Internal Guidelines for Reducing Lymph Node Contour Variability in Total Marrow and Lymph Node Irradiation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. TMLI Procedure
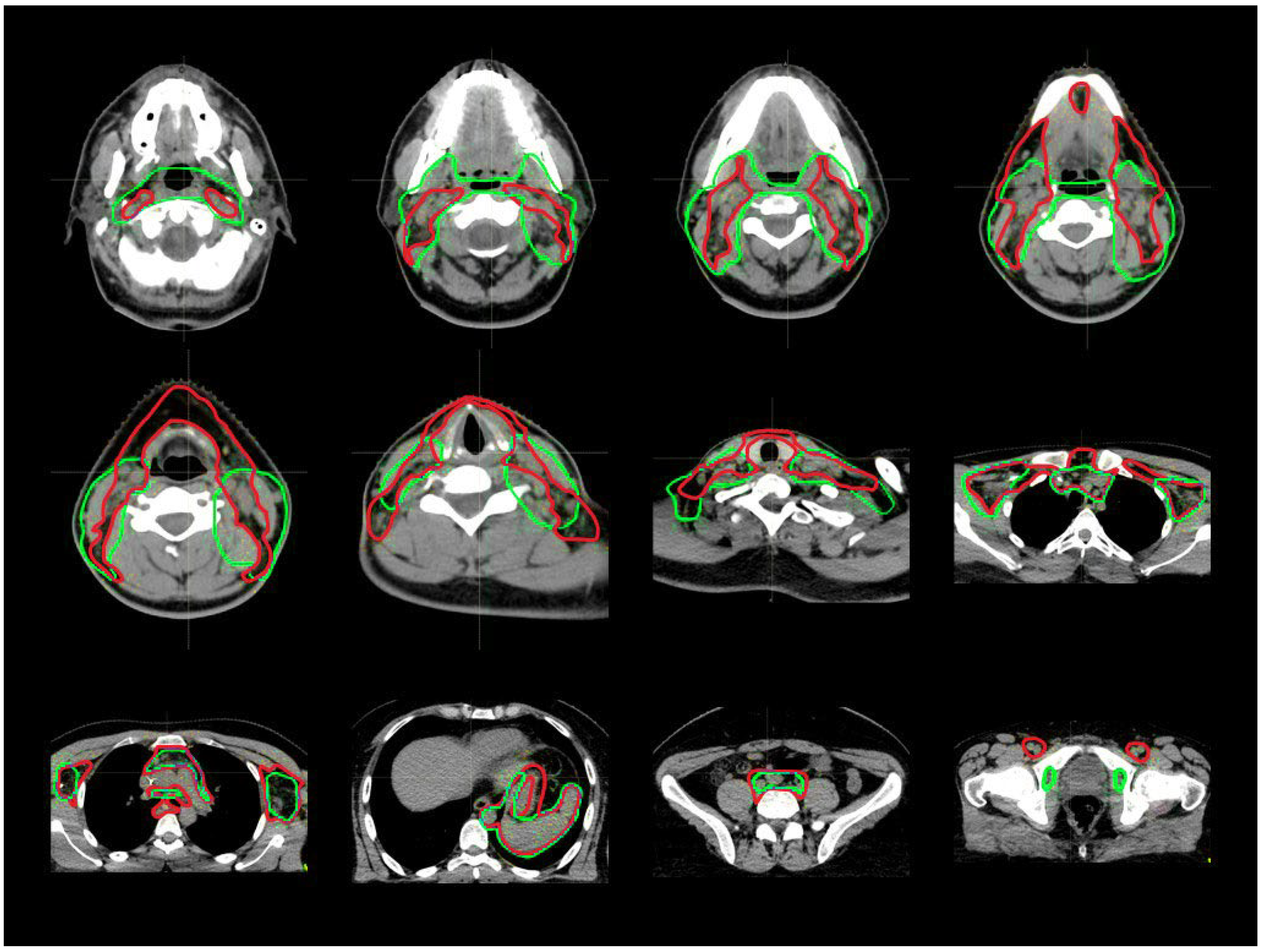
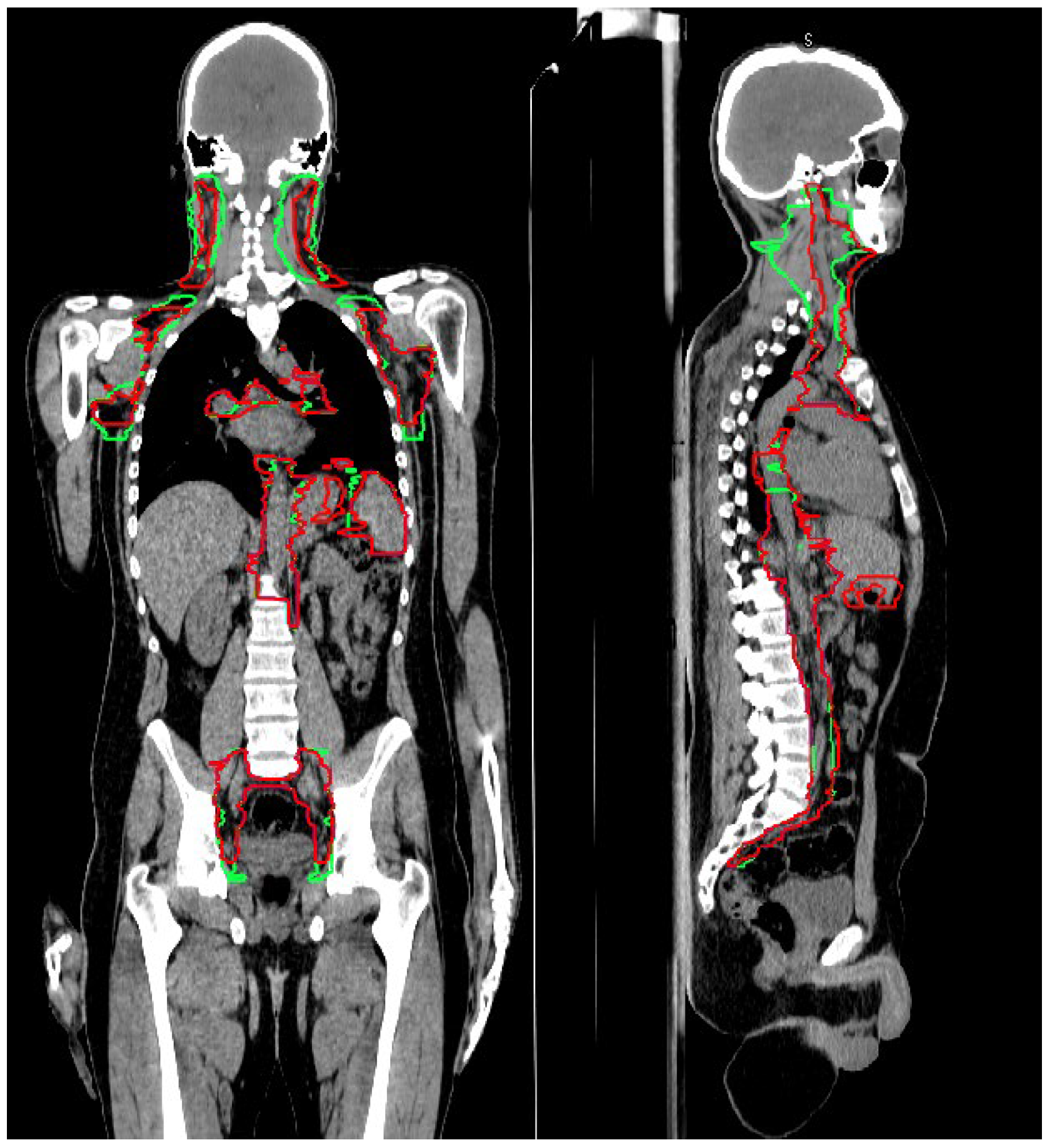
2.2. Target Definition Guidelines
2.3. Guideline Evaluation
2.4. Data Analysis
2.4.1. Topological Evaluation
2.4.2. Dosimetric Evaluation
2.4.3. Statistical Tests
3. Results
3.1. CTV_LN Inter-/Intraobserver Contouring Variability
3.2. Target Coverage and Dose Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, E.D.; Lochte, H.L.; Ferrebee, J.W. Irradiation of the entire body and marrow transplantation: Some observations and comments. Blood 1959, 14, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cosset, J.M.; Socie, G.; Dubray, B.; Girinsky, T.; Fourquet, A.; Gluckman, E. Single dose versus fractionated total body irradiation before bone marrow transplantation: Radiobiological and clinical considerations. Int. J. Radiat. Oncol. Biol. Phys. 1994, 30, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E. Late effects in children receiving total body irradiation for bone marrow transplantation. Radiother. Oncol. 1990, 18 (Suppl. 1), 82–87. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A.; Baldomero, H. Trends of hematopoietic stem cell transplantation in the third millennium. Curr. Opin. Hematol. 2009, 16, 420–426. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L. Total body irradiation: Guidelines from the international lymphoma radiation oncology group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 521–529. [Google Scholar] [CrossRef]
- Peters, C.; Dalle, J.-H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total body irradiation or chemotherapy conditioning in childhood ALL: A multinational, randomized, noninferiority phase III study. JCO 2021, 39, 295–307. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. BJR 2011, 84, 967–996. [Google Scholar] [CrossRef]
- Intensity Modulated Radiation Therapy Collaborative Working Group. Intensity-modulated radiotherapy: Current status and issues of interest. Inte. J. Radiat. Oncol. Biol. Phys. 2001, 51, 880–914. [Google Scholar] [CrossRef]
