Constitutive Expression of a Cytotoxic Anticancer Protein in Tumor-Colonizing Bacteria
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Culture Conditions
2.2. Cell Lines and Animal Experiments
2.3. Bacterial RNA and cDNA Library Preparation
2.4. Quantitative Polymerase Chain Reaction (Real-Time PCR)
2.5. RNA Sequencing Analysis
2.6. Bacterial Division Analysis
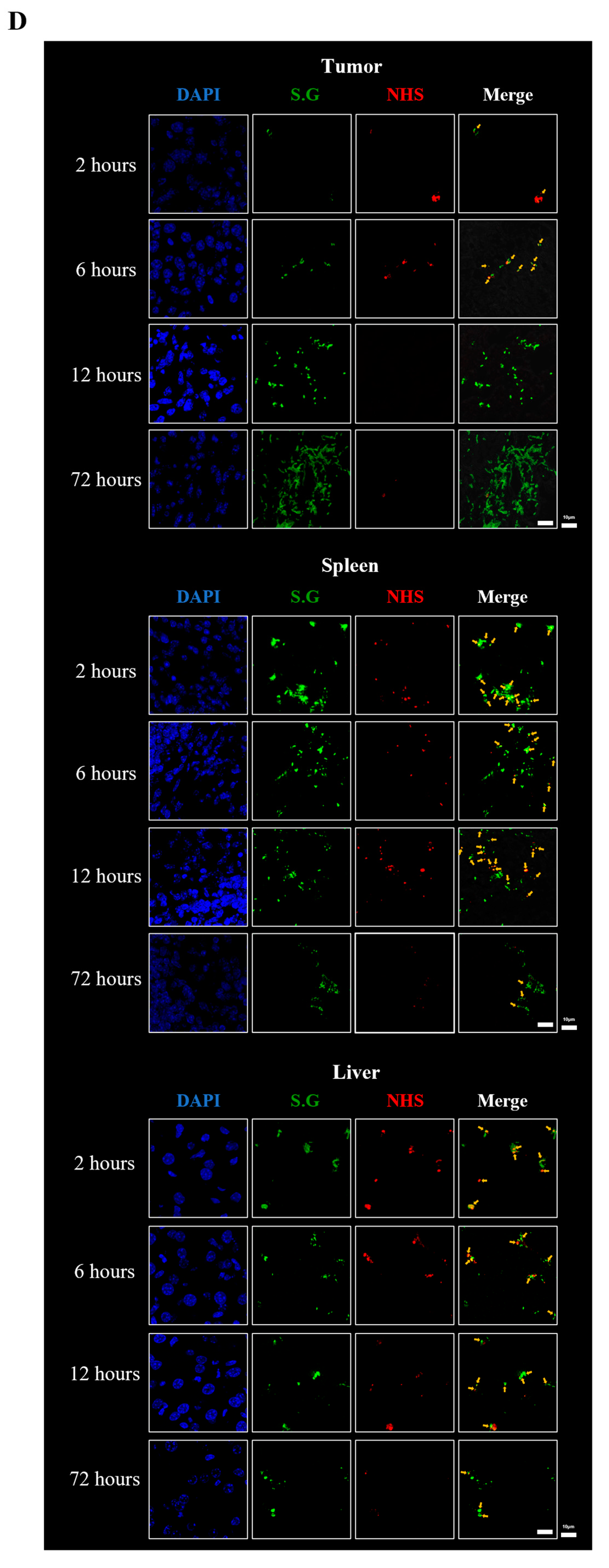
2.7. Immunofluorescent Staining and Confocal Microscope
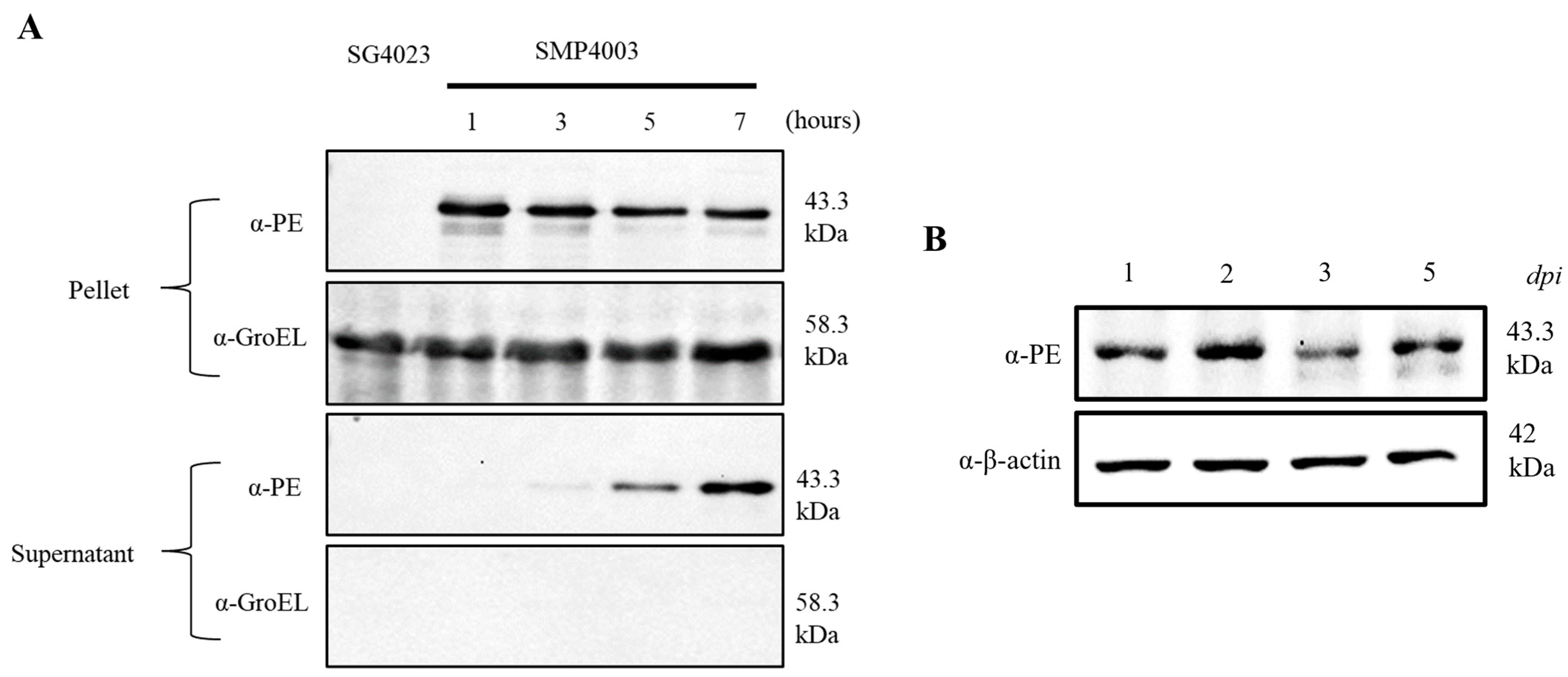
2.8. Western Blot Analysis
2.9. Statistical Analysis
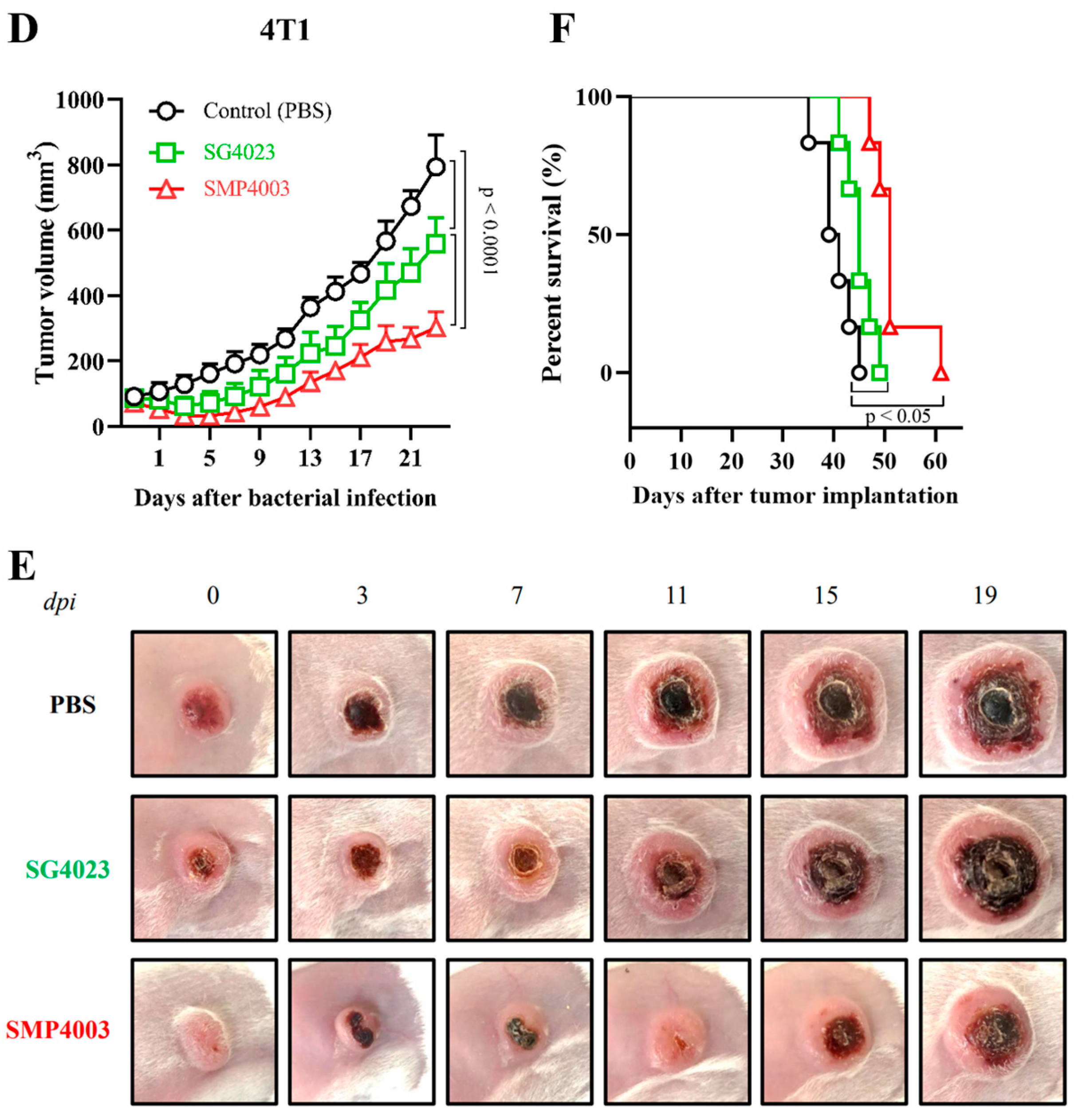
3. Results
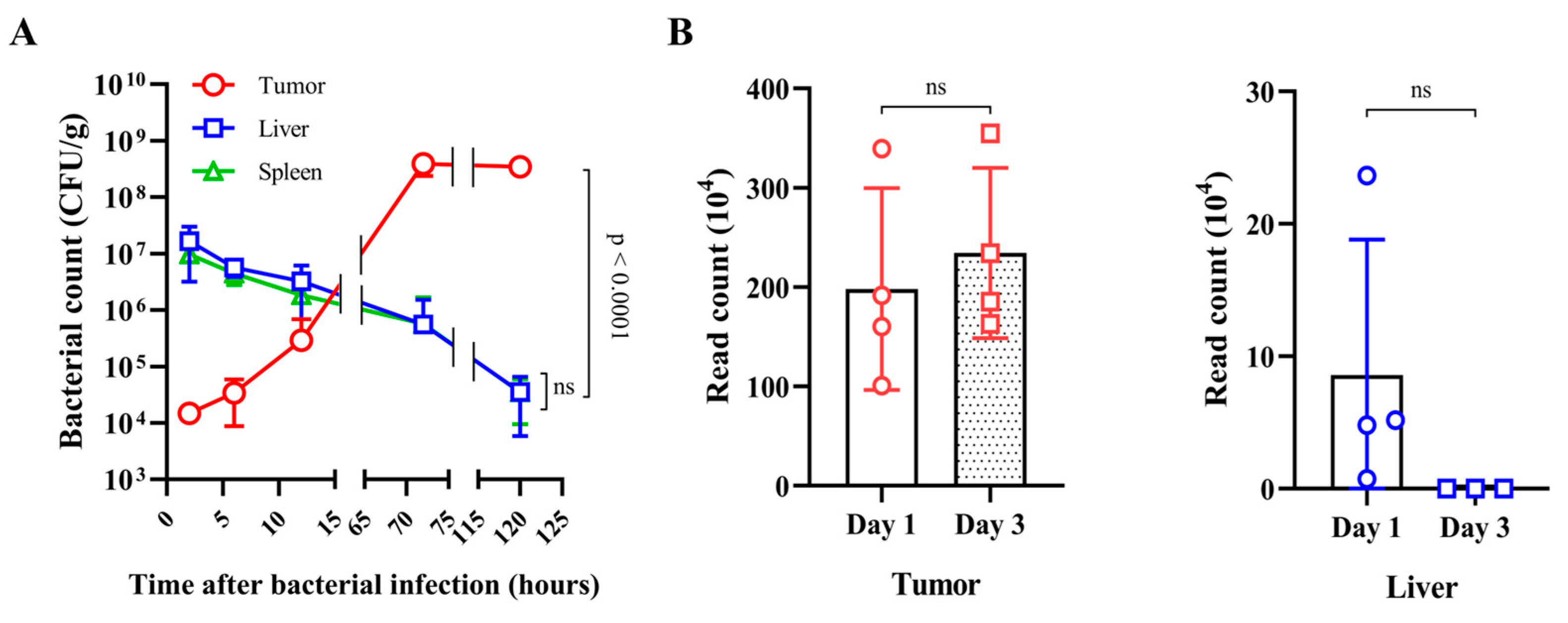
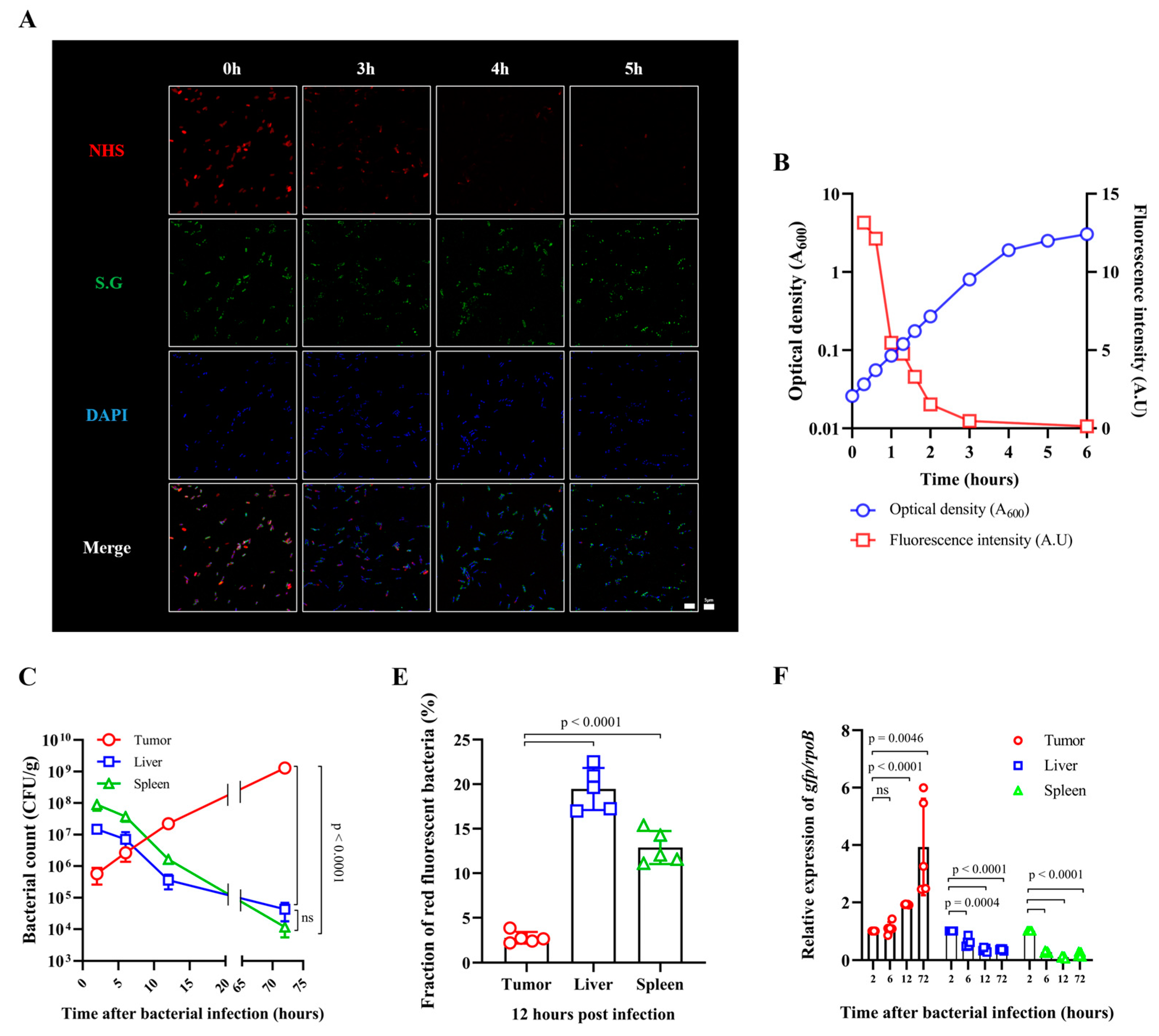
3.1. Fate of E. coli Injected into Tumor-Bearing Mice
3.2. Fate of ppGpp-Defective S. Gallinarum Injected into Tumor-Bearing Mice
3.3. Expression of an Immunotoxin under the Control of the Ribosomal RNA Promoter (rrnB P1)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, R.K.; Forbes, N.S. Can Engineered Bacteria Help Control Cancer? Proc. Natl. Acad. Sci. USA 2001, 98, 14748–14750. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.S. Engineering the Perfect (Bacterial) Cancer Therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumor-Targeting Bacteria Engineered to Fight Cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.M.; Srikanth, C.V.; McCormick, B.A. Targeting Tumors with Salmonella typhimurium—Potential for Therapy. Oncotarget 2011, 1, 721–728. [Google Scholar] [CrossRef]
- Hong, E.-H.; Chang, S.-Y.; Lee, B.-R.; Pyun, A.-R.; Kim, J.-W.; Kweon, M.-N.; Ko, H.-J. Intratumoral Injection of Attenuated Salmonella Vaccine Can Induce Tumor Microenvironmental Shift from Immune Suppressive to Immunogenic. Vaccine 2013, 31, 1377–1384. [Google Scholar] [CrossRef]
- Ashraf, S.; Kong, W.; Wang, S.; Yang, J.; Curtiss, R. Protective Cellular Responses Elicited by Vaccination with Influenza Nucleoprotein Delivered by a Live Recombinant Attenuated Salmonella Vaccine. Vaccine 2011, 29, 3990–4002. [Google Scholar] [CrossRef]
