion criteria:
- (a).
- Pathologically confirmed HCC after R0 resection or LT;
- (b).
- Single tumor without satellite nodules and a lesion diameter ≤ 5 cm;
- (c).
- Available for the pathological assessment of MVI;
- (d).
- Receipt of preoperative hepatic CECT scan < 1 month;
- (e).
- With well-preserved clinical and imaging information for reevaluation.
The following were the exclusion criteria.
- (a).
- Lack of hepatic CECT within 1 month before resection or LT;
- (b).
- Patients with recurrent HCC;
- (c).
- Presence of extrahepatic metastases or macrovascular invasion;
- (d).
- With multiple liver tumors;
- (e).
- Clinical or pathological information was not available;
- (f).
- With poor imaging quality that did not match the region of interest (ROI) definition;
- (g).
- Patient received cancer-related preoperative treatments, including TACE, radiofrequency ablation (RFA), chemotherapy, targeted therapy, immunotherapy, or other antitumor treatments.
All patients were randomly allocated to either the training cohort (n = 448, MVI positive 120) or the validation cohort (n = 111, MVI positive 29) at a rough ratio of 4:1.
2.2. Clinical Characteristics and Pathological Examination
Baseline clinical information was collected for each patient, including patients’ age, sex, maximum diameter of tumors, HBV-DNA, hepatitis B surface antigen (HBsAg), and tumor markers (serum alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), protein induced by vitamin K absence or antagonist-II (PIVKA II), etc.).
Information about pathology type and MVI was obtained under the microscope. Diagnosis of MVI was based on the HCC diagnosis and treatment guide [26]. MVI hazard levels consisted of M0 (no MVI), M1 (low risk, no more than 5 MVI inside 1 cm of tumor or adjacent to tumor), and M2 (high risk, greater than 5 MVI or MVI in non-tumor adjacent tissues). MVI negative was defined as M0, and MVI positive was described as including M1 and M2.
2.3. CT Data Collection
The preoperative CECT of HCC patients was retrieved from the system of picture archiving and communication in our hospital. All data were carried out on a 256-slice scanner (Brilliance iCT; Philips, Amsterdam, The Netherlands). The reconstruction of CECT images adopted the standard kernel, which has a slice thickness of 1.0 mm and an interval of 1.0 mm.
We used an automatic pump syringe to inject iodinated contrast medium and saline. CT scans were carried out at 25–35 s (AP), 50–70 s (portal vein phase), and 120–160 s (VP) after contrast injection.
2.4. Tumor Segmentation
The VP of CECT demonstrated better visibility of the target tumor lesion and was therefore chosen to quantify cancer features in this study. Window Width (WW) was set to 400 and Window Level (WL) was 40 to obtain proper and clear images for the surgeon [27]. The tumor segmentation on CT data was manually implemented by three hepatobiliary surgeons working independently (Cao Linping, Zhang Weicheng, and Zhong Xun) (all of them had eight years of HCC diagnosis experience) with ITK-SNAP software (3.8.0) [28], and the results were reviewed by a senior hepatobiliary surgeon (Wu Jian) with twenty years of HCC diagnosis experience. For the purpose of extracting radiomics features, we focused on the whole region of the primary lesion and contoured the domain covering it in the slice with the maximum tumor cross-sectional area.
2.5. Data Preprocessing
CT scans with Hounsfield units’ raw intensities were stored in Data Interchange Standard for Biomedical Imaging (DICOM) format. In order to fully utilize the information contained in the tumor region, we selected the 2D slice with the maximum tumor area from 3D CT images. Finally, the whole ROI image was resized to 224 × 224 pixels by bilinear interpolation to obtain more detailed information.
2.6. Data Augmentation
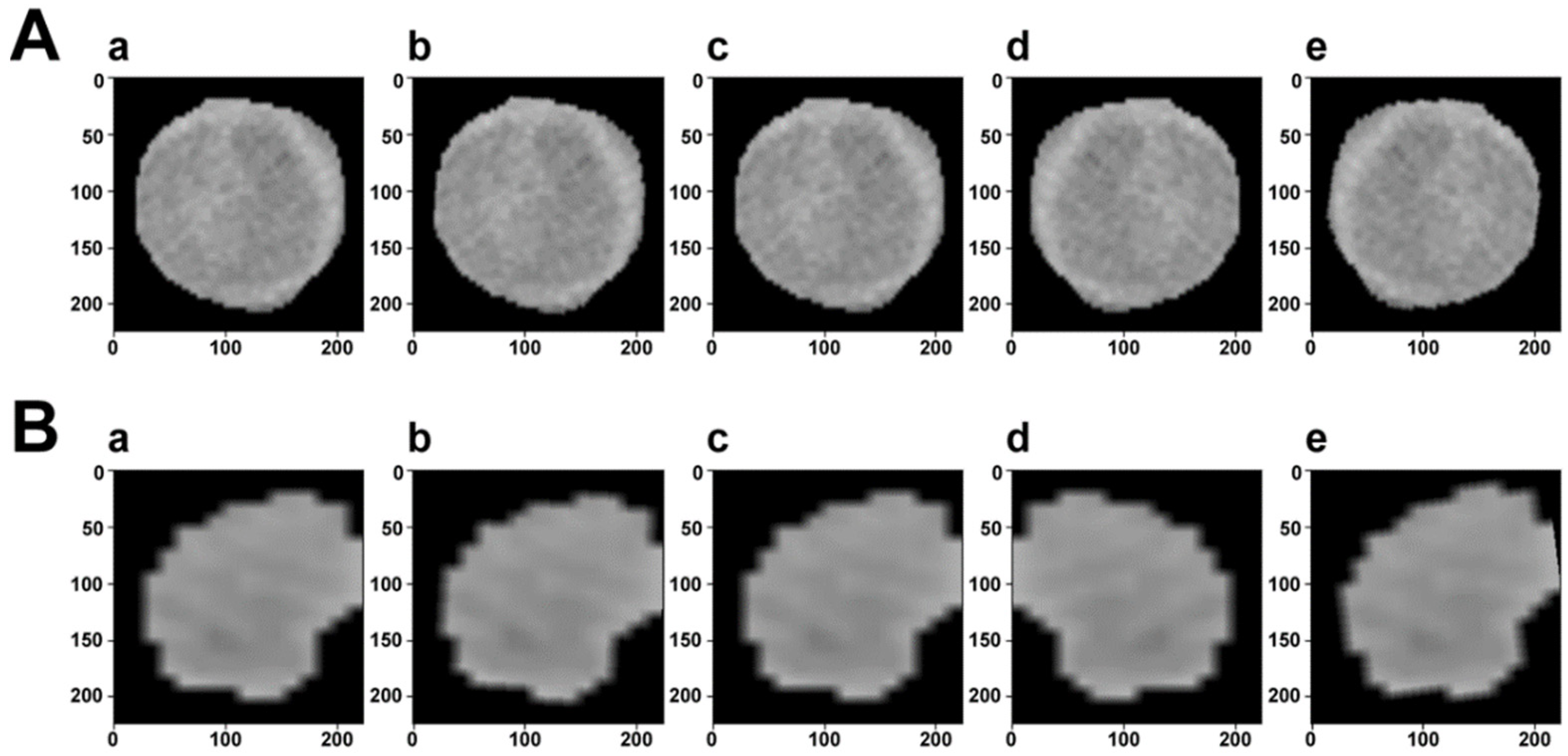
Data enhancement is a technology that enhances the quantity and quality of limited data. It can fundamentally reduce overfitting and improve the generalization of deep learning models. During the training process, we implemented different types of data enhancement, as shown in Figure 1. Because image transformations increased the diversity of data, it was conducive to overcoming problems such as scale and background, and building a more robust DL model. Experiments showed that the MVI state recognition performance of DL models had been significantly improved.
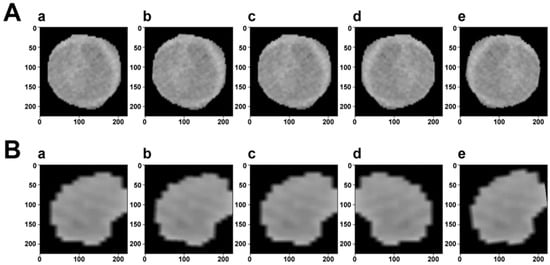
Figure 1.
Visualization of data augmentation. The first row (A) is the ROI of a sample with MVI, while the second row (B) is without MVI. In each row, (a) is the original image. (b–d) Are generated after random rotation, random rotation crop, and random horizontal flip transformation, respectively. (e) Is the composition of the previous transformed images.
