p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Cohort
2.2. Clinical Characteristics
2.3. Evaluation of p16 and Rb1 Immunohistochemistry
2.4. FISH Analysis
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population
- -
- adult-type diffuse gliomas, including 63 IDH-wt glioblastomas (GBM) (considered WHO grade 4 tumors), 15 IDH-mutant astrocytomas (four grade 2, five grade 3, and six grade 4 cases), and 27 IDH-mutant 1p/19q codeleted oligodendrogliomas (OG) (10 grade 2 and 17 grade 3 cases)
- -
- 18 high-grade pediatric-type diffuse gliomas, including nine H3K27-altered gliomas, one hemispheric H3.3 G34-mutant glioma, and eight wt hemispheric gliomas
- -
- 47 circumscribed gliomas, including 36 pilocytic astrocytomas (PA) (considered WHO grade 1 tumors), 10 gangliogliomas (GGLs) (grade 1), and one pleomorphic xanthoastrocytoma (PXA) (with anaplastic features of WHO grade 3)
- -
- three low-grade pediatric gliomas including two subependymal giant-cell astrocytomas (WHO grade 1) and one angiocentric glioma (also grade 1)
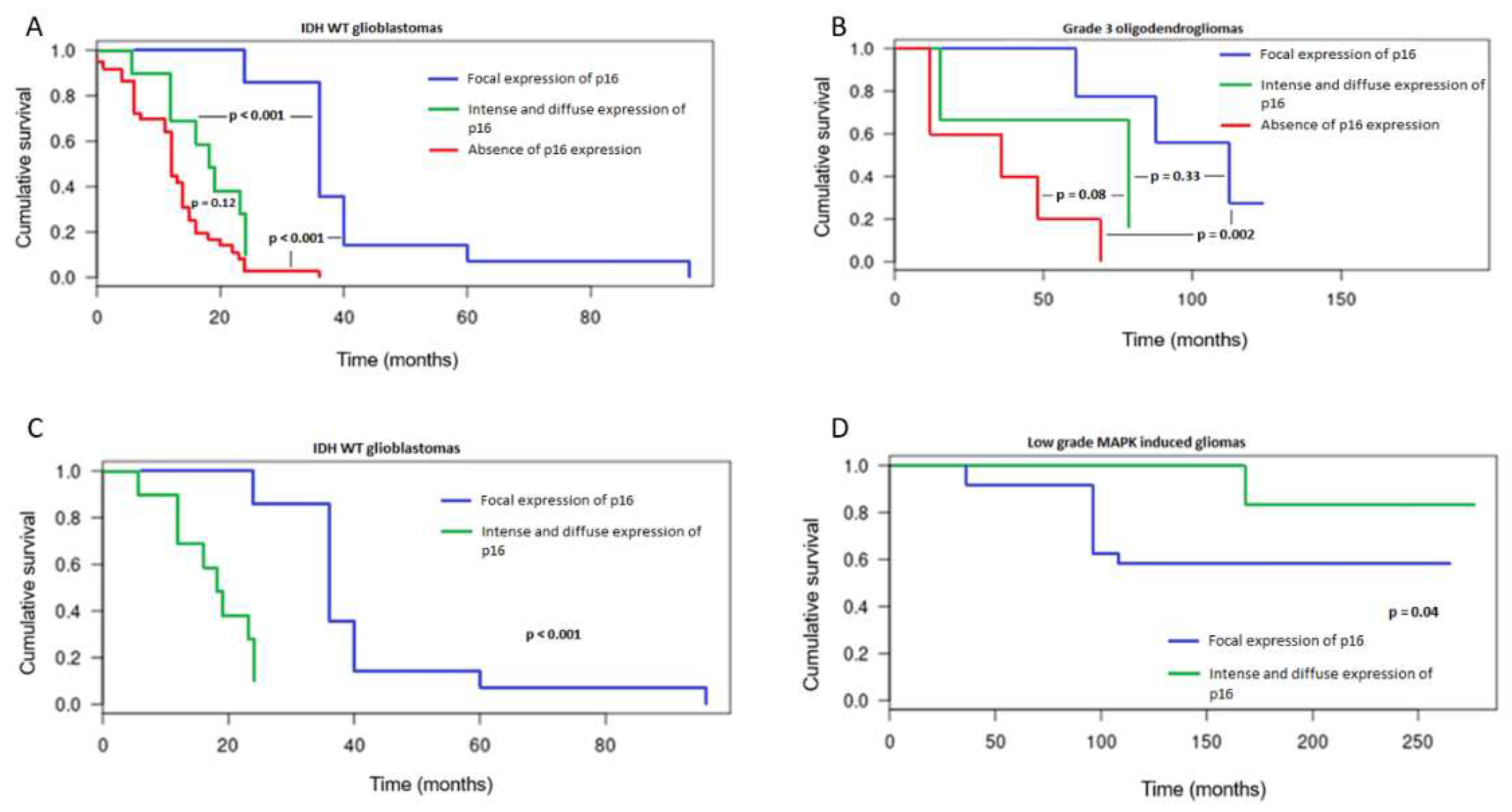
3.2. Patterns of Immunohistochemical p16 Expression and Prognostic Implications
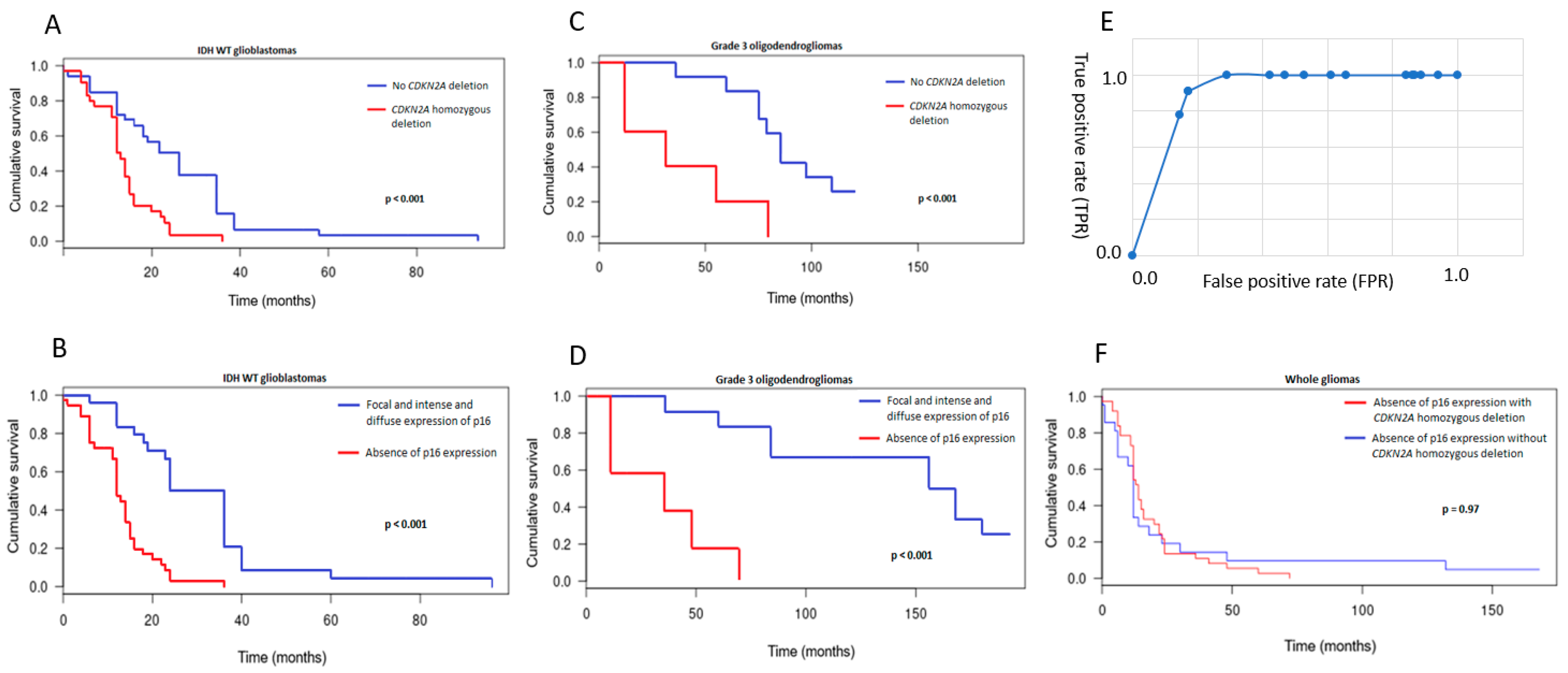
3.3. CDKN2A Status and Prognostic Implications