- Grille, S.; Moreno, M.; Bascuas, T.; Marqués, J.M.; Muñoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella Enterica Serovar Typhimurium Immunotherapy for B-Cell Lymphoma Induces Broad Anti-Tumour Immunity with Therapeutic Effect. Immunology 2014, 143, 428–437. [Google Scholar] [CrossRef]
- Yun, M.; Pan, S.; Jiang, S.-N.; Nguyen, V.H.; Park, S.-H.; Jung, C.-H.; Kim, H.-S.; Min, J.-J.; Choy, H.E.; Hong, Y. Effect of Salmonella Treatment on an Implanted Tumor (CT26) in a Mouse Model. J. Microbiol. 2012, 50, 502–510. [Google Scholar] [CrossRef]
- Kim, J.-E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.-V.; Zheng, J.H.; Yun, M.; Park, S.-G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella Typhimurium Suppresses Tumor Growth via the Pro-Inflammatory Cytokine Interleukin-1β. Theranostics 2015, 5, 1328–1342. [Google Scholar] [CrossRef]
- Leschner, S.; Weiss, S. Salmonella—Allies in the Fight against Cancer. J. Mol. Med. 2010, 88, 763–773. [Google Scholar] [CrossRef]
- Leschner, S.; Westphal, K.; Dietrich, N.; Viegas, N.; Jablonska, J.; Lyszkiewicz, M.; Lienenklaus, S.; Falk, W.; Gekara, N.; Loessner, H.; et al. Tumor Invasion of Salmonella Enterica Serovar Typhimurium Is Accompanied by Strong Hemorrhage Promoted by TNF-α. PLoS ONE 2009, 4, e6692. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Kasnitz, N.; Kocijancic, D.; Trittel, S.; Riese, P.; Guzman, C.A.; Leschner, S.; Weiss, S. Induction of CD4+ and CD8+ Anti-Tumor Effector T Cell Responses by Bacteria Mediated Tumor Therapy. Int. J. Cancer 2015, 137, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Demaria, O.; Gassart, A.D.; Coso, S.; Gestermann, N.; Domizio, J.D.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. STING Activation of Tumor Endothelial Cells Initiates Spontaneous and Therapeutic Antitumor Immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Wen, Y.; Fu, J.; Wang, H.; Ma, Z.; Shi, Y.; Wang, B. Suppression of Established Hepatocarcinoma in Adjuvant Only Immunotherapy: Alum Triggers Anti-Tumor CD8+ T Cell Response. Sci. Rep. 2015, 5, 17695. [Google Scholar] [CrossRef]