2.7. Deep Learning Models
There are two mainstream DL-based strategies for feature extraction, unsupervised and supervised learning methods. We first used contrastive learning for unsupervised feature extraction, which discovers hidden patterns without the need for human intervention. On the other hand, we developed different supervised learning models: MVI-TR and ResNets family. Labels are introduced in these models which further improve the performance of MVI status prediction.
2.7.1. Contrastive Learning
As a self-supervised learning method, contrastive learning is effective if and only if similar instances are relatively close and dissimilar instances are farther apart in the representation space [29].
Let with trainable parameters be a backbone encoder. Referring to the work by [30], the backbone was pretrained on ImageNet [31]. Representation of images can be optimized independently of downstream tasks. If supervised learning is performed, the extracted information is related to the current target. We added a classifier to predict MVI status, after the pretrained backbone .
The framework of contrastive learning was shown in Figure 2. The backbone encoder extracts representation vectors from augmented data examples. A projection head of multi-layer perceptron (MLP) is applied to map representation vectors into the space of the contrastive loss. In the inference period, we threw away the projection head and used encoder for the downstream classification task.
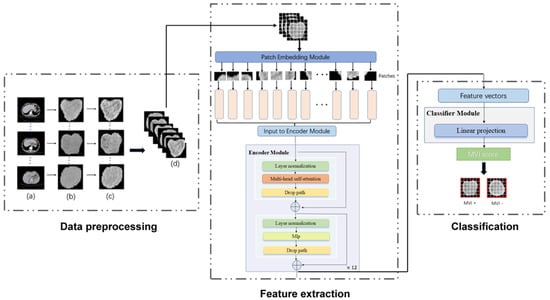
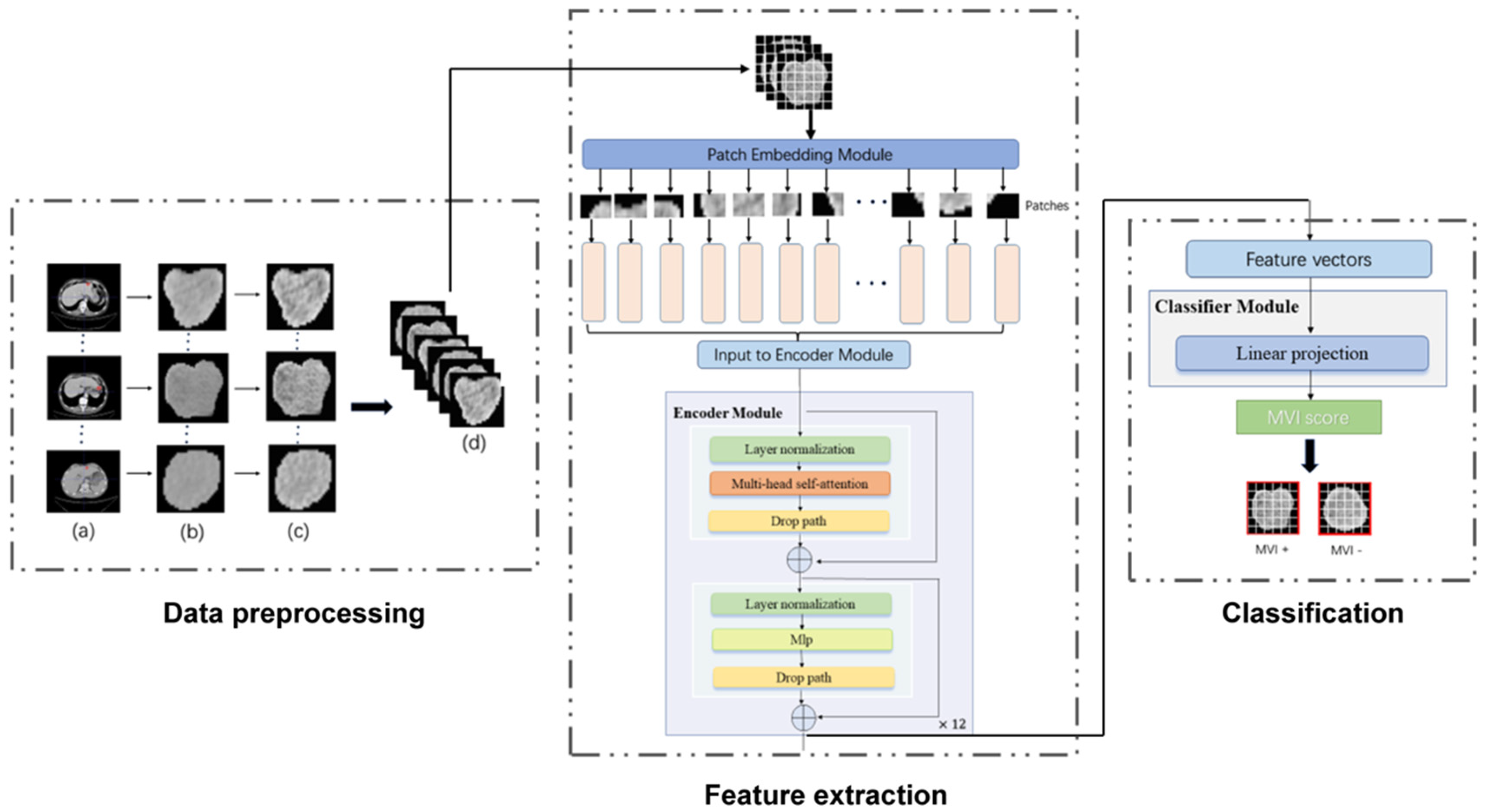
Figure 2.
The workflow of our MVI−TR model. The entire process was divided into three parts: data preprocessing, feature extraction, and final classification. As for the data preprocessing, the region of tumor was drawn in the origin CT, as shown in (a). (b) were the cropped images that were resized into 224 224. (c) were the result after histogram equalization. Then (d) showed data augmentation, such as random rotation and horizontal flip. During feature extraction, a ROI was fed into the patch embedding module and then encoded in a feature vector of 768D through 12 encoder blocks. The MVI status was predicted by a linear projection on this 768D feature vector.
2.7.2. MVI-TR: A Transformer-Based Model
Transformer has been widely used in computer vision (VIT) and has achieved great success [32]. Inspired by this, we also introduced a multi-head self-attention (MSA) mechanism to extract features that reflect the relationship among different regions. As illustrated in Figure 2, MVI-TR was composed of three main components, which were stated in detail as follows:
(1) Patch Embedding module. This module divided the ROI image into a patch sequence. Then, each patch was flattened to a 1D vector by convolutional operation, and it was regarded as a token in natural language processing (NLP).
(2) Encoder module. This module consisted of 12 blocks, sharing similar structures but different parameters. We used MSA to capture the relationship between the different parts and global information. The MLP was introduced to lift to a higher dimension space and then project back to the target dimension.
(3) Classifier module. This module predicted MVI status with features extracted from the previous Encoder module.
Since parameters in different attention heads were not shared, the model was encouraged to learn the correlation among different representation subspaces, so as to better obtain the global information. As a result, the ability to predict MVI status was markedly improved.
2.7.3. ResNets Family
We performed a couple of classic ResNet-like deep convolutional neural network (DCNN) architectures, ResNet18, ResNet50, and ResNet101, which are still the state-of-the-art of convolutional networks in the tasks of image classification.
ResNet18 outputs a 512D vector as features by eight convolutional blocks, and we chose a simple fully connected (FC) layer as the classifier to predict MVI status based on the features. Resnet50 and ResNet101 have the same shortcut connections as ResNet18, but with more residual blocks. For classifiers of Resnet50 and ResNet101, we adopted the convolutional and average pooling operators to derive the final probability prediction of MVI status, instead of FC layers. This further reduced the burden of computation during the inference period.
2.8. Regularization Techniques
Considering that our dataset was limited, we performed some effective ways to alleviate the overfitting of DL-based models:
(1) Normalization Operation. During the training period, we introduced some regularization approaches, including batch-normalization, layer-normalization, as well as a dropout for MSA, which are effective techniques to prevent deep neural networks from overfitting.
(2) Label smoothing. This technique permitted DL models to predict training samples too confidently, which was better for the improvement of generalization capability. With the noise distribution represented by , we generated the new ground truth label as
where ε ∈ (0,1) is a weighted factor, is the original one-hot encoded label.
(3) Drop path. This was an extension of dropout strategy. In MVI-TR, this approach adopted randomly dropping the operands of join layers to regularize the co-adaptation of sub-paths. Such regularization can avoid co-adaptation of paralleling paths and allow the extraction of high-performance in sub-networks with the fixed depth [33].
