Simple Summary
The effectiveness of neoadjuvant treatment has led to an expansion of its indications from locally advanced to highly chemo-sensitive early-stage breast cancers, aiming to increase conservative treatments, in place of more invasive surgery, and to improve long term outcomes. At the same time, the continuous development of diagnostic techniques necessitates continuous updating, due to their importance in tumoral staging and in the prediction of the response to treatment, as well as in surgical planning. With our review, we sought to discuss the strengths of the various imaging modalities; in particular, the role of magnetic resonance imaging, which is still the center of scientific debate in this setting. Moreover, we analyzed the evolution of surgical approaches to breast cancer in patients treated with neoadjuvant chemotherapy.
Abstract
Neoadjuvant chemotherapy (NACT) today represents a cornerstone in the treatment of locally advanced breast cancer and highly chemo-sensitive tumors at early stages, increasing the possibilities of performing more conservative treatments and improving long term outcomes. Imaging has a fundamental role in the staging and prediction of the response to NACT, thus aiding surgical planning and avoiding overtreatment. In this review, we first examine and compare the role of conventional and advanced imaging techniques in preoperative T Staging after NACT and in the evaluation of lymph node involvement. In the second part, we analyze the different surgical approaches, discussing the role of axillary surgery, as well as the possibility of non-operative management after-NACT, which has been the subject of recent trials. Finally, we focus on emerging techniques that will change the diagnostic assessment of breast cancer in the near future.
Keywords:
neoadjuvant chemotherapy (NACT); locally advanced breast cancer (LABC); magnetic resonance imaging (MRI); breast-conservative surgery (BCS); conservative mastectomy with reconstruction (CMR); oncoplastic surgery (OPS); sentinel lymph node biopsy technique (SLNB); axillary lymphadenectomy (AL); selective axillary dissection (SAD); clipped lymph node (CL) 1. Introduction
Neoadjuvant chemotherapy (NACT) today represents a cornerstone in the treatment of locally advanced breast cancer (LABC) and highly chemo-sensitive tumors, such as the triple-negative (TN) and HER2-positive subtypes, even at early stages [1,2,3,4,5]. Indications for NACT include clinical parameters (i.e., tumor size and phenotype), lymph node involvement, and high-grade disease. NACT has a key role in reducing tumor size, increasing the possibility of performing breast-conservative surgery (BCS) over conservative mastectomy with reconstruction (CMR) [6,7], and so reducing the need for axillary lymph node dissection [2,8,9]. Another benefit of NACT is evaluating the in vivo response, to consequently allow a tailored adjuvant chemotherapy [2,10,11].
The challenge of BCS is to obtain clear margins, in order to decrease the incidence of loco-regional recurrences and, at the same time, to preserve as far as possible the healthy tissue, for the best aesthetic result [1]. Currently no specific guidelines support the choice of the best type of surgery for post-NACT patients. Some authors have tried to define which factors can influence the therapeutic choice in the neoadjuvant setting, with multifocality disease, extensive microcalcifications, and a lobular histotype predictive for mastectomy [12,13,14,15].
Even on the axillary side, surgery is now able to propose more conservative treatments instead of lymphadenectomy, even in patients node-positive at diagnosis [16,17,18].
There is, furthermore, a great variability in the response of breast cancer to NACT: an accurate evaluation of the residual disease is crucial to ensure the best assessment of patients, reducing morbidity and the necessity for further surgical procedures. Nonetheless, the detection of a pathologically complete response (pCR), strictly linked to prognosis, could help clinicians to orient towards tailored treatments [19,20,21,22,23].
For these reasons, imaging techniques have a crucial role in surgical planning in post-NACT patients, in order to predict pathological response and disease extension.
Physical examination, mammography (MX), ultrasound (US), and magnetic resonance imaging (MRI) are different techniques used to evaluate the residual tumor burden. The diagnostic accuracy of each of these is based on the ability to discriminate cancerous cells from the fibrosis resulting from biopsy procedures and chemotherapy, as well as from necrosis and fragmentation [24,25].
2. Role of Conventional Techniques in Preoperative T Staging after NACT
2.1. Mammography
The two principal features of mammography linked to a tumoral response after neoadjuvant therapies are changes in mass dimension and density, and the disappearance of microcalcifications. Unfortunately, these signs do not have a high accuracy in predicting pCR, making this technique unsuitable for preoperative staging after treatment. Some retrospective studies proved that mammography measurement had little consistency compared to pathological results in patients following NACT, with a mean concordance correlation coefficient (CCC) of only 0.55 [26,27].