- Patel, P.; Aydogan, B.; Koshy, M.; Mahmud, D.; Oh, A.; Saraf, S.L.; Quigley, J.G.; Khan, I.; Sweiss, K.; Mahmud, N.; et al. Combination of linear accelerator–based intensity-modulated total marrow irradiation and myeloablative fludarabine/busulfan: A phase I study. Biol. Blood Marrow Transplant. 2014, 20, 2034–2041. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Forman, S.; Somlo, G.; Rosenthal, J.; Liu, A.; Schultheiss, T.; Radany, E.; Palmer, J.; Stein, A. Dose escalation of total marrow irradiation with concurrent chemotherapy in patients with advanced acute leukemia undergoing allogeneic hematopoietic cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 148–156. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Rosenthal, J.; Liu, A.; Schultheiss, T.; Forman, S.; Somlo, G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 273–279. [Google Scholar] [CrossRef]
- Patel, P.R.; Rondelli, D. Total marrow and lymphoid irradiation to rescue refractory leukemia. Biol. Blood Marrow Transplant. 2017, 23, 536–537. [Google Scholar] [CrossRef]
- Haraldsson, A.; Wichert, S.; Engström, P.E.; Lenhoff, S.; Turkiewicz, D.; Warsi, S.; Engelholm, S.; Bäck, S.; Engellau, J. Organ sparing total marrow irradiation compared to total body irradiation prior to allogeneic stem cell transplantation. Eur. J. Haematol. 2021, 107, 393–407. [Google Scholar] [CrossRef]
- Warrell, G.R.; Colussi, V.C.; Swanson, W.L.; Caimi, P.F.; Mansur, D.B.; Lima, M.J.G.; Pereira, G.C. Organ sparing of linac-based targeted marrow irradiation over total body irradiation. J. Appl. Clin. Med. Phys. 2019, 20, 69–79. [Google Scholar] [CrossRef]
- Haraldsson, A.; Engellau, J.; Lenhoff, S.; Engelholm, S.; Bäck, S.; Engström, P.E. Implementing safe and robust total marrow irradiation using helical tomotherapy—A practical guide. Phys. Med. 2019, 60, 162–167. [Google Scholar] [CrossRef]
- Schultheiss, T.E.; Wong, J.; Liu, A.; Olivera, G.; Somlo, G. Image-guided total marrow and total lymphatic irradiation using helical tomotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1259–1267. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Filippi, A.R.; Scorsetti, M.; Hui, S.; Muren, L.P.; Mancosu, P. Total marrow and total lymphoid irradiation in bone marrow transplantation for acute leukaemia. Lancet Oncol. 2020, 21, e477–e487. [Google Scholar] [CrossRef]
- Ogawa, H.; Konishi, T.; Najima, Y.; Kito, S.; Hashimoto, S.; Kato, C.; Sakai, S.; Kanbara, Y.; Atsuta, Y.; Konuma, R.; et al. Phase I trial of myeloablative conditioning with 3-day total marrow and lymphoid irradiation for leukemia. Cancer Sci. 2023, 114, 596–605. [Google Scholar] [CrossRef]
- Stein, A.; Palmer, J.; Tsai, N.-C.; Al Malki, M.M.; Aldoss, I.; Ali, H.; Aribi, A.; Farol, L.; Karanes, C.; Khaled, S.; et al. Phase I trial of total marrow and lymphoid irradiation transplantation conditioning in patients with relapsed/refractory acute leukemia. Biol. Blood Marrow Transplant. 2017, 23, 618–624. [Google Scholar] [CrossRef]