2.9. Implementation
During the training process, 448 patients were randomly selected to compose the training cohort, with 120 MVI positive patients and 328 MVI negative patients. The remaining 111 patients constituted the validation cohort, with 29 MVI positive patients and 82 MVI negative patients. When different deep learning models were trained, the same data augmentation configuration was applied on every epoch. Given that the majority of medical images had simple structure and strong similarity, we applied three main transformations, refer to Ma’s work [34], as follows:
- (1)
- Rotation at a randomly selected angle from −10 degrees to 10 degrees;
- (2)
- Cutting the image with a random size from 0.8 to 1.0 and a random longitudinal ratio from 0.95 to 1.05;
- (3)
- Rolling the image horizontally with a probability of 1/2.
For supervised learning, MVI-TR and baseline ResNet-like models were all optimized through the SGD algorithm, with the same hyper-parameter configuration (a learning rate of 1/1000, a weight decay of 1/100,000, and a momentum of 0.9). We used different small batches (batch size 8, 12, and 16) to train these models and noted that, in most cases, the performance with the mini-batch of 8 was the best. The classical categorical cross-entropy loss guided the optimization of all the models. To obtain the optimal parameters, we trained over 3000 epochs on these supervised learning models. Therefore, models with higher accuracy and AUC in the validation cohort were selected for evaluation in the following parts.
3. Results
3.1. Demographic Characteristics
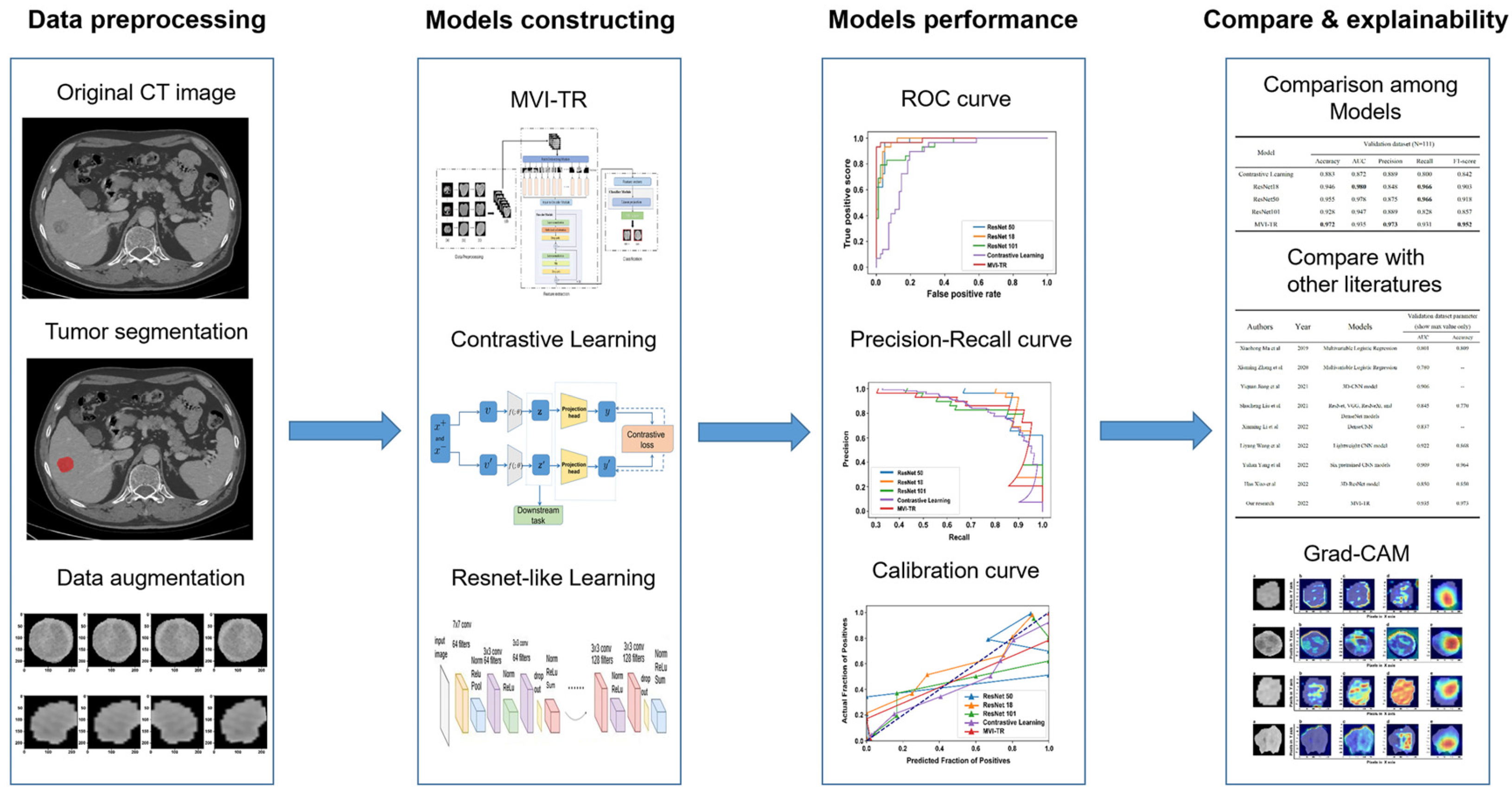
Tumor segmentation, data preprocessing and augmentation, feature extraction, and performance evaluation were all parts of the pipeline (Figure 3). In this study, 559 patients met the inclusion standard and were enrolled. They were randomly divided into the training cohort (448 patients) and the validation cohort (111 patients). The demographic characteristics of patients were compared between the training and validation cohorts (Table 1). For clinical outcomes and baseline variables, there were no significant differences between the training and validation cohorts (all p > 0.05).
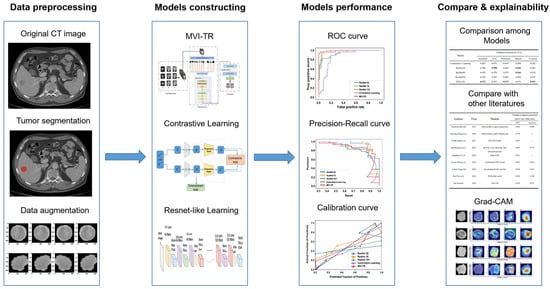
Figure 3.
The workflow of this study. The workflow of our work included tumor segmentation, data augmentation, features extraction, model construction, and performance evaluation.

Table 1.
Demographic characteristics of patients in the training and validation cohorts.
3.2. Performance of MVI Prediction Models
To assess the predictive performance of the models, the following metrics were chosen: accuracy precision, AUC, receiver operating characteristic (ROC), recalling rate, and F1-score. More information is provided in Table 2.

Table 2.
The predictive performance of the proposed models.
Contrastive learning showed great performance in the training cohort, with both accuracy and AUC of nearly 1.0. However, in the validation cohort, it did not show better performance than the other methods, and only yielded an accuracy of 0.883, precision of 0.889, AUC of 0.872, recalling rate of 0.8, and F1-score of 0.842. A possible reason is that AUC is sensitive to the relative relationship between positive and negative classes. It was difficult for contrastive learning to predict whether MVI existed or not, which indicates that the contrastive learning model lacked confidence.
For supervised deep learning models, the ResNet18 model archived the highest score of AUC (0.997) in the training cohort, but its accuracy was lower than ResNet50 and MVI-TR. Additionally, although ResNet50 and ResNet101 attained better performance in the training dataset, they had lower precision in the validation dataset. This suggests that they might be worse in generalization and suffered from overfitting. Moreover, MVI-TR attained the highest accuracy (0.972), precision (0.973), and F1-score (0.952) in the validation cohort, with improved generalizability and robustness by the introduction of MSA and MLP. It outperformed other state-of-the-art methods with the highest accuracy and AUC. Further details are discussed in Section 4.