In a prospective study published in 2020, the diagnostic accuracy of MX in predicting pCR post treatment was reported as having a sensitivity of 0.65, specificity of 0.81, positive predictive value (PPV) of 0.52, and negative predictive value (NPV) of 0.88; with an agreement rate of around 40% compared to histopathological assessment [28].
Mammography is a cornerstone in the diagnosis of breast cancer, thanks to its ability to detect microcalcifications; conversely, this feature is not helpful for evaluating the residual tumor burden. Kim et al. proved that the extent of the microcalcification poorly correlates with tumoral residua, because it could also characterize the NACT-induced necrosis [29]. Even if microcalcifications do not disappear post-NACT, MRI shows a better accuracy than MX [30].
Feliciano et al. emphasized that, if residual microcalcifications are found at the end of the treatment, it would be advisable to have surgery to remove them. In fact, though microcalcifications are not related to the persistence of tumoral cells for around 45% of cases, the absence of contrast enhancement in MRI imaging does not provide sufficient accuracy to avoid excision [31].
While in pre-treatment staging, mammography is recommended by the American College of Radiology (ACR) with a grade 9, as well as US and MRI; after-treatment, MX is downgraded to grade 8 and US to grade 7, contrary to MRI which remains at the highest grade [32].
In conclusion, there are multiple limitations to MX employment after NACT: MX does not adequately assess multicenter lesions; MX is not able to distinguish between lesions and changes in the tumor bed related to NACT, i.e., necrosis; microcalcifications are not useful for predicting the persistence of tumoral residua after therapy.
2.2. Ultrasound
Ultrasound has many advantages: it is a low-cost and non-invasive imaging modality that does not employ ionizing radiation. It allows the description of important tumor features such as the dimensions, morphology, and margins; and with some additional technologies, i.e., color-Doppler and elastography, it is also possible to evaluate tumor vascularization and stiffness [33]. For these reasons, the Chinese Anti-Cancer Association recommends US every two cycles of neoadjuvant treatment, in order to assess tumor response [34].
Concerning post-treatment evaluation, US is an effective technique, especially when the residual tumor is larger than 7 mm [35,36]. However, a reduction in vascular supply does not contribute to the assessment of the response [37]. In two studies, the US accuracy in predicting tumoral residual burden was 59.6% to 80%; conversely, for mammography it was 31.7% to 71% [38,39]. Keune et al. found that the correlation between the absence of lesions depicted on MX and US after treatment and pCR is of around 80% [26]. Furthermore, it is less accurate than MX for preoperatively detecting the size of ductal carcinoma in situ (DCIS) [40].
Elastography contributes to pretreatment staging with the assessment of tumor stiffness, which has been shown to be strongly correlated with tumor response; conversely, there is a lack of data for its usefulness post-treatment [41,42].
Likewise, we need further scientific evidence for the use of contrast-enhanced US (CEUS) in this field, although Cao et al. suggested that modifications in time–intensity curves could have a role in predicting tumoral response to NACT [43].
3. Role of MRI in Preoperative T Staging after NACT
The refinement of magnetic resonance technique has led to its increased use in the field of breast cancer, due to its high sensitivity and high contrast resolution. MRI actually has many indications (i.e., the screening of women with a greater lifetime risk for breast cancer [44,45], discrepancy between clinical and imaging evaluation [46], study of breast silicone implants [47]) and, in the NACT setting, has the role of staging and monitoring the response to neoadjuvant treatment [46].
Compared to physical examination and other conventional imaging techniques (MX and US), MRI has a better performance in the assessment of tumor response in breast cancer patients undergoing NACT, because of its superiority in identifying tumor residua and in predicting pathologic complete response (pCR) [43,48,49,50,51,52,53,54]. Although the latter is not strictly necessary to perform a BCS, a better tumor response is linked to a better chance of success [55,56].
According to the European Society of Breast Cancer Specialists (EUSOMA), MRI should be performed in the preoperative evaluation of women who have received a new diagnosis of breast cancer oriented towards a BCS before the first course of NACT (but MRI must not significantly delay therapy initiation), for a greater anatomical definition of the index lesion and to assess the presence of any additional cancer foci [57].
The American College of Radiology (ACR) recommends MRI as being more sensitive and more specific compared to other methods, stressing the importance of a pre-treatment MRI to better estimate volumetric changes [32].