- Kuhn, S.; Yang, J.; Hyde, E.J.; Harper, J.L.; Kirman, J.R.; Ronchese, F. IL-1βR-Dependent Priming of Antitumor CD4+ T Cells and Sustained Antitumor Immunity after Peri-Tumoral Treatment with MSU and Mycobacteria. Oncoimmunology 2015, 4, e1042199. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Luna, M.A.; Luria-Pérez, R. Cancer Immunotherapy: Priming the Host Immune Response with Live Attenuated Salmonella Enterica. J. Immunol. Res. 2018, 2018, 2984247. [Google Scholar] [CrossRef]
- Bakowski, M.A.; Cirulis, J.T.; Brown, N.F.; Finlay, B.B.; Brumell, J.H. SopD Acts Cooperatively with SopB during Salmonella enterica Serovar Typhimurium Invasion. Cell. Microbiol. 2007, 9, 2839–2855. [Google Scholar] [CrossRef]
- Hurley, D.; McCusker, M.P.; Fanning, S.; Martins, M. Salmonella-Host Interactions—Modulation of the Host Innate Immune System. Front. Immunol. 2014, 5, 481. [Google Scholar] [CrossRef]
- Min, J.-J.; Nguyen, V.H.; Kim, H.-J.; Hong, Y.; Choy, H.E. Quantitative Bioluminescence Imaging of Tumor-Targeting Bacteria in Living Animals. Nat. Protoc. 2008, 3, 629–636. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Kim, H.-S.; Ha, J.-M.; Hong, Y.; Choy, H.E.; Min, J.-J. Genetically Engineered Salmonella typhimurium as an Imageable Therapeutic Probe for Cancer. Cancer Res. 2010, 70, 18–23. [Google Scholar] [CrossRef]
- Yu, Y.A.; Shabahang, S.; Timiryasova, T.M.; Zhang, Q.; Beltz, R.; Gentschev, I.; Goebel, W.; Szalay, A.A. Visualization of Tumors and Metastases in Live Animals with Bacteria and Vaccinia Virus Encoding Light-Emitting Proteins. Nat. Biotechnol. 2004, 22, 313–320. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, J.H.; Lim, D.; Hong, Y.; Lim, H.-J.; Kim, G.-J.; Shin, S.; Lee, J.-J.; Yun, M.; Harris, R.A.; et al. L-Asparaginase Delivered by Salmonella typhimurium Suppresses Solid Tumors. Mol. Ther.-Oncolytics 2015, 2, 15007. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Kim, K.; Lim, D.; Jeong, K.; Hong, Y.; Nguyen, V.H.; Kim, T.-H.; Ryu, S.; Lim, J.-A.; Kim, J.I.; et al. Anti-Tumoral Effect of the Mitochondrial Target Domain of Noxa Delivered by an Engineered Salmonella typhimurium. PLoS ONE 2014, 9, e80050. [Google Scholar] [CrossRef]
- Jiang, S.-N.; Park, S.-H.; Lee, H.J.; Zheng, J.H.; Kim, H.-S.; Bom, H.-S.; Hong, Y.; Szardenings, M.; Shin, M.G.; Kim, S.-C.; et al. Engineering of Bacteria for the Visualization of Targeted Delivery of a Cytolytic Anticancer Agent. Mol. Ther. 2013, 21, 1985–1995. [Google Scholar] [CrossRef]
- Lim, D.; Kim, K.S.; Kim, H.; Ko, K.-C.; Song, J.J.; Choi, J.H.; Shin, M.; Min, J.; Jeong, J.-H.; Choy, H.E. Anti-Tumor Activity of an Immunotoxin (TGFα-PE38) Delivered by Attenuated Salmonella typhimurium. Oncotarget 2017, 8, 37550–37560. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Song, M.; Park, S.-I.; Cho, K.-O.; Rhee, J.H.; Choy, H.E. Salmonella Enterica Serovar Gallinarum Requires PpGpp for Internalization and Survival in Animal Cells. J. Bacteriol. 2008, 190, 6340–6350. [Google Scholar] [CrossRef]
- Kim, K.; Jeong, J.H.; Lim, D.; Hong, Y.; Yun, M.; Min, J.-J.; Kwak, S.-J.; Choy, H.E. A Novel Balanced-Lethal Host-Vector System Based on GlmS. PLoS ONE 2013, 8, e60511. [Google Scholar] [CrossRef]
- Ross, W.; Thompson, J.F.; Newlands, J.T.; Gourse, R.L. E. Coli Fis Protein Activates Ribosomal RNA Transcription in Vitro and In Vivo. EMBO J. 1990, 9, 3733–3742. [Google Scholar] [CrossRef]
- Rollenhagen, C.; Sörensen, M.; Rizos, K.; Hurvitz, R.; Bumann, D. Antigen Selection Based on Expression Levels during Infection Facilitates Vaccine Development for an Intracellular Pathogen. Proc. Natl. Acad. Sci. USA 2004, 101, 8739–8744. [Google Scholar] [CrossRef] [PubMed]