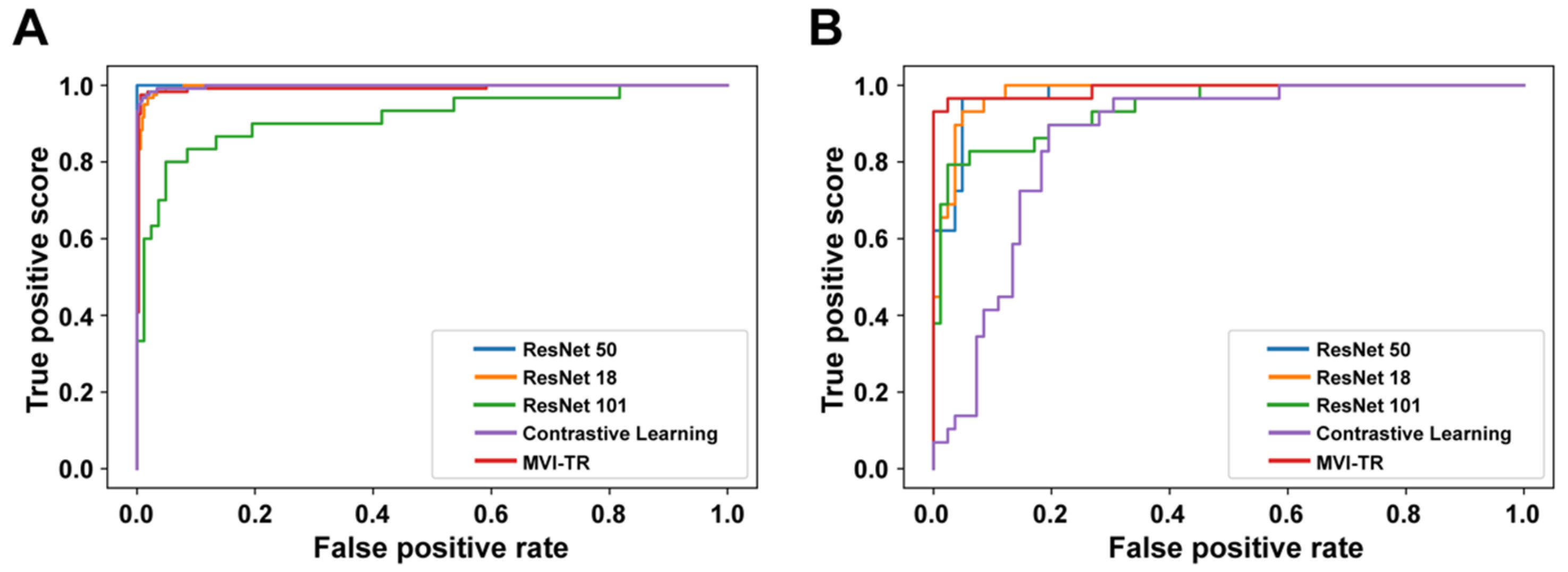
A receiver operating characteristic (ROC) illustrates how well a classification model performs at all classification levels. Figure 4 displays the ROC curves for the contrastive learning model, MVI-TR, and baseline ResNet-like models. The formulas for calculating the true positive rate (TPR) and the false positive rate (FPR) were Equations (1) and (2), respectively,
where TP, FN, FP, and TN denote the numbers of true positive, false negative, false positive, and true negative, respectively. To essentially show the superiority of MVI-TR, we calculated the number of model parameters and floating-point operations (FLOPs) for all models. The results showed MVI-TR with the most parameters had a large model development capability, Table 3.
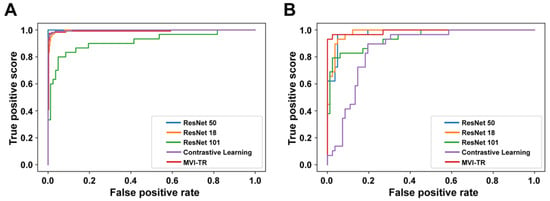
Figure 4.
The receiver operating characteristic (ROC) curves of 5 different models. Receiver operating characteristic (ROC) curves of the contrastive learning model, MVI-TR, and baseline ResNet-like models. (A) ROC curves on the training cohort. (B) ROC curves on the validation cohort.

Table 3.
Parameters’ number and floating-point operations (FLOPs) of different deep learning models.
3.3. The Advantages of MVI-TR
In this section, we provided the illustration to show that MVI-TR had the superior capability of discrimination and calibration.
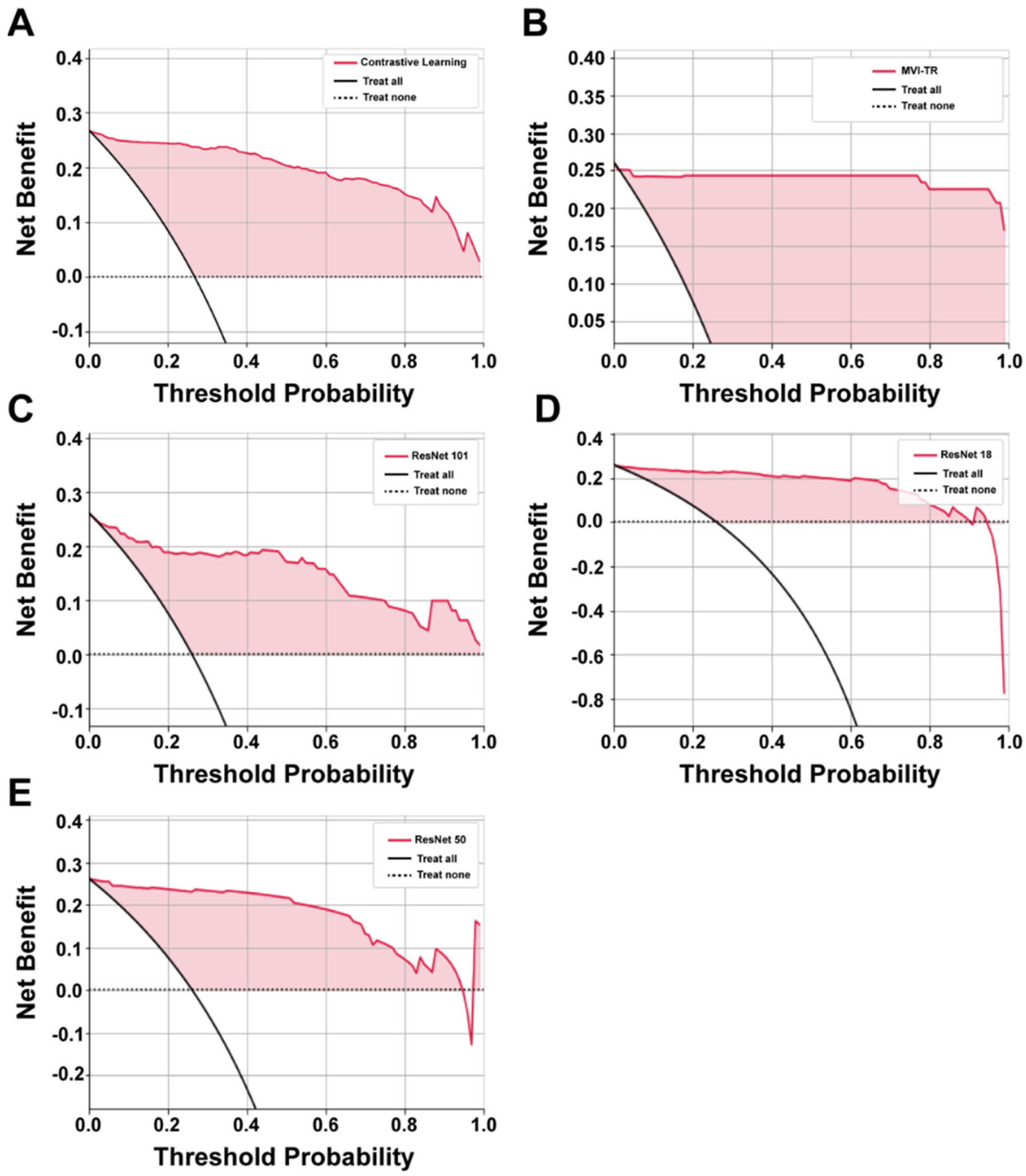
3.3.1. Decision Curve Analysis (DCA)
It is of great value to apply a DCA for evaluating clinical predictive models and diagnostic tests. The reason for this is that the ROC curve can only measure the diagnostic accuracy while failing to take into account the clinical utility as a specific model. The net benefit is calculated with the following equations [35]
where is the threshold probability, and N is the number of samples from the validation cohort. Net benefit is the sum of the net benefit treated and the net benefit untreated [36].
The benefits of all the deep learning models were higher than the extreme curves, which meant they performed well in predicting MVI status, as shown in Figure 5. In particular, in a large range, the benefits of MVI-TR are much higher than the others, so its optional ranges are relatively large and safe.
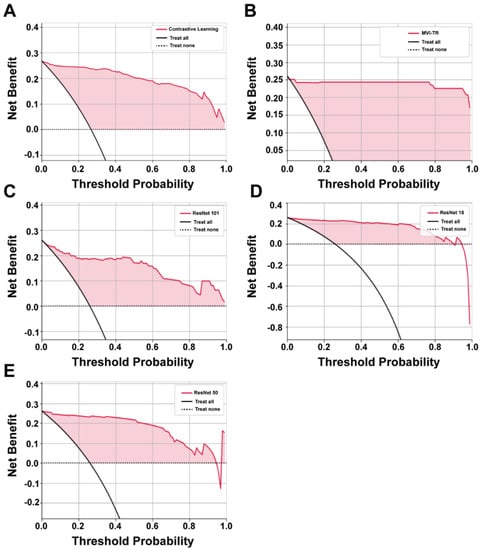
Figure 5.