The best timing for post-NACT breast MRI, according to EUSOMA, should be two weeks after the last NACT cycle and within two weeks before surgery (treatment delay due to post-NACT MRI should not be longer than 1 month) [57].
Evaluation of the Extent of Residual Disease
Various studies have demonstrated that MRI is the best method to assess residual malignancy, with around 90% sensitivity and from 60% to 100% of specificity, especially in multifocal and multicentric tumors, [43,48,49,50,51,52,53,54], even if sometimes it is likely to underestimate or overestimate the response [53,54,58,59,60,61].
Depending on the tumor subtype, MRI is more or less accurate: in fact, its accuracy is greater with invasive lobular carcinoma, HER2-positive, and TN tumors, and lower for the luminal A and B subtypes [62,63,64,65,66,67,68,69,70].
Pre-treatment non-mass enhancement and low nuclear grade are two other factors that affect MRI accuracy [71]. It should also be emphasized that MRI may overestimate the size of the residual tumor burden when there is an in situ component or when the response to treatment manifests as an area of fibrosis with scattered foci of contrast enhancement [72,73].
Additionally, the chemotherapeutic regimen can influence MRI features, making the evaluation more challenging; estrogen receptors (ER) modulators, antiangiogenic and taxane-containing agents may in fact lead to an underestimation [74,75].
Therefore, the ACRIN 6657 trial demonstrated that MRI was more accurate compared to other techniques (i.e., MX and physical examination), notably allowing measurement of the longest diameter of tumoral residua, which is closest to the final pathological size, and identifying pCR. Moreover, this trial concluded that the type of enhancement found at MRI, namely mass/non-mass and single or multiple, may have a great influence on the assessment; on the other side, little importance was given to the histology, presence of ductal carcinoma in situ (DCIS), and breast density at MX [76].
4. Evaluation of Lymph Node Involvement
The evaluation of node involvement is crucial for a correct assessment and must be carried out both at the time of diagnosis and after the conclusion of neoadjuvant treatment.
This valuation is important, even though imaging techniques do not allow the identification of “isolated tumor cells” (small aggregates of cells no larger than 0.2 mm or single cancer cells or a small clump of cells with less than 200 cells in one single histological section, pN0i+) or “micrometastases” (aggregate of contiguous tumor cells larger than 0.2 mm and/or more than 200 cells, but not larger than 2 mm, pNmi) [77,78], considering that these two conditions do not affect survival [79,80]. Although US is an operator-dependent method, at baseline, it is a very reliable imaging technique for the evaluation of the axilla, with a specificity of 88–98% and sensibility of 26–76% [81], due to its high resolution for evaluation of changes in the cortical zone of lymph nodes [82], and for the assessment of Berg levels I and II [32]; US also allows the realization of diagnostic insight into nodes with features of malignancy, guiding the execution of biopsies (fine needle aspiration or core biopsy).
Meanwhile, MRI is necessary for a better characterization of Berg level III, internal mammary chains, supraclavicular lymph nodes, and for a comparison between the two axillary regions [32,83,84].
After neoadjuvant treatment, ultrasound was confirmed to be the best technique to evaluate the response and residual axillary disease, with around 70% sensitivity [85]. Alvarado and colleagues, in a study conducted on 150 women, observed that a normalized morphology post-NACT in previously node-positive disease is linked to better pathological response rates [86].
Shear wave elastography can be an additional evaluation tool to support ultrasound in the assessment of axillary status after NACT; in fact, a study conducted on 201 patients with pathologically-proven node-positive breast cancer suggested that the combination of the two imaging modalities can improve both the sensitivity and accuracy [87].
Some studies evaluated the accuracy of MRI in assessing lymph node status in patients treated with NACT; however, with discordant results, predominantly related to tumor histotype and molecular characteristics. Abel et al. conducted a retrospective study of a patient population with invasive lobular carcinoma (ILC) demonstrating that the accuracy of lymph node assessment was low compared to patients with invasive ductal carcinoma (IDC) [88]. In 2021, Samiei et al. performed a systematic review and metanalysis to compare the diagnostic performance of the different imaging techniques for the evaluation of axillary lymph node response after NACT in node-positive patients. Thirteen studies were included, representing a total of 2380 analyzed patients. Their conclusion was that ultrasound and MRI are limited for this assessment, identifying axillary residual disease in 77% and 78% and pCR in 50% and 58%, respectively [89].
For these reasons, if nodes were metastatic at baseline, it is recommended to perform a sentinel node biopsy or axillary node dissection [16,85,90].