- Sarina, B.; Mancosu, P.; Navarria, P.; Bramanti, S.; Mariotti, J.; De Philippis, C.; Clerici, E.; Franzese, C.; Mannina, D.; Valli, V.; et al. Nonmyeloablative conditioning regimen including low-dose total marrow/lymphoid irradiation before haploidentical transplantation with post-transplantation cyclophosphamide in patients with advanced lymphoproliferative diseases. Transplant. Cell. Ther. 2021, 27, 492.e1–492.e6. [Google Scholar] [CrossRef]
- Mancosu, P.; Navarria, P.; Muren, L.P.; Castagna, L.; Reggiori, G.; Clerici, E.; Sarina, B.; Bramanti, S.; De Philippis, C.; Tomatis, S.; et al. Development of an immobilization device for total marrow irradiation. Pract. Radiat. Oncol. 2021, 11, e98–e105. [Google Scholar] [CrossRef] [PubMed]
- Mancosu, P.; Navarria, P.; Castagna, L.; Reggiori, G.; Sarina, B.; Tomatis, S.; Alongi, F.; Nicolini, G.; Fogliata, A.; Cozzi, L.; et al. Interplay effects between dose distribution quality and positioning accuracy in total marrow irradiation with volumetric modulated arc therapy: TMI by VMAT: Positioning uncertainties. Med. Phys. 2013, 40, 111713. [Google Scholar] [CrossRef] [PubMed]
- Lambri, N.; Dei, D.; Hernandez, V. Evaluation of plan complexity and dosimetric plan quality of total marrow and lumphoid irradiation using volumetric modulated arc therapy. J. Appl. Clin. Med. Phys. 2023. [Google Scholar] [CrossRef]
- Lambri, N.; Dei, D.; Hernandez, V.; Castiglioni, I.; Clerici, E.; Crespi, L.; De Philippis, C.; Loiacono, D.; Navarria, P.; Reggiori, G.; et al. Automatic planning of the lower extremities for total marrow irradiation using volumetric modulated arc therapy. Strahlenther. Onkol. 2022. [Google Scholar] [CrossRef]
- Fogliata, A.; Cozzi, L.; Clivio, A.; Ibatici, A.; Mancosu, P.; Navarria, P.; Nicolini, G.; Santoro, A.; Vanetti, E.; Scorsetti, M. Preclinical assessment of volumetric modulated arc therapy for total marrow irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 628–636. [Google Scholar] [CrossRef]
- Mancosu, P.; Navarria, P.; Castagna, L.; Roggio, A.; Pellegrini, C.; Reggiori, G.; Fogliata, A.; Lobefalo, F.; Castiglioni, S.; Alongi, F.; et al. Anatomy driven optimization strategy for total marrow irradiation with a volumetric modulated arc therapy technique. J. Appl. Clin. Med. Phys. 2012, 13, 138–147. [Google Scholar] [CrossRef]