The decision curve analysis (DCA) curves of 5 different models. (A–E) Show the DCA curves of contrastive learning, MVI-TR, ResNet101, ResNet18, ResNet50, respectively.
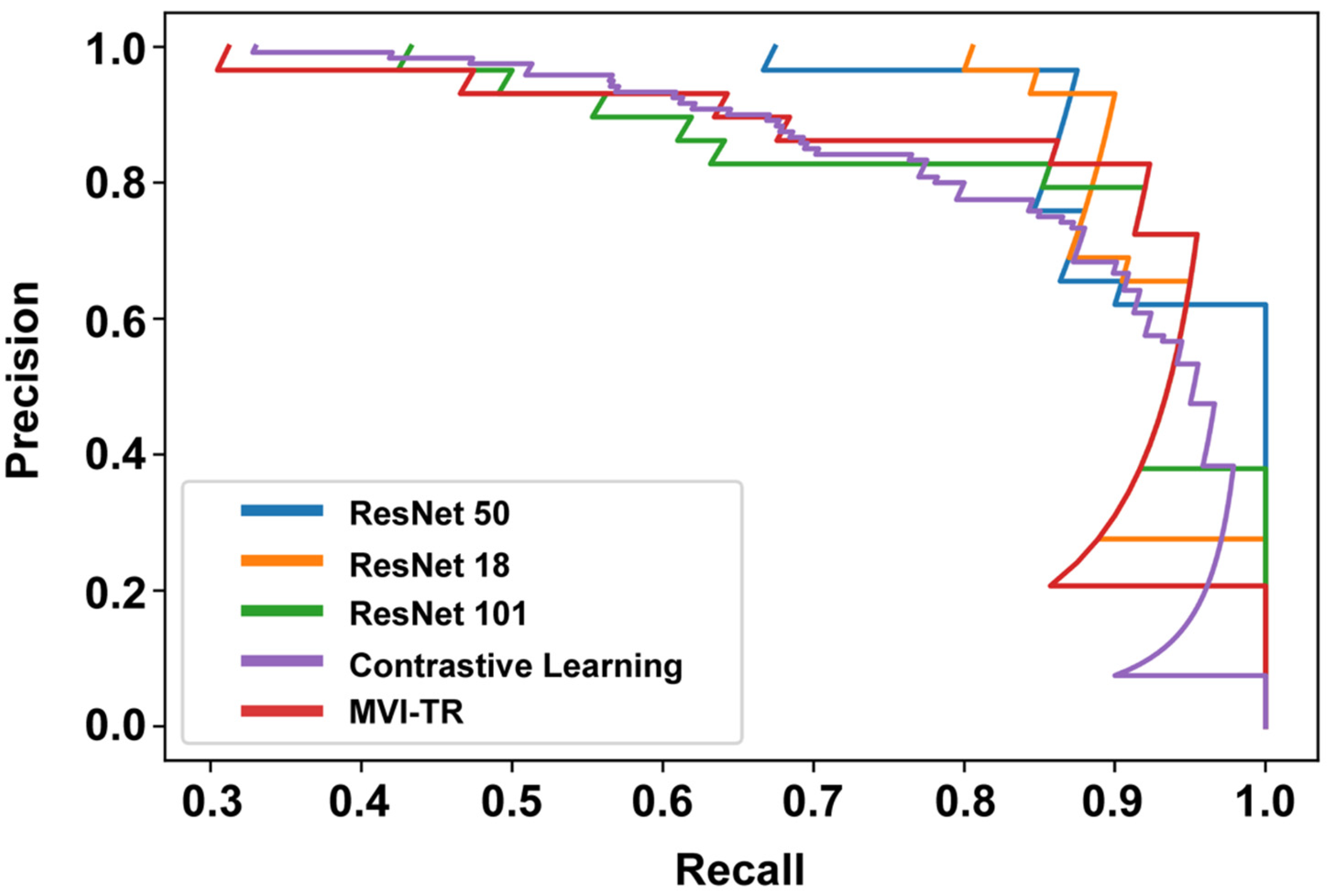
3.3.2. Precision-Recall (PR) Curve
The PR curve is particularly useful in reporting Information Retrieval (IR) results. IR involves searching a pool of documents to find things that are relevant to a particular user query. Different from the ROC curve, the precision-recall curve can help highlight how relevant the retrieved results are, which is more significant for accurately judging whether a patient has MVI or not. The effective algorithm should have both high precision and high recall. However, most DL-based algorithms often involve a trade-off between the two. Therefore, a favorable PR curve has a greater AUC. In Figure 6, the classification results of MVI-TR, corresponding to the red line, had a better performance.
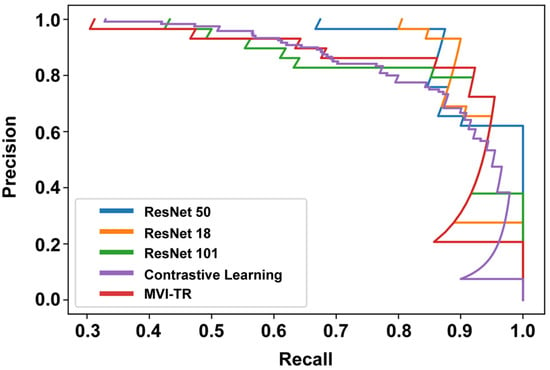
Figure 6.
The precision-recall (PR) curves of 5 different models on the validation cohort.
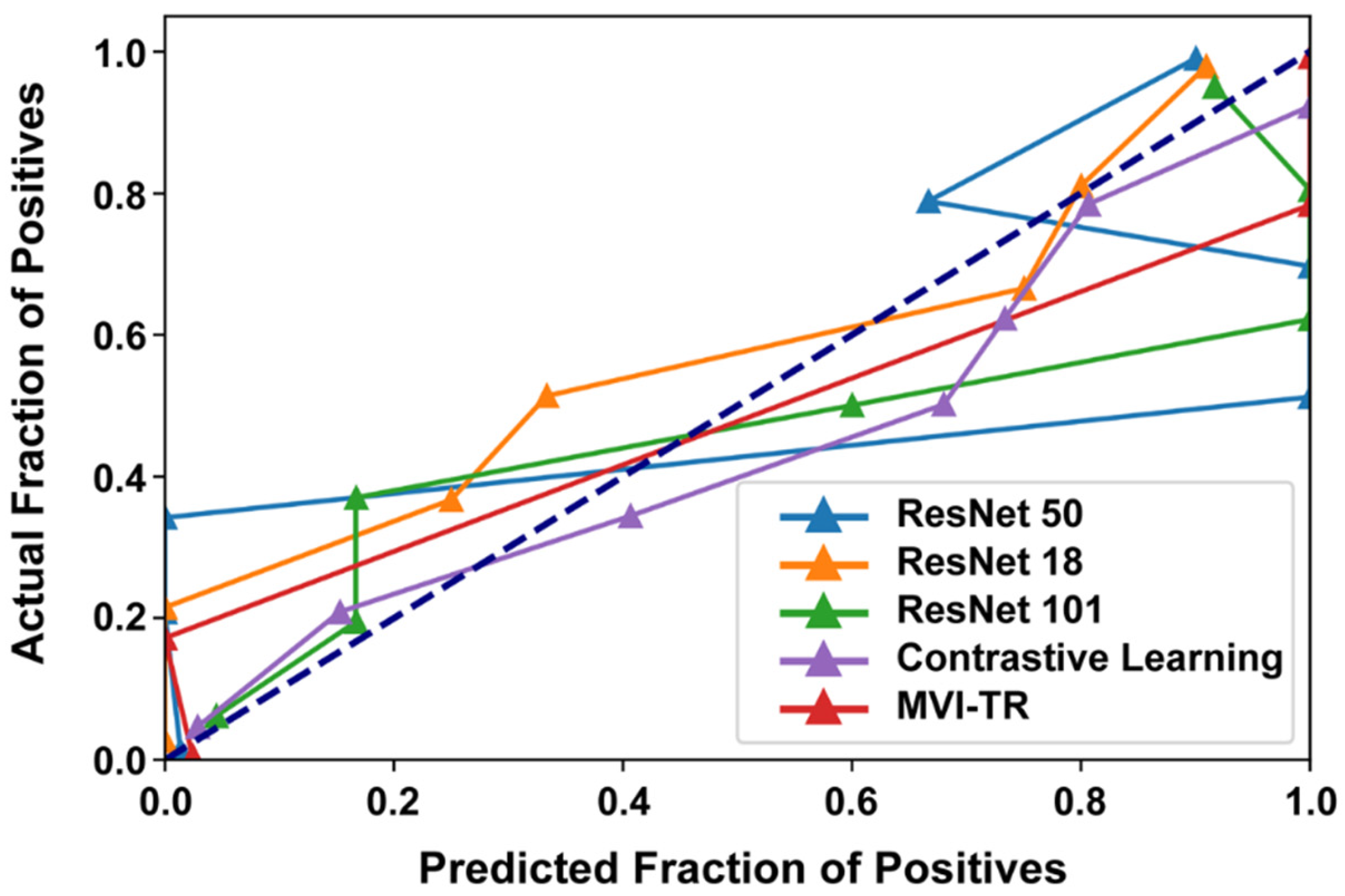
3.3.3. Calibration Curve
Generally, classifiers having a linear probability of predicting the label of each class are called calibration. Therefore, a calibration curve is often used to evaluate the calibration degree of a classifier. It helps to understand the “sure” probabilities of a classification model in predicting MVI status. Additionally, it interprets how decisive a classification model is. When the calibration curve approaches the diagonal, the model achieves better performance. From Figure 7, we noted that ResNet50 and ResNet101 were not very well calibrated, tending to overfit. While MVI-TR, the contrastive learning model, and ResNet18 were closer to the perfect calibrated model’s curve (dotted curve).