- Bachman, B.J. Derivations and Genotypes of Some Mutant Derivatives of Escherichia Coli K-12. In Escherichia coli and Salmonella: Cellular and Molecular Biology; Neidhardt, F., Ed.; ASM Press: Washington, DC, USA, 1996; Volume 2, pp. 2460–2488. [Google Scholar]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The Complete Genome Sequence of Escherichia Coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Keener, J.; Nomura, M. Regulation of Ribosome Synthesis. In Escherichia coli and Salmonella: Cellular and Molecular Biology; Neidhardt, F., Ed.; ASM Press: Washington, DC, USA, 1996; pp. 1417–1431. [Google Scholar]
- Nilsson, L.; Verbeek, H.; Vijgenboom, E.; van Drunen, C.; Vanet, A.; Bosch, L. FIS-Dependent Trans Activation of Stable RNA Operons of Escherichia coli under Various Growth Conditions. J. Bacteriol. 1992, 174, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Taketo, M.; Ishihama, A. Autogenous Regulation of RNA Polymerase Beta Subunit Synthesis in Vitro. J. Biol. Chem. 1978, 253, 4501–4504. [Google Scholar] [CrossRef] [PubMed]
- Tedin, K.; Norel, F. Comparison of ΔrelA Strains of Escherichia coli and Salmonella enterica Serovar Typhimurium Suggests a Role for PpGpp in Attenuation Regulation of Branched-Chain Amino Acid Biosynthesis. J. Bacteriol. 2001, 183, 6184–6196. [Google Scholar] [CrossRef]
- Lönnbro, P.; Nordenfelt, P.; Tapper, H. Isolation of Bacteria-Containing Phagosomes by Magnetic Selection. BMC Cell Biol. 2008, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Kim, K.S.; Jeong, J.-H.; Marques, O.; Kim, H.-J.; Song, M.; Lee, T.-H.; Kim, J.I.; Choi, H.-S.; Min, J.-J.; et al. The Hepcidin-Ferroportin Axis Controls the Iron Content of Salmonella-Containing Vacuoles in Macrophages. Nat. Commun. 2018, 9, 2091. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Belin, D.; Carson, M.J.; Beckwith, J. Tight Regulation, Modulation, and High-Level Expression by Vectors Containing the Arabinose PBAD Promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef]
- Andersson, Y.; Juell, S.; Fodstad, Ø. Downregulation of the Antiapoptotic MCL-1 Protein and Apoptosis in MA-11 Breast Cancer Cells Induced by an Anti-Epidermal Growth Factor Receptor-Pseudomonas Exotoxin a Immunotoxin. Int. J. Cancer 2004, 112, 475–483. [Google Scholar] [CrossRef]