- Jabbour, S.K.; Hashem, S.A.; Bosch, W.; Kim, T.K.; Finkelstein, S.E.; Anderson, B.M.; Ben-Josef, E.; Crane, C.H.; Goodman, K.A.; Haddock, M.G.; et al. Upper abdominal normal organ contouring guidelines and atlas: A Radiation Therapy Oncology Group consensus. Pract. Radiat. Oncol. 2014, 4, 82–89. [Google Scholar] [CrossRef]
- Lynch, R.; Pitson, G.; Ball, D.; Claude, L.; Sarrut, D. Computed tomographic atlas for the new international lymph node map for lung cancer: A radiation oncologist perspective. Pract. Radiat. Oncol. 2013, 3, 54–66. [Google Scholar] [CrossRef]
- Offersen, B.V.; Boersma, L.J.; Kirkove, C.; Hol, S.; Aznar, M.C.; Biete Sola, A.; Kirova, Y.M.; Pignol, J.-P.; Remouchamps, V.; Verhoeven, K.; et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer. Radiother. Oncol. 2015, 114, 3–10. [Google Scholar] [CrossRef]
- Grégoire, V.; Ang, K.; Budach, W.; Grau, C.; Hamoir, M.; Langendijk, J.A.; Lee, A.; Le, Q.-T.; Maingon, P.; Nutting, C.; et al. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother. Oncol. 2014, 110, 172–181. [Google Scholar] [CrossRef]
- Lengelé, B.; Scalliet, P. Anatomical bases for the radiological delineation of lymph node areas. Part III: Pelvis and lower limbs. Radiother. Oncol. 2009, 92, 22–33. [Google Scholar] [CrossRef]
- Jensen, L.G.; Stiller, T.; Wong, J.Y.C.; Palmer, J.; Stein, A.; Rosenthal, J. Total Marrow Lymphoid Irradiation/Fludarabine/ Melphalan Conditioning for Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 301–307. [Google Scholar] [CrossRef]
- Rosenthal, J.; Wong, J.; Stein, A.; Qian, D.; Hitt, D.; Naeem, H.; Dagis, A.; Thomas, S.H.; Forman, S. Phase 1/2 trial of total marrow and lymph node irradiation to augment reduced-intensity transplantation for advanced hematologic malignancies. Blood 2011, 117, 309–315. [Google Scholar] [CrossRef]
- Aristei, C.; Saldi, S.; Pierini, A.; Ruggeri, L.; Piccinelli, S.; Ingrosso, G.; Martelli, M.F.; Velardi, A. Total marrow/lymphoid irradiation in the conditioning regimen for haploidentical T-cell depleted hematopoietic stem cell transplantation for acute myeloid leukemia. The Perugia experience. In Total Marrow Irradiation, 1st ed.; Wong, J., Hui, S.K., Eds.; Springer Nature: New York, NY, USA, 2020; pp. 111–122. [Google Scholar]
- Arslan, S.; Al Malki, M.M. Total marrow and lymphoid irradiation in patient undergoing haplo-identical transplants. In Total Marrow Irradiation, 1st ed.; Wong, J., Hui, S.K., Eds.; Springer Nature: New York, NY, USA, 2020; pp. 123–134. [Google Scholar]