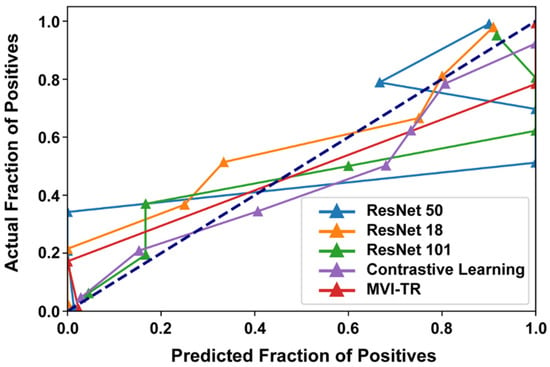
Figure 7.
The calibration curves of 5 different models on the validation cohort.
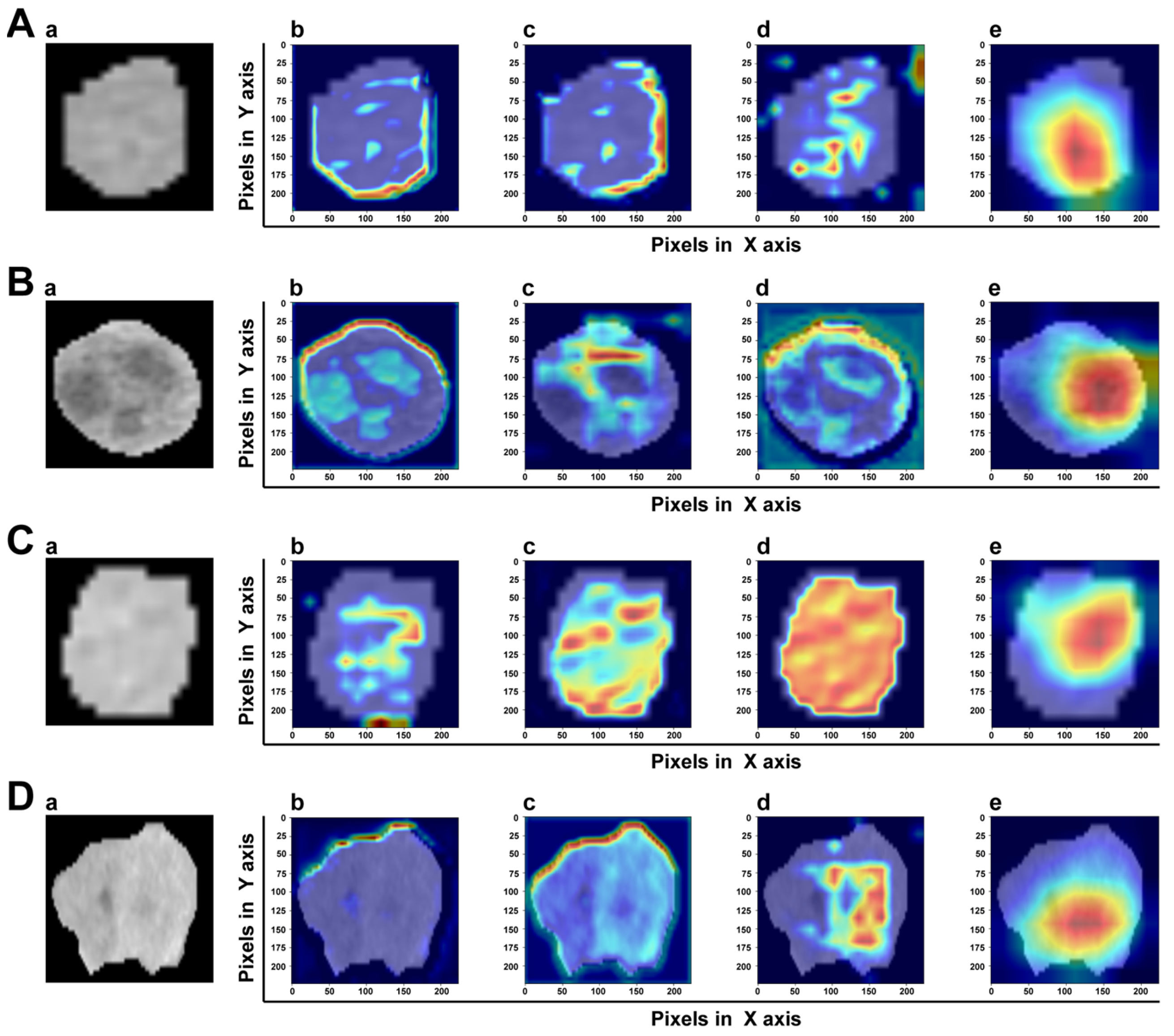
3.4. Model Explainability
A good DL model should provide high reliability and obtain the trust of physicians and patients. The gradient-weighted class activation map (Grad-CAM) can determine which essential features of an input ROI have more influence on the final prediction. In the transformer-based model, the intensities of the heatmap were set as the gradients computed by the backpropagation algorithm. Figure 8 shows the heatmap of two samples with or without MVI. It was clear that MVI-TR focused on some subdomains in the tumor areas that were highly correlated with MVI. In a sense, this provided reasonable explanations for understanding how MVI-TR performed better. It also showed that MVI-TR was unbiased, i.e., MVI-TR predicted MVI status from the information provided by the tumor, not others.
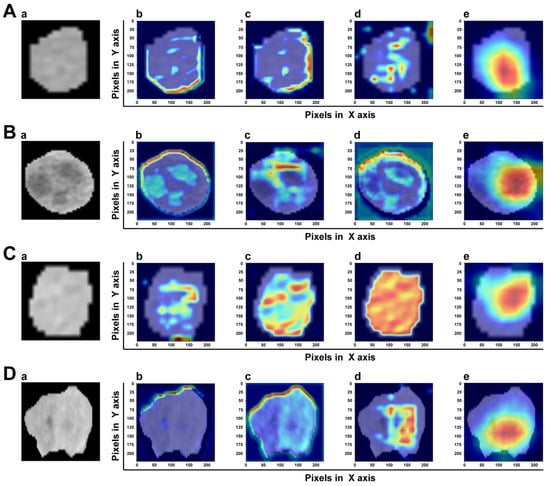
Figure 8.
The visualization of attention which MVI-TR paid in the input ROIs by Grad-CAM. The top two rows (A,B) are the ROIs of two samples with MVI, while the bottom two rows (C,D) are without MVI. In each row, (a) is the original image. (b–d) Support for the class with MVI according to various visualizations for features of different layers in MVI-TR. (e) Is the final feature map to predict the MVI status.
4. Discussion
Early-stage HCC still has a high possibility of recurrence after timely surgical resection or LT (about 50–70%) [37,38,39]. MVI and other biomarkers are reliable predictors of both overall and progression-free survival in HCC [40]. As mentioned above, the precise evaluation of MVI is very important to improve the individualized treatment strategy, reduce the recurrence for high-risk patients, and intervene in time because of the high recurrence rate after resection or LT. Partial HCC patients with MVI have benefited from TACE treatment before resection or LT [41]. Our predictive model was designed to accurately screen for MVI in HCC. As a routine preoperative method, CECT can be used as a radiological feature for MVI status prediction and MVI-related parameter generation. Our central data confirms the accuracy of the prediction models. Therefore, for patients with positive predictive outcomes, preoperative interventional therapy might be necessary to enhance clinical prognosis.