3.4. Comparison between p16 Immunohistochemistry and CDKN2A FISH Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serra, S.; Chetty, R. p16. J. Clin. Pathol. 2018, 71, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Stott, F.J.; Bates, S.; James, M.C.; McConnell, B.B.; Starborg, M.; Brookes, S.M.; Palmero, I.; Ryan, K.M.; Hara, E.; Vousden, K.H.; et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998, 17, 5001–5014. [Google Scholar] [CrossRef]
- Hume, S.; Dianov, G.L.; Ramadan, K. A unified model for the G1/S cell cycle transition. Nucleic Acids Res. 2020, 48, 12483–12501. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E. INK4a/ARF: A multifunctional tumor suppressor locus. Mutat. Res. 2005, 576, 22–38. [Google Scholar] [CrossRef]
- Le, L.P.; Nielsen, G.P.; Rosenberg, A.E.; Thomas, D.; Batten, J.M.; Deshpande, V.; Schwab, J.; Duan, Z.; Xavier, R.J.; Hornicek, F.J.; et al. Recurrent chromosomal copy number alterations in sporadic chordomas. PLoS ONE 2011, 6, e18846. [Google Scholar] [CrossRef]
- E Schmidt, E.; Ichimura, K.; Messerle, K.R.; Goike, H.M.; Collins, V.P. Infrequent methylation of CDKN2A(MTS1/p16) and rare mutation of both CDKN2A and CDKN2B(MTS2/p15) in primary astrocytic tumours. Br. J. Cancer 1997, 75, 2–8. [Google Scholar] [CrossRef]