- Al Malki, M.M.; Palmer, J.; Tsai, N.-C.; Mokhtari, S.; Hui, S.; Tsai, W.; Aldoss, I.; Ali, H.; Aribi, A.; Cao, T.; et al. Total marrow and lymphoid irradiation as conditioning in haploidentical transplant with posttransplant cyclophosphamide. Blood Adv. 2022, 6, 4098–4106. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Liu, A.; Han, C.; Dandapani, S.; Schultheiss, T.; Palmer, J.; Yang, D.; Somlo, G.; Salhotra, A.; Hui, S.; et al. Total marrow irradiation (TMI): Addressing an unmet need in hematopoietic cell transplantation—A single institution experience review. Front. Oncol. 2022, 12, 1003908. [Google Scholar] [CrossRef]
- Kim, J.H.; Stein, A.; Tsai, N.; Schultheiss, T.E.; Palmer, J.; Liu, A.; Rosenthal, J.; Forman, S.J.; Wong, J.Y.C. Extramedullary relapse following total marrow and lymphoid irradiation in patients undergoing allogeneic hematopoietic cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 75–81. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Kan, H.; Zhao, M.; Xue, X.; Yan, B.; An, H.; Shen, J.; Bartlett, J.; Lu, W.; et al. Automatic segmentation of target structures for total marrow and lymphoid irradiation in bone marrow transplantation. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, Scotland, UK, 11–15 July 2022; pp. 5025–5029. [Google Scholar]
- Vinod, S.K.; Min, M.; Jameson, M.G.; Holloway, L.C. A review of interventions to reduce inter-observer variability in volume delineation in radiation oncology. J. Med. Imaging Radiat. Oncol. 2016, 60, 393–406. [Google Scholar] [CrossRef]
- Genovesi, D.; Ausili Cèfaro, G.; Trignani, M.; Vinciguerra, A.; Augurio, A.; Di Tommaso, M.; Perrotti, F.; De Paoli, A.; Olmi, P.; Valentini, V.; et al. Interobserver variability of clinical target volume delineation in soft-tissue sarcomas. Cancer/Radiothér. 2014, 18, 89–96. [Google Scholar] [CrossRef]
- Piva, C.; Genovesi, D.; Filippi, A.R.; Balducci, M.; Barra, S.; Buglione, M.; Busetto, M.; Ciammella, P.; Franzone, P.; De Sanctis, V.; et al. Interobserver variability in clinical target volume delineation for primary mediastinal B-cell lymphoma. Pract. Radiat. Oncol. 2015, 5, 383–389. [Google Scholar] [CrossRef]
- Grégoire, V.; Levendag, P.; Ang, K.K.; Bernier, J.; Braaksma, M.; Budach, V.; Chao, C.; Coche, E.; Cooper, J.S.; Cosnard, G.; et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother. Oncol. 2003, 69, 227–236. [Google Scholar] [CrossRef]
- Matzinger, O.; Gerber, E.; Bernstein, Z.; Maingon, P.; Haustermans, K.; Bosset, J.F.; Gulyban, A.; Poortmans, P.; Collette, L.; Kuten, A. EORTC-ROG expert opinion: Radiotherapy volume and treatment guidelines for neoadjuvant radiation of adenocarcinomas of the gastroesophageal junction and the stomach. Radiother. Oncol. 2009, 92, 164–175. [Google Scholar] [CrossRef]