- Gao, W.; Tang, Z.; Zhang, Y.; Feng, M.; Qian, M.; Dimitrov, D.S.; Ho, M. Immunotoxin Targeting Glypican-3 Regresses Liver Cancer via Dual Inhibition of Wnt Signaling and Protein Synthesis. Nat. Commun. 2015, 6, 6536. [Google Scholar] [CrossRef]
- Chandramohan, V.; Sampson, J.H.; Pastan, I.H.; Bigner, D.D. Immunotoxin Therapy for Brain Tumors. In Translational Immunotherapy of Brain Tumors; Sampson, J.H., Ed.; Academic Press: San Diego, CA, USA, 2017; Chapter 10; pp. 227–260. ISBN 9780128024201. [Google Scholar]
- Michalska, M.; Wolf, P. Pseudomonas Exotoxin A: Optimized by Evolution for Effective Killing. Front. Microbiol. 2015, 6, 963. [Google Scholar] [CrossRef]
- Pastan, I.; Hassan, R.; FitzGerald, D.J.; Kreitman, R.J. Immunotoxin Therapy of Cancer. Nat. Rev. Cancer 2006, 6, 559–565. [Google Scholar] [CrossRef]
- Simon, N.; Fitzgerald, D. Immunotoxin Therapies for the Treatment of Epidermal Growth Factor Receptor-Dependent Cancers. Toxins 2016, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.M.; Grandis, J.R. Pharmacokinetic and Pharmacodynamic Properties of EGFR Inhibitors under Clinical Investigation. Cancer Treat. Rev. 2004, 30, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.S.; Munn, L.L.; Fukumura, D.; Jain, R.K. Sparse Initial Entrapment of Systemically Injected Salmonella typhimurium Leads to Heterogeneous Accumulation within Tumors. Cancer Res. 2003, 63, 5188–5193. [Google Scholar] [PubMed]
- Noller, H.F.; Nomura, M. Chapter 9—Molecular Architecture and Assembly of Ribosomes. In Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology; Neidhardt, F., Ed.; American Society for Microbiology: Washington, DC, USA, 1986; Volume 1, pp. 104–125. [Google Scholar]