- Cottone, L.; Eden, N.; Usher, I.; Lombard, P.; Ye, H.; Ligammari, L.; Lindsay, D.; Brandner, S.; Pižem, J.; Pillay, N.; et al. Frequent alterations in p16/CDKN2A identified by immunohistochemistry and FISH in chordoma. J. Pathol. Clin. Res. 2020, 6, 113–123. [Google Scholar] [CrossRef]
- Appay, R.; Dehais, C.; Maurage, C.-A.; Alentorn, A.; Carpentier, C.; Colin, C.; Ducray, F.; Escande, F.; Idbaih, A.; Kamoun, A.; et al. CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas. Neuro Oncol. 2019, 21, 1519–1528. [Google Scholar] [CrossRef]
- Marker, D.F.; Pearce, T.M. Homozygous deletion of CDKN2A by fluorescence in situ hybridization is prognostic in grade 4, but not grade 2 or 3, IDH-mutant astrocytomas. Acta Neuropathol. Commun. 2020, 8, 169. [Google Scholar] [CrossRef]
- Brat, D.J.; Reuss, D.E.; Von Deimling, A. Luis DN Chapter 2: Astrocytoma, IDH-mutant. In WHO Classification of Tumours Editorial Board, Central nervous system tumours; WHO classification of tumours series; 5th ed.; Volume 6, International Agency for Research on Cancer: Lyon, France, 2021; Available online: https://publications.iarc.fr/601 (accessed on 20 June 2022).