- Robin, S.; Jolicoeur, M.; Palumbo, S.; Zilli, T.; Crehange, G.; De Hertogh, O.; Derashodian, T.; Sargos, P.; Salembier, C.; Supiot, S.; et al. Prostate bed delineation guidelines for postoperative radiation therapy: On behalf of the francophone group of urological radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Ciardo, D.; Argenone, A.; Boboc, G.I.; Cucciarelli, F.; De Rose, F.; De Santis, M.C.; Huscher, A.; Ippolito, E.; La Porta, M.R.; Marino, L.; et al. Variability in axillary lymph node delineation for breast cancer radiotherapy in presence of guidelines on a multi-institutional platform. Acta Oncol. 2017, 56, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Fong, A.; McVicar, N.; Smith, S.; Giambattista, J.; Wells, D.; Kolbeck, C.; Giambattista, J.; Gondara, L.; Alexander, A. Comparing deep learning-based auto-segmentation of organs at risk and clinical target volumes to expert inter-observer variability in radiotherapy planning. Radiother. Oncol. 2020, 144, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Stouthandel, M.E.J.; Veldeman, L.; Van Hoof, T. Call for a multidisciplinary effort to map the lymphatic system with advanced medical imaging techniques: A review of the literature and suggestions for future anatomical research. Anat. Rec. 2019, 302, 1681–1695. [Google Scholar] [CrossRef]
- Lobefalo, F.; Bignardi, M.; Reggiori, G.; Tozzi, A.; Tomatis, S.; Alongi, F.; Fogliata, A.; Gaudino, A.; Navarria, P.; Cozzi, L.; et al. Dosimetric impact of inter-observer variability for 3D conformal radiotherapy and volumetric modulated arc therapy: The rectal tumor target definition case. Radiat. Oncol. 2013, 8, 176. [Google Scholar] [CrossRef]
- White, I.; Hunt, A.; Bird, T.; Settatree, S.; Soliman, H.; Mcquaid, D.; Dearnaley, D.; Lalondrelle, S.; Bhide, S. Interobserver variability in target volume delineation for CT/MRI simulation and MRI-guided adaptive radiotherapy in rectal cancer. BJR 2021, 94, 20210350. [Google Scholar] [CrossRef]
- Kumar, S.; Holloway, L.; Boxer, M.; Yap, M.L.; Chlap, P.; Moses, D.; Vinod, S. Variability of gross tumour volume delineation: MRI and CT based tumour and lymph node delineation for lung radiotherapy. Radiother. Oncol. 2022, 167, 292–299. [Google Scholar] [CrossRef]
| Region | Lymph Nodes |
|---|---|
| H&N | VIIa + VIIb + II + Ib + V + III + Ia + VIa + VIb + IVa + IVb |
| axilla | I + II + III + IV |
| mediastinum | 1–8 + 10 |
| abdomen | I + II + III |