5. Surgical Approach after NACT
5.1. Breast Surgery: Conserving Therapy or Not?
Surgery must be adapted to the response to neoadjuvant treatment and may consist of a total mastectomy, oncoplastic surgery, or breast-conservative surgery.
Over the years, the assessment of early-stage breast cancer has changed; initially, the first-line treatment was represented by mastectomy. For about 30 years, however, thanks to numerous randomized controlled trials and meta-analysis, [91,92,93,94,95,96], BCS has proven to be a reliable alternative, due to an equivalent long-term survival between the two treatments.
The aim of BCS is to achieve clear margins, to lower the risk of loco-regional disease, while achieving the best aesthetic outcome and preserving healthy tissue [1].
The risk of recurrence is high when the excision regards only the primary tumor. Various randomized controlled trials have demonstrated that radiation therapy affects the risk of local recurrence when associated with BCS: in 2005, the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) estimated that there was a 70% proportional reduction in loco-regional recurrences compared with BCS alone, with a 10-year risk of approximately 10%, and a minimal statistically significant reduction in mortality for women who received radiation therapy [97]. Moreover, an improvement in disease-free interval, as well as in overall survival, is obtained with the addition of adjuvant radiotherapy or hormonal therapy in the assessment of patients with early breast cancer.
Due to the large amount of data in the literature that supporting the safety of BCS over mastectomy in the treatment of breast cancer, women can choose between treatments, even following NACT [98,99,100].
Before choosing a breast-conserving treatment after neoadjuvant therapy, multiple clinical and histological criteria must be carefully evaluated, to ensure that the patient can be subjected to treatment without entailing an increased risk of loco-regional recurrence of disease; in particular, treatment should be proposed considering the size of the residual tumor, possible multifocality, the extension of suspicious microcalcifications associated with in situ carcinoma diagnosed on the biopsy, the volume ratio of the residual tumor to breast volume, and the localization of the tumor [98,99,101,102,103,104,105,106] (Figure 1). Straver et al., in a study conducted on 208 women, noticed that patients where better suited for BCS then mastectomy when MRI depicted a maximum size of the lesion not exceeding 30 mm on pretreatment, a dimensional reduction after treatment, and in HER2-positive and triple-negative subtypes [60].
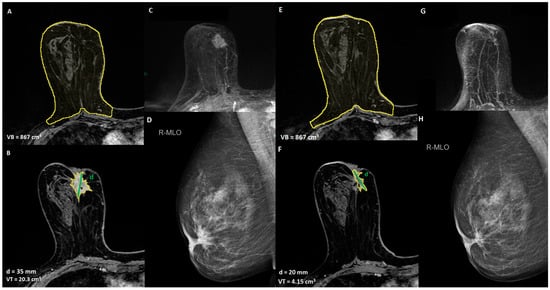
Figure 1.
A 52-year-old women with unifocal HER2 positive invasive ductal carcinoma, infiltrating the nipple areola complex, with indication of breast-conservative surgery after NACT: (A,B) T1-weighted post-contrast MRI to define the extension of the disease and breast volume, VB = breast volume, VT = tumor volume, d = diameter of the lesion; (C) maximum intensity projection (MIP) pre-NACT image; (D) mediolateral oblique mammogram of the lesion, (E,F) T1-weighted post-contrast MRI to assess the response to NACT, (G) maximum intensity projection (MIP) post-NACT image, (H) mediolateral oblique mammogram of the lesion post-NACT.
In order to optimize oncological and aesthetic outcomes in patients with large or multifocal tumors desiring breast conservation, oncoplastic surgery (OPS) is an option. The indication for OPS is a non-optimal response after NACT, for which a BCS with safe margins would either seem impossible or lead to major deformity [107]. Mastectomy remains indicated in patients with multicentric disease, widespread microcalcifications, or pathogenic variants of BRCA 1/2 genes [108,109,110] (Figure 2).
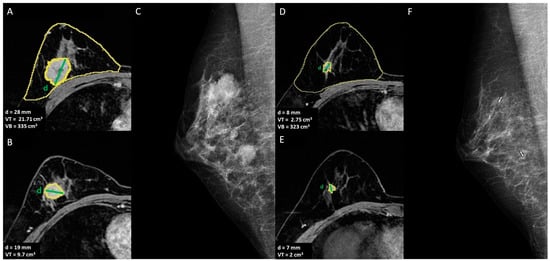
Figure 2.