- Purkait, S.; Jha, P.; Sharma, M.C.; Suri, V.; Sharma, M.; Kale, S.S.; Sarkar, C. CDKN2A deletion in pediatric versus adult glioblastomas and predictive value of p16 immunohistochemistry. Neuropathology 2013, 33, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kang, J.; Lim, K.Y.; Kim, H.; Kim, S.-I.; Won, J.K.; Park, C.-K.; Park, S.-H. The prognostic significance of p16 expression pattern in diffuse gliomas. J. Pathol. Transl. Med. 2021, 55, 102–111. [Google Scholar] [CrossRef]
- Reis, G.F.; Pekmezci, M.; Hansen, H.M.; Rice, T.; Marshall, R.E.; Molinaro, A.M.; Phillips, J.J.; Vogel, H.; Wiencke, J.K.; Wrensch, M.R.; et al. CDKN2A loss is associated with shortened overall survival in lower-grade (World Health Organization Grades II-III) astrocytomas. J. Neuropathol. Exp. Neurol. 2015, 74, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, S.; Cavalla, P.; Bosone, I.; Mauro, A.; Schiffer, D. CDKN2A/p16 inactivation in the prognosis of oligodendrogliomas. Int. J. Cancer 2000, 88, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.T.-S.; Santos, G.D.C.; Hwang, D.M.; Ludkovski, O.; Pintilie, M.; Squire, J.; Tsao, M.-S. FISH assay development for the detection of p16/CDKN2A deletion in malignant pleural mesothelioma. J. Clin. Pathol. 2010, 63, 630–634. [Google Scholar] [CrossRef]
- E Damsky, W.; Bosenberg, M. Melanocytic nevi and melanoma: Unraveling a complex relationship. Oncogene 2017, 36, 5771–5792. [Google Scholar] [CrossRef]
- Raabe, E.H.; Lim, K.S.; Kim, J.M.; Meeker, A.; Mao, X.-G.; Nikkhah, G.; Maciaczyk, J.; Kahlert, U.; Jain, D.; Bar, E.; et al. BRAF activation induces transformation and then senescence in human neural stem cells: A pilocytic astrocytoma model. Clin. Cancer Res. 2011, 17, 3590–3599. [Google Scholar] [CrossRef]
- Biernat, W.; Tohma, Y.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Alterations of cell cycle regulatory genes in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 1997, 94, 303–309. [Google Scholar] [CrossRef]
- Watanabe, T.; Yokoo, H.; Yokoo, M.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Concurrent inactivation of RB1 and TP53 pathways in anaplastic oligodendrogliomas. J. Neuropathol. Exp. Neurol. 2001, 60, 1181–1189. [Google Scholar] [CrossRef]