| pelvis | I-VII + superficial and deep groin |
| CTV_LN Comparison | Comparison Abbreviation | Explanation |
|---|---|---|
| GL_RO1 vs. Old | A | After-GL vs. before-GL |
| GL_RO1 vs. GL_RO2 | B | Inter-observer-variability |
| GL_RO1a vs. GL_RO1b | C | Intra-observer-variability |
| Before GL [cm3] | After GL [cm3] | |
|---|---|---|
| CTV_LN_Tot | 2176 ± 600 [1312–3194] | 2370 ± 672 [1412–3511] |
| CTV_LN_H&N | 332 ± 128 [197–558] | 559 ± 111 [180–544] |
| CTV_LN_Thorax | 501 ± 178 [254–801] | 532 ± 200 [265–853] |
| CTV_LN_Abdominal | 710 ± 196 [454–1057] | 721 ± 260 [582–1407] |
| CTV_LN_Pelvis | 612 ± 175 [350–898] | 590 ± 177 [349–871] |
| DSC | p-Value (DSC) | Mean DA [mm] | HD [mm] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LN levels | A | B | C | A vs. B | A vs. C | B vs. C | A | B | C | A | B | C |
| Tot | 0.82 ± 0.09 | 0.97 ± 0.01 | 0.98 ± 0.02 | 0.03 | <0.01 | 1.00 | 0.4 ± 0.2 | 0.1 ± 0.1 | 0.03 ± 0.02 | 7 ± 1 | 2 ± 2 | 1.9 ± 0.3 |
| H&N | 0.69 ± 0.15 | 0.88 ± 0.04 | 0.96 ± 0.03 | 0.27 | <0.01 | 0.13 | 0.5 ± 0.4 | 0.1 ± 0.1 | 0.02 ± 0.01 | 7 ± 7 | 2 ± 1 | 0.9 ± 0.3 |
| Thorax | 0.77 ± 0.15 | 0.97 ± 0.01 | 0.97 ± 0.02 | 0.18 | 0.02 | 1.00 | 0.5 ± 0.5 | 0.1 ± 0.2 | 0.03 ± 0.01 | 6 ± 7 | 2 ± 2 | 1.4 ± 0.5 |
| Abdomen | 0.82 ± 0.08 | 0.98 ± 0.01 | 0.97 ± 0.01 | 0.05 | 0.02 | 0.35 | 0.7 ± 0.4 | 0.1 ± 0.2 | 0.03 ± 0.01 | 8 ± 6 | 1 ± 2 | 1.6 ± 0.6 |
| Pelvis | 0.88 ± 0.09 | 0.96 ± 0.01 | 0.95 ± 0.03 | 0.27 | 0.16 | 0.80 | 0.2 ± 0.2 | 0.1 ± 0.2 | 0.06 ± 0.04 | 3 ± 2 | 1 ± 1 | 1.6 ± 0.5 |
| D90 | D80 | |||||||||||
| CTV_LN | A | B | C | A | B | C | ||||||
| RO1 | Old | RO1 | RO2 | RO1a | RO1b | RO1 | Old | RO1 | RO2 | RO1a | RO1b | |
| Tot | 1.01 ± 0.11 | 1.03 ± 0.06 | 1.04 ± 0.02 | 1.04 ± 0.02 | 1.04 ± 0.01 | 1.04 ± 0.01 | 1.04 ± 0.06 | 1.05 ± 0.05 | 1.06 ± 0.02 | 1.060.02 | 1.06 ± 0.01 | 1.06 ± 0.01 |
| H&N | 0.98 ± 0.09 | 1.03 ± 0.05 | 1.05 ± 0.02 | 1.05 ± 0.02 | 1.04 ± 0.01 | 1.04 ± 0.01 | 1.03 ± 0.06 | 1.05 ± 0.05 | 1.08 ± 0.02 | 1.08 ± 0.02 | 1.06 ± 0.02 | 1.06 ± 0.02 |
| Thorax | 1.03 ± 0.09 | 1.04 ± 0.07 | 1.05 ± 0.02 | 1.05 ± 0.02 | 1.04 ± 0.02 | 1.04 ± 0.02 | 1.05 ± 0.07 | 1.05 ± 0.07 | 1.07 ± 0.02 | 1.07 ± 0.02 | 1.06 ± 0.02 | 1.06 ± 0.02 |
| Abdomen | 0.92 ± 0.18 | 1.03 ± 0.07 | 1.03 ± 0.02 | 1.03 ± 0.01 | 1.04 ± 0.01 | 1.04 ± 0.01 | 1.02 ± 0.11 | 1.05 ± 0.07 | 1.05 ± 0.02 | 1.06 ± 0.01 | 1.05 ± 0.01 | 1.05 ± 0.01 |
| Pelvis | 1.04 ± 0.06 | 1.04 ± 0.06 | 1.06 ± 0.02 | 1.06 ± 0.02 | 1.06 ± 0.02 | 1.05 ± 0.02 | 1.05 ± 0.06 | 1.06 ± 0.06 | 1.07 ± 0.02 | 1.07 ± 0.02 | 1.07 ± 0.02 | 1.07 ± 0.02 |