A prediction model, MVI-TR, was proposed in this exploration for predicting the absence of MVI in early-stage HCC patients (tumors size ≤ 5 cm), which showed good discrimination in both training and validation cohorts. MVI-TR of CECT data may be applied to individualized treatment guidance for HCC patients in the future.
Previous work mainly used traditional machine learning algorithms. MVI was predicted by the extraction of radiomics and the combination of clinical data. Some work adopted the LASSO algorithm to select radiomics features [34] and performed multivariable logistic regression to predict MVI status. The introduction of DL radiomics made it possible to identify MVI noninvasively and efficiently [13,25,42]. A recent study [24] combined segmentation with prediction and proposed a light-weight transformer for the segmentation and CNN for the prediction. Yang et al. [25] applied six pretrained CNN algorithms (pretrained using the ImageNet database) for the DL feature extraction on the sequences of NC, AP, and VP, which contained more valuable information than a single image. A 3D-ResNet network was also applied in MVI prediction, which improved the AUC to 0.85 [43]. Table 4 lists the performance of MVI-TR compared with previous DL work.

Table 4.
The performance of MVI-TR compared with previous deep learning work.
We introduced MSA to capture the relationship between different parts of the tumor region, which attained the best precision compared with other baseline Resnet-like models. The previous parameters were not shared while the results were finally stitched together. This allowed the model to learn relevant information in different presentation subspaces. In short, each attention head focused only on a single subspace, independent of the others. The core idea was to extract richer feature information. MSA expanded the model’s ability to focus on different locations of tumor images. Finally, by Grad-CAM, it was confirmed that MSA paid more attention to tumor regions, instead of others.
Traditional radiological features gave a better explanation for the prediction in practice and were easy to understand; however, the choosing of the features relied on the experience of radiologists. This might bring subjectivity into predictions, while MVI-TR extracted features automatically. As a consequence, the use of MVI-TR reduced bias caused by individual selection and variable factors in experience.
Our model had better robustness, which could deal with the problem of class imbalance well. We trained the model on imbalanced training data, in which there were 410 samples with MVI negative status and 149 samples with MVI positive status.
There were some limitations of this research. First, the DL-based models involved were only verified using data from a single center. The performance measurement of this model in multi-center, large cohorts, and different model scanning conditions would be of significant value in future research. Second, we chose 2D maximum tumor area for our radiomics study, which may lose information on 3D segmented tumors. Third, this study did not conduct a postoperative patient follow-up.
5. Conclusions
The MVI-TR derived from CECT yielded better performance for the prediction of MVI in HCC patients, compared with other DL-based models. Thus, it can help to professionally choose the optimal treatment strategies and patient management.
Author Contributions
Conception, L.C., J.W. and K.L.; data collection, W.Z., X.Z., Y.M., K.D. and D.W.; data analysis, Q.W., Y.H., T.P. and Y.C.; funding acquisition, L.C.; manuscript preparation, J.H., L.C. and Q.W.; final approval and agreement to be accountable for all aspects of the work, L.C., Q.W., J.H., Y.H., W.Z., X.Z., Y.C., Y.M., K.D., D.W., T.P., J.W. and K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Zhejiang Province (NO. LQ21H160025).
Institutional Review Board Statement
This study was approved by the ethics committee of The First Affiliated Hospital, Zhejiang University School of Medicine (2022–1131).
Informed Consent Statement
As our research is a retrospective study and all privacy information have been hidden during our research, the ethical board of our hospital approved the waiver of the consent form. So we choose “Patient consent was waived due to retrospective study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AFP | Alpha-fetoprotein |
| AP | Arterial phase |
| AUC | Area under the receiver operating characteristic curve |
| CA19-9 | Carbohydrate antigen 19-9 |
| CAD | Computer-aided diagnosis |
| CEA | Carcinoembryonic antigen |
| CECT | Contrast-enhanced Computed Tomography |
| CNN | Convolutional Neural Network |
| DCNN | Deep convolutional neural network |
| DL | Deep learning |
| FC | Fully connected |
| FNN | Feed-forward neural network |
| Grad-CAM | Gradient-weighted class activation mapping |
| HBsAg | Hepatitis B surface antigen |
| HCC | Hepatocellular cancer |
| LT | Liver transplantation |
| MLP | Multi-layer perceptron |
| MSA | Multi-head self-attention |
| MVI | Microvascular invasion |
| MVI-TR | Transformer-based end-to-end DL model |
| NC | Non-contrast |
| NLP | Natural language processing |
| PIVKA II | Protein induced by vitamin K absence/antagonist-II |
| ResNets | Residual networks |
| RFA | Radiofrequency ablation |
| ROI | Region of Interest |
| TACE | Transcatheter arterial chemoembolization |
| VP | Venous phase |
| WL | Window Level |
| WW | Window Width |
References
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Sutter, O.; Nahon, P.; Ganne-Carrié, N.; Séror, O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J. Hepatol. 2018, 68, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Bruix, J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 203–217. [Google Scholar] [CrossRef]
- Lim, K.C.; Chow, P.K.; Allen, J.C.; Siddiqui, F.J.; Chan, E.S.; Tan, S.B. Systematic review of outcomes of liver resection for early hepatocellular carcinoma within the Milan criteria. Br. J. Surg. 2012, 99, 1622–1629. [Google Scholar] [CrossRef]
- Hong, S.B.; Choi, S.H.; Kim, S.Y.; Shim, J.H.; Lee, S.S.; Byun, J.H.; Park, S.H.; Kim, K.W.; Kim, S.; Lee, N.K. MRI Features for Predicting Microvascular Invasion of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2021, 10, 94–106. [Google Scholar] [CrossRef]
- Lee, S.; Kang, T.W.; Song, K.D.; Lee, M.W.; Rhim, H.; Lim, H.K.; Kim, S.Y.; Sinn, D.H.; Kim, J.M.; Kim, K.; et al. Effect of Microvascular Invasion Risk on Early Recurrence of Hepatocellular Carcinoma After Surgery and Radiofrequency Ablation. Ann. Surg 2021, 273, 564–571. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, L.; Ren, Y.; Li, Y.; Yuan, F.; Cao, M.; Zhang, Y.; Deng, M.; Yao, Z. Identification and Validation of a Prognostic Model Based on Three MVI-Related Genes in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2022, 18, 261–275. [Google Scholar] [CrossRef]
- Wang, H.; Feng, L.H.; Qian, Y.W.; Cao, Z.Y.; Wu, M.C.; Cong, W.M. Does microvascular invasion in Barcelona Clinic Liver Cancer stage A multinodular hepatocellular carcinoma indicate early-stage behavior? Ann. Transl. Med. 2019, 7, 428. [Google Scholar] [CrossRef]
- Degroote, H.; Piñero, F.; Costentin, C.; Notarpaolo, A.; Boin, I.F.; Boudjema, K.; Baccaro, C.; Chagas, A.L.; Bachellier, P.; Ettorre, G.M.; et al. International study on the outcome of locoregional therapy for liver transplant in hepatocellular carcinoma beyond Milan criteria. JHEP Rep. 2021, 3, 100331. [Google Scholar] [CrossRef]
- Lei, Z.; Li, J.; Wu, D.; Xia, Y.; Wang, Q.; Si, A.; Wang, K.; Wan, X.; Lau, W.Y.; Wu, M.; et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg. 2016, 151, 356–363. [Google Scholar] [CrossRef]