- Aoki, K.; Nakamura, H.; Suzuki, H.; Matsuo, K.; Kataoka, K.; Shimamura, T.; Motomura, K.; Ohka, F.; Shiina, S.; Yamamoto, T.; et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro Oncol. 2018, 20, 66–77. [Google Scholar] [CrossRef]
- Korshunov, A.; Ryzhova, M.; Hovestadt, V.; Bender, S.; Sturm, D.; Capper, D.; Meyer, J.; Schrimpf, D.; Kool, M.; Northcott, P.A.; et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015, 129, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Coutant, M.; Lhermitte, B.; Guérin, E.; Chammas, A.; Reita, D.; Sebastia, C.; Douzal, V.; Gabor, F.; Salmon, A.; Chenard, M.; et al. Retrospective and integrative analyses of molecular characteristics and their specific imaging parameters in pediatric grade 1 gliomas. Pediatr. Blood Cancer. 2022, 69, e29575. [Google Scholar] [CrossRef] [PubMed]
- Ryall, S.; Tabori, U.; Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
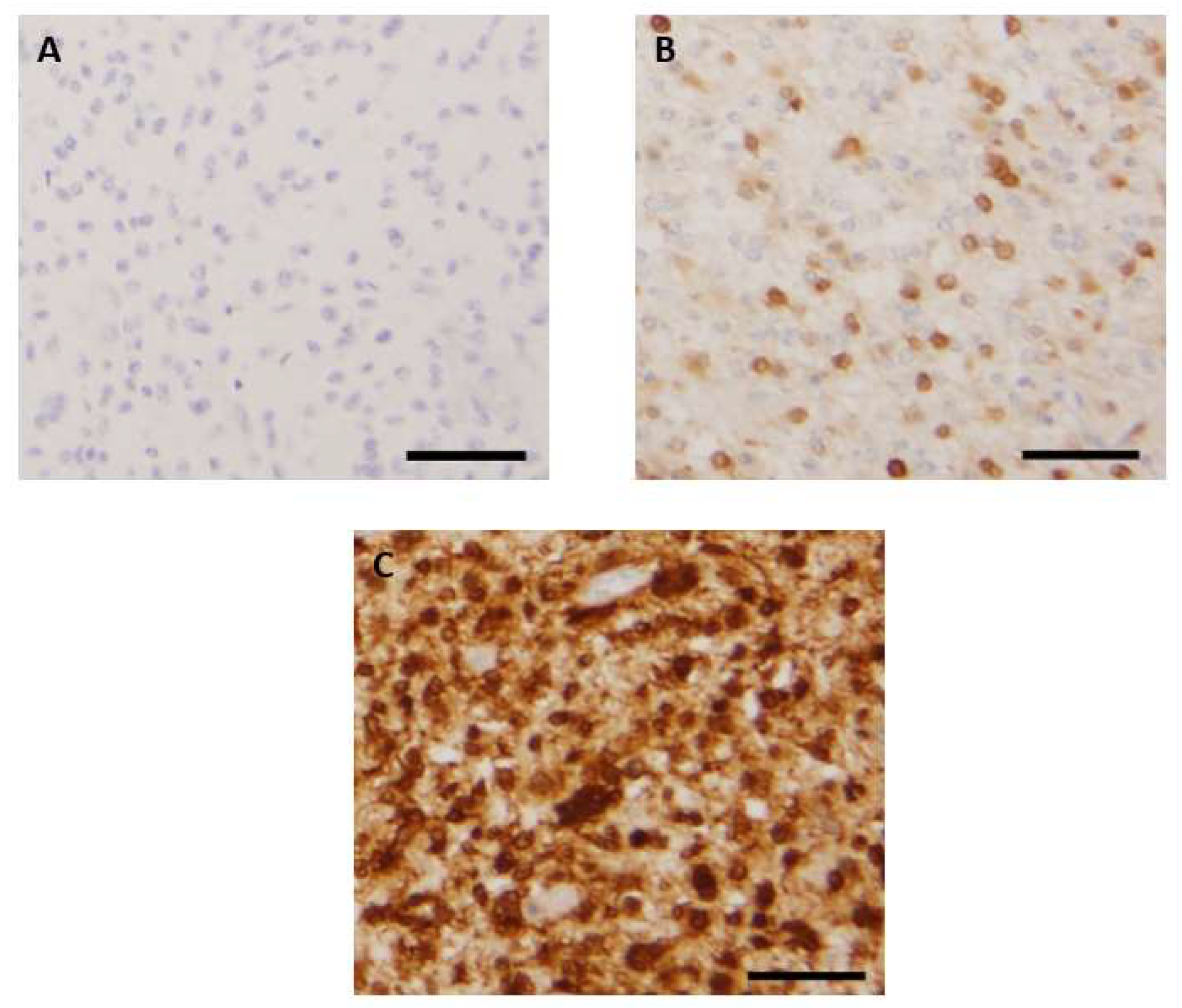

| Characteristics | Number (%) (n = 173) | Number of Deceased Patients (%) |
|---|---|---|
| Median age (years) | 36.5 (1–79) | |
| Sex | ||
| Male | 95 (55) | |
| Female | 78 (45) | |
| Adult-type diffuse gliomas | ||
| IDH-mutant astrocytoma, grade 2 | 4 (2) | 1 (25) |
| IDH-mutant astrocytoma, grade 3 | 5 (3) | 4 (80) |
| IDH-mutant astrocytoma, grade 4 | 6 (3) | 6 (100) |
| IDH mutant and 1p/19q codeleted oligodendroglioma, grade 2 | 10 (6) | 3 (30) |
| IDH mutant and 1p/19q codeleted oligodendroglioma, grade 3 | 17 (10) | 16 (94) |
| IDH-wt glioblastoma, grade 4 | 63 (36) | 63 (100) |
| Circumscribed gliomas | ||
| Pilocytic astrocytoma | 36 (21) | 3 (8) |
| Fusion KIAA1549 :: BRAF | 27 | |
| BRAF V600E mutation | 1 | |
| NF1 mutation | 1 | |
| FGFR1 duplication | 1 | |
| NOS | 6 | |
| Pleomorphic xanthoastrocytoma with anaplasia | 1 (1) | 0 (0) |
| Glioneuronal tumors | ||
| Ganglioglioma | 10 (6) | 0 (0) |
| Fusion KIAA1549 :: BRAF | 2 | |
| BRAF V600E mutation | 8 | |
| Other low-grade pediatric gliomas | ||
| Subependymal giant-cell astrocytoma | 2 (1) | 0 (0) |
| Angiocentric glioma with MYBL1 alteration | 1 (1) | 0 (0) |
| High-grade pediatric-type diffuse gliomas | ||
| Diffuse midline glioma H3K27-altered | 9 (5) | 9 (100) |
| High-grade diffuse pediatric glioma, H3 and IDH-wt | 8 (4) | 8 (100) |
| Diffuse hemispheric glioma H3 G34-mutant | 1 (1) | 1 (100) |