| V95 | V90 | |||||||||||
| A | B | C | A | B | C | |||||||
| CTV_LN | RO1 | Old | RO1 | RO2 | RO1 | RO1b | RO1 | Old | RO1 | RO2 | RO1 | RO1b |
| Tot | 0.94 ± 0.05 | 0.99 ± (<<) | 0.99 ± (<<) | 0.99 ± 0.01 | 1.00 ± (<<) | 0.99 ± (<<) | 0.96 ± 0.04 | 1.00 ± (<<) | 0.99 ± (<<) | 0.99 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) |
| H&N | 0.91 ± 0.05 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.99 ± (<<) | 0.99 ± (<<) | 0.93 ± 0.04 | 1.00 ± (<<) | 0.99 ± (<<) | 1.00 ± 0.01 | 1.00 ± (<<) | 1.00 ± (<<) |
| Thorax | 0.99 ± 0.05 | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 0.99 ± (<<) | 0.99 ± 0.03 | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) |
| Abdomen | 0.89 ± 0.09 | 1.00 ± (<<) | 1.00 ± 0.01 | 1.00 ± 0.01 | 1.00 ± (<<) | 1.00 ± (<<) | 0.91 ± 0.08 | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) |
| Pelvis | 0.99 ± 0.03 | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± 0.01 | 1.00 ± (<<) | 0.99 ± (<<) | 0.99 ± 0.03 | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) | 1.00 ± (<<) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dei, D.; Lambri, N.; Stefanini, S.; Vernier, V.; Brioso, R.C.; Crespi, L.; Clerici, E.; Bellu, L.; De Philippis, C.; Loiacono, D.; et al. Internal Guidelines for Reducing Lymph Node Contour Variability in Total Marrow and Lymph Node Irradiation. Cancers 2023, 15, 1536. https://doi.org/10.3390/cancers15051536
Dei D, Lambri N, Stefanini S, Vernier V, Brioso RC, Crespi L, Clerici E, Bellu L, De Philippis C, Loiacono D, et al. Internal Guidelines for Reducing Lymph Node Contour Variability in Total Marrow and Lymph Node Irradiation. Cancers. 2023; 15(5):1536. https://doi.org/10.3390/cancers15051536
Chicago/Turabian StyleDei, Damiano, Nicola Lambri, Sara Stefanini, Veronica Vernier, Ricardo Coimbra Brioso, Leonardo Crespi, Elena Clerici, Luisa Bellu, Chiara De Philippis, Daniele Loiacono, and et al. 2023. "Internal Guidelines for Reducing Lymph Node Contour Variability in Total Marrow and Lymph Node Irradiation" Cancers 15, no. 5: 1536. https://doi.org/10.3390/cancers15051536
APA StyleDei, D., Lambri, N., Stefanini, S., Vernier, V., Brioso, R. C., Crespi, L., Clerici, E., Bellu, L., De Philippis, C., Loiacono, D., Navarria, P., Reggiori, G., Bramanti, S., Rodari, M., Tomatis, S., Chiti, A., Carlo-Stella, C., Scorsetti, M., & Mancosu, P. (2023). Internal Guidelines for Reducing Lymph Node Contour Variability in Total Marrow and Lymph Node Irradiation. Cancers, 15(5), 1536. https://doi.org/10.3390/cancers15051536