- Kim, H.L.; An, J.; Park, J.A.; Park, S.H.; Lim, Y.S.; Lee, E.K. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High-Risk Patients With Cirrhosis. Hepatology 2019, 69, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.L.; Liu, Q.P.; Sun, S.W.; Zhang, J.; Zhu, F.P.; Yang, G.; Yan, X.; Zhang, Y.D.; Liu, X.S. Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J. Hepatol. 2019, 70, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Peng, H.; Dong, D.; Fang, M.J.; Li, L.; Tang, L.L.; Chen, L.; Li, W.F.; Mao, Y.P.; Fan, W.; Liu, L.Z.; et al. Prognostic Value of Deep Learning PET/CT-Based Radiomics: Potential Role for Future Individual Induction Chemotherapy in Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2019, 25, 4271–4279. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Coroller, T.P.; Grossmann, P.; Zeleznik, R.; Kumar, A.; Bussink, J.; Gillies, R.J.; Mak, R.H.; Aerts, H. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med. 2018, 15, e1002711. [Google Scholar] [CrossRef]
- Chen, W.; Hou, X.; Hu, Y.; Huang, G.; Ye, X.; Nie, S. A deep learning- and CT image-based prognostic model for the prediction of survival in non-small cell lung cancer. Med. Phys. 2021, 48, 7946–7958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Fang, M.; Dong, D.; Wang, X.; Ke, X.; Zhang, L.; Hu, C.; Guo, L.; Guan, X.; Zhou, J.; et al. Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiother. Oncol. 2020, 145, 13–20. [Google Scholar] [CrossRef]
- Kim, H.; Goo, J.M.; Lee, K.H.; Kim, Y.T.; Park, C.M. Preoperative CT-based Deep Learning Model for Predicting Disease-Free Survival in Patients with Lung Adenocarcinomas. Radiology 2020, 296, 216–224. [Google Scholar] [CrossRef]
- Wang, K.; Xiang, Y.; Yan, J.; Zhu, Y.; Chen, H.; Yu, H.; Cheng, Y.; Li, X.; Dong, W.; Ji, Y.; et al. A deep learning model with incorporation of microvascular invasion area as a factor in predicting prognosis of hepatocellular carcinoma after R0 hepatectomy. Hepatol. Int. 2022, 16, 1188–1198. [Google Scholar] [CrossRef]
- Sun, L.; Sun, Z.; Wang, C.; Cheng, S.; Wang, K.; Huang, M. PCformer: An MVI recognition method via classification of the MVI boundary according to histopathological images of liver cancer. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2022, 39, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, Q.; Chen, Y.; Zhu, Y.; Zhang, Y.; Cao, D.; Zhou, W.; Liang, X.; Yang, Y.; Lin, L.; et al. A novel multimodal deep learning model for preoperative prediction of microvascular invasion and outcome in hepatocellular carcinoma. Eur J. Surg Oncol. 2023, 49, 156–164. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, X.; Yu, G.; Hou, J.; Xu, H.; Ren, A.; Wang, Z.; Yang, D.; Yang, Z. Can a proposed double branch multimodality-contribution-aware TripNet improve the prediction performance of the microvascular invasion of hepatocellular carcinoma based on small samples? Front. Oncol. 2022, 12, 1035775. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, M.; Li, R.; Xu, X.; Zhu, C.; Feng, X. MVI-Mind: A Novel Deep-Learning Strategy Using Computed Tomography (CT)-Based Radiomics for End-to-End High Efficiency Prediction of Microvascular Invasion in Hepatocellular Carcinoma. Cancers 2022, 14, 2956. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Zhou, C.; Ma, X. Deep learning radiomics based on contrast enhanced computed tomography predicts microvascular invasion and survival outcome in early stage hepatocellular carcinoma. Eur. J. Surg. Oncol. 2022, 48, 1068–1077. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, H.C.; Wang, Z.; Cong, W.M.; Wang, J.H.; Zeng, M.S.; Yang, J.M.; Bie, P.; Liu, L.X.; Wen, T.F.; et al. Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 2018, 7, 235–260. [Google Scholar] [CrossRef]
- Gao, R.; Zhao, S.; Aishanjiang, K.; Cai, H.; Wei, T.; Zhang, Y.; Liu, Z.; Zhou, J.; Han, B.; Wang, J.; et al. Deep learning for differential diagnosis of malignant hepatic tumors based on multi-phase contrast-enhanced CT and clinical data. J. Hematol. Oncol. 2021, 14, 154. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Chen, T.; Kornblith, S.; Norouzi, M.; Hinton, G. A Simple Framework for Contrastive Learning of Visual Representations. In Proceedings of the International Conference on Machine Learning (ICML), Electronic Network, 13–18 July 2020. [Google Scholar]
- Dangovski, R.; Jing, L.; Loh, C.; Han, S.; Srivastava, A.; Cheung, B.; Agrawal, P.; Soljačić, M. Equivariant Contrastive Learning. arXiv 2021, arXiv:2111.00899. [Google Scholar]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F.F. ImageNet: A Large-Scale Hierarchical Image Database. In Proceedings of the IEEE-Computer-Society Conference on Computer Vision and Pattern Recognition Workshops, Miami Beach, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar]
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An Image is Worth 16 × 16 Words: Transformers for Image Recognition at Scale. arXiv 2020, arXiv:2010.11929. [Google Scholar]
- Larsson, G.; Maire, M.; Shakhnarovich, G. FractalNet: Ultra-Deep Neural Networks without Residuals. arXiv 2016, arXiv:1605.07648. [Google Scholar]
- Ma, X.; Wei, J.; Gu, D.; Zhu, Y.; Feng, B.; Liang, M.; Wang, S.; Zhao, X.; Tian, J. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur. Radiol. 2019, 29, 3595–3605. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Zhang, Z.; Rousson, V.; Lee, W.C.; Ferdynus, C.; Chen, M.; Qian, X.; Guo, Y. Decision curve analysis: A technical note. Ann. Transl Med. 2018, 6, 308. [Google Scholar] [CrossRef]
- Lim, C.; Bhangui, P.; Salloum, C.; Gómez-Gavara, C.; Lahat, E.; Luciani, A.; Compagnon, P.; Calderaro, J.; Feray, C.; Azoulay, D. Impact of time to surgery in the outcome of patients with liver resection for BCLC 0-A stage hepatocellular carcinoma. J. Hepatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, G.; Bekki, Y.; Lourdusamy, V.; Schwartz, M. Surgical Treatments of Hepatobiliary Cancers. Hepatology 2021, 73 (Suppl. S1), 128–136. [Google Scholar] [CrossRef]
- Wei, X.; Su, R.; Yang, M.; Pan, B.; Lu, J.; Lin, H.; Shu, W.; Wang, R.; Xu, X. Quantitative proteomic profiling of hepatocellular carcinoma at different serum alpha-fetoprotein level. Transl. Oncol. 2022, 20, 101422. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, S.; Xiao, H.; Wang, Y.; Li, J.; Mei, J.; Chen, Z.; Zhou, Q.; Feng, S.; Chen, M.; et al. Microvascular Invasion as a Predictor of Response to Treatment with Sorafenib and Transarterial Chemoembolization for Recurrent Intermediate-Stage Hepatocellular Carcinoma. Radiology 2019, 292, 237–247. [Google Scholar] [CrossRef]
- Wang, L.; Ke, Q.; Lin, K.; Chen, J.; Wang, R.; Xiao, C.; Liu, X.; Liu, J. Not All Hepatocellular Carcinoma Patients with Microvascular Invasion After R0 Resection Could Be Benefited from Prophylactic Transarterial Chemoembolization: A Propensity Score Matching Study. Cancer Manag. Res. 2020, 12, 3815–3825. [Google Scholar] [CrossRef]
- Liu, S.C.; Lai, J.; Huang, J.Y.; Cho, C.F.; Lee, P.H.; Lu, M.H.; Yeh, C.C.; Yu, J.; Lin, W.C. Predicting microvascular invasion in hepatocellular carcinoma: A deep learning model validated across hospitals. Cancer Imaging 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Guo, Y.; Zhou, Q.; Chen, Q.; Du, Q.; Chen, S.; Fu, S.; Lin, J.; Li, D.; Song, X.; et al. Prediction of microvascular invasion in hepatocellular carcinoma with expert-inspiration and skeleton sharing deep learning. Liver Int. 2022, 42, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ruan, S.; Xiao, W.; Shao, J.; Tian, W.; Liu, W.; Zhang, Z.; Wan, D.; Huang, J.; Huang, Q.; et al. Contrast-enhanced CT radiomics for preoperative evaluation of microvascular invasion in hepatocellular carcinoma: A two-center study. Clin. Transl. Med. 2020, 10, e111. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Cao, S.E.; Cao, S.; Chen, J.N.; Wang, G.Y.; Shi, W.Q.; Deng, Y.N.; Cheng, N.; Ma, K.; Zeng, K.N.; et al. Preoperative identification of microvascular invasion in hepatocellular carcinoma by XGBoost and deep learning. J. Cancer Res. Clin. Oncol. 2021, 147, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, Z.; Du, H.; Geng, Z.; Li, Z.; Qin, S.; Zhang, X.; Liang, J.; Zhang, X.; Liang, W.; et al. Deep convolutional neural network for preoperative prediction of microvascular invasion and clinical outcomes in patients with HCCs. Eur. Radiol. 2022, 32, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, S.W.; Liu, Q.P.; Xu, X.; Zhang, Y.; Zhang, Y.D. TED: Two-stage expert-guided interpretable diagnosis framework for microvascular invasion in hepatocellular carcinoma. Med. Image Anal. 2022, 82, 102575. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).