| Deceased patients | 114 (66) | |
| Operated tumor recurrence | 33 (19) | |
| Surgical specimens | ||
| Biopsy | 10 (6) | |
| Resection | 163 (94) |
| Diagnostic | Number of Cases | Absence of p16 Expression (Nb) | Focal p16 Expression (Nb) | P16 Overexpression (Nb) |
|---|---|---|---|---|
| IDH-mutant astrocytoma, grade 2 | 4 | 0 | 4 | 0 |
| IDH-mutant astrocytoma, grade 3 | 5 | 2 | 2 | 1 |
| IDH-mutant astrocytoma, grade 4 | 6 | 2 | 3 | 1 |
| IDH-mutant, 1p/19q codeleted oligodendroglioma, grade 2 | 10 | 0 | 10 | 0 |
| IDH-mutant, 1p/19q codeleted oligodendroglioma, grade 3 | 17 | 5 | 10 | 2 |
| IDH-wt glioblastoma, grade 4 | 63 | 36 | 17 | 10 |
| Pilocytic Astrocytoma, grade 1 | 36 | 1 | 30 | 5 |
| Anaplastic PXA, grade 3 | 1 | 1 | 0 | 0 |
| Ganglioglioma, grade 1 | 10 | 0 | 5 | 5 |
| Other low grade gliomas, grade 1 | 3 | 0 | 3 | 0 |
| Diffuse midline glioma, H3K27-altered, grade 4 | 9 | 6 | 1 | 2 |
| Diffuse pediatric-type glioma, H3 and IDH wt, grade 4 | 8 | 3 | 3 | 2 |
| Diffuse hemispheric glioma, H3-G34-mutant, grade 4 | 1 | 0 | 1 | 0 |
| Total | 173 | 56 | 89 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geyer, L.; Wolf, T.; Chenard, M.-P.; Cebula, H.; Schott, R.; Noel, G.; Guerin, E.; Pencreach, E.; Reita, D.; Entz-Werlé, N.; et al. p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances. Cancers 2023, 15, 1512. https://doi.org/10.3390/cancers15051512
Geyer L, Wolf T, Chenard M-P, Cebula H, Schott R, Noel G, Guerin E, Pencreach E, Reita D, Entz-Werlé N, et al. p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances. Cancers. 2023; 15(5):1512. https://doi.org/10.3390/cancers15051512
Chicago/Turabian StyleGeyer, Lucas, Thibaut Wolf, Marie-Pierre Chenard, Helene Cebula, Roland Schott, Georges Noel, Eric Guerin, Erwan Pencreach, Damien Reita, Natacha Entz-Werlé, and et al. 2023. "p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances" Cancers 15, no. 5: 1512. https://doi.org/10.3390/cancers15051512
APA StyleGeyer, L., Wolf, T., Chenard, M.-P., Cebula, H., Schott, R., Noel, G., Guerin, E., Pencreach, E., Reita, D., Entz-Werlé, N., & Lhermitte, B. (2023). p16 Immunohistochemical Expression as a Surrogate Assessment of CDKN2A Alteration in Gliomas Leading to Prognostic Significances. Cancers, 15(5), 1512. https://doi.org/10.3390/cancers15051512