- Dunn, D.L.; Barke, R.A.; Knight, N.B.; Humphrey, E.W.; Simmons, R.L. Role of Resident Macrophages, Peripheral Neutrophils and Translymphatic Absorption in Bacterial Clearance from the Peritoneal Cavity. Infect. Immun. 1985, 49, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Loessner, H.; Leschner, S.; Endmann, A.; Westphal, K.; Wolf, K.; Kochruebe, K.; Miloud, T.; Altenbuchner, J.; Weiss, S. Drug-Inducible Remote Control of Gene Expression by Probiotic Escherichia coli Nissle 1917 in Intestine, Tumor and Gall Bladder of Mice. Microbes Infect. 2009, 11, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Loessner, H.; Endmann, A.; Leschner, S.; Westphal, K.; Rohde, M.; Miloud, T.; Hämmerling, G.; Neuhaus, K.; Weiss, S. Remote Control of Tumour-Targeted Salmonella enterica Serovar Typhimurium by the Use of l-Arabinose as Inducer of Bacterial Gene Expression In Vivo. Cell. Microbiol. 2007, 9, 1529–1537. [Google Scholar] [CrossRef]
- Leschner, S.; Deyneko, I.V.; Lienenklaus, S.; Wolf, K.; Bloecker, H.; Bumann, D.; Loessner, H.; Weiss, S. Identification of Tumor-Specific Salmonella typhimurium Promoters and Their Regulatory Logic. Nucleic Acids Res. 2012, 40, 2984–2994. [Google Scholar] [CrossRef]
- Petroianu, A.; Barbosa, A.A. Quantitative Studies on Macrophage Phagocytosis in Whole Spleen and in the Remnat of Subtotal Splenectomy. Med. Sci. Res. 1991, 19, 373–375. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Strains | Description | References |
|---|---|---|
| Escherichia coli | ||
| MG1655 | Wild type (with defects in ilvG, rfb50, and rph-1) | ATCC [30,31] |
| EMP4002 | MG1655, prrnBP1-gfpOVA, Ampr | This work |
| Salmonella enterica serovar Gallinarum | ||
| SG4021 | Wild-type isolate, clinical | |
| SG4023 | SG4021, ΔrelA, ΔspoT | [26] |
| SG4030 | SG4023, ΔrelA, ΔspoT, ΔglmS::Kanr | This work |
| SMP4001 | SG4023, prrnBP1-gfpOVA, Ampr | This work |
| SMP4003 | SG4030, prrnBP1-psp-TP, glmS+, Ampr | This work |
| SMP4004 | SG4030, pSEC-TGFα-PE38, glmS+, Ampr | This work |
| Plasmids | ||
| prrnBP1-gfpOVA | gfpOVA under control of PrrnB P1 in pBR322 | This work |
| prrnBP1-psp-TP | psp-TP under control of PrrnB P1 in pBAD24 | This work (Figure S1) |
| pSEC-TGFα-PE38 | psp-TP under control of ParaBAD in pBAD24 | [25] |
| Construction | Name and Direction * | Sequence ** |
|---|---|---|
| prrnBP1-gfpOVA | GFP-vector-FW | 5′-CGGAATAACTCCCTATAATGCGCCACCACTTCTAGATTTAAGAAGGAGATATACATATGA-3′ |
| GFP-vector-RV | 5′-AACGCTGTAAAACGGGCAATAATTGTTCAGCGCATGCACCATTCCTTGCGGCG-3′ | |
| rrnB1-insert-FW | 5′-CGCCGCAAGGAATGGTGCATGCGCTGAACAATTATTGCCCGTTTTACAGCGTT-3′ | |
| rrnB1-insert-RV | 5′-TCATATGTATATCTCCTTCTTAAATCTAGAAGTGGTGGCCATTATAGGGAGTTATTCCG-3′ | |
| Seq-GFP-FW | 5′-ATAAGTGCGGCGACGATAGTCAT -3′ | |
| prrnBP1-psp-TP | psp-vector-FW | 5′AATAACTCCCTATAATGCGCCACCACTATGGGTTTGAAGATGAAGAAAAGATCAG-3′ |
| psp-vector-RV | 5′-AACGCTGTAAAACGGGCAATAATTGTTCAGCCTCTGAATGGCGGGAGTATGAAAA-3′ | |
| rrnB2-insert-FW | 5′-CCATACTTTTCATACTCCCGCCATTCAGAGGCTGAACAATTATTG CCCGTTTTAC-3′ | |
| rrnB2-insert-RV | 5′-GCCTGATCTTTTCTTCATCTTCAAACCCATAGTGGTGGCGCATTATAGGG-3′ | |
| Seq-psp-FW | 5′-AAAATCGAGATAACCGTTGGCC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, P.-T.; Lim, D.; So, E.; Kim, H.Y.; Duysak, T.; Tran, T.-Q.; Song, M.; Jeong, J.-H.; Choy, H.E. Constitutive Expression of a Cytotoxic Anticancer Protein in Tumor-Colonizing Bacteria. Cancers 2023, 15, 1486. https://doi.org/10.3390/cancers15051486
Mai P-T, Lim D, So E, Kim HY, Duysak T, Tran T-Q, Song M, Jeong J-H, Choy HE. Constitutive Expression of a Cytotoxic Anticancer Protein in Tumor-Colonizing Bacteria. Cancers. 2023; 15(5):1486. https://doi.org/10.3390/cancers15051486
Chicago/Turabian StyleMai, Phuong-Thu, Daejin Lim, EunA So, Ha Young Kim, Taner Duysak, Thanh-Quang Tran, Miryoung Song, Jae-Ho Jeong, and Hyon E. Choy. 2023. "Constitutive Expression of a Cytotoxic Anticancer Protein in Tumor-Colonizing Bacteria" Cancers 15, no. 5: 1486. https://doi.org/10.3390/cancers15051486
APA StyleMai, P.-T., Lim, D., So, E., Kim, H. Y., Duysak, T., Tran, T.-Q., Song, M., Jeong, J.-H., & Choy, H. E. (2023). Constitutive Expression of a Cytotoxic Anticancer Protein in Tumor-Colonizing Bacteria. Cancers, 15(5), 1486. https://doi.org/10.3390/cancers15051486