A 42-year-old women with multicentric invasive TN ductal carcinoma with indication of mastectomy after NACT: (A,B) T1-weighted post-contrast MRI to define the extension of the disease and breast volume, VB = breast volume, VT = tumor volume, d = diameter of the lesion; (C) mediolateral oblique mammogram shows the two lesions, (D,E) T1-weighted post-contrast MRI to assess the response to NACT, (F) mediolateral oblique mammogram of the two lesions post-NACT.
Although MRI is widely used to stage breast cancer in countries with a developed health care system, the current literature does not have a unitary view on the benefit in defining surgical planning brought by its routine preoperative use [111,112]. The fear is that MRI can overestimate the extent of the disease, leading to an increased number of unnecessary mastectomies [113]. In this regard, although NACT patients were excluded from the updated studies, it was demonstrated that preoperative MRI leads to a reduction in the rate of reoperation after conserving surgery, despite a slight increase in the rate of mastectomies [114,115].
If surgical margins are proven to be involved by the tumor in the pathological examination of resected specimens, it may be necessary for the patient to undergo re-excision surgery and, subsequently, radiotherapy. Mastectomy should be performed if clean margins cannot be obtained. A meta-analysis conducted in 2016 on eight trials, with a total of 3215 patients analyzed, stated that breast conserving surgery after NACT showed no significant difference in terms of the prevalence of local recurrence and five-year local recurrence-free survival rate when compared to mastectomy, thus allowing the possibility of performing a more conservative treatment without a loss in oncological outcomes [100]. Furthermore, conservative treatment is linked to greater aesthetic satisfaction of patients and less psycho-social morbidity [116,117,118].
Consequently, the choice between treatments must be made carefully on a case-by-case basis and must always aim to improve the patient’s outcome.
For these reasons, imaging, and in particular MRI, has a large role in the staging before and after neoadjuvant treatment, thus leading the surgical planning.
5.2. Axillary Surgery: Sentinel Lymph Node Biopsy, Lymphadenectomy, or Selective Axillary Dissection?
Axillary lymph node metastasis is an important prognostic factor in breast cancer and guides treatment planning.
At present, women with node-positive breast cancer often undergo NACT, which leads to elimination of lymph nodal disease in 40–70% of cases [119]. On the other hand, in women with clinically negative nodes pre-NACT, the use of the sentinel lymph node biopsy technique (SLNB) can accurately predict the axillary status in the post-treatment assessment [120,121,122]. The aim of SLNB is to overcome the standard surgical approach of axillary lymphadenectomy (AL) in node-positive breast cancer patients at diagnosis, preventing complications from the most radical surgical techniques [123,124,125].
Nevertheless, in this last cohort of patients, SLNB was proven to be not accurate in restaging axilla and, therefore, in selecting patients with complete lymph node disease regression after NACT, with a false negative rate (FNR) of 12.6–24.3 % [126,127]. To lower FNR, it is necessary to remove one or more lymph nodes in addition to sentinel lymph nodes; the greater the removal, the lesser the FNR [128]. The surgeon selects the lymph nodes to be removed by assessing their macroscopic and clinical characteristics during surgery, as well as their proximity to the sentinel lymph node. To better guide surgeon’s decision of which lymph node to remove beyond the sentinel nodes, several studies have proposed different modalities to help refine SLNB accuracy in the post-NACT setting: mandatory use of immunohistochemistry [127]; use of a dual mapping technique (both blue dye and radiolabeled colloid mapping agents) [16,126]; or the placement, before beginning of NACT, of a clip in the axillary node, which is proven to be metastatic at a core needle biopsy (CNB) [129,130,131,132]. In about 20% of patients, such a metastatic lymph node is not the same as the sentinel lymph nodes, because NACT can determine a modification of lymphatic drainage from breast neoplasm [129,131]. Therefore, if after NACT the clipped metastatic lymph node is not the same as the sentinel lymph nodes, it can be removed during surgery and histologically analyzed. The removal of the clipped lymph node (CL) was proved to reduce FNR of SLNB [131]; even in a subgroup analysis of the cohort of the ACOSOG Z1071 trial [129], patients with a metastatic clipped axillary node had less FNR than those without a clip. It has also been proposed that the SLNB associated with the removal of CL, the so called “selective axillary dissection” (SAD) (Figure 3), can therefore enable the selection of the lymph nodes, to allow analysis in a more correct and repeatable way, increasing the accuracy in the evaluation of lymph nodes pathological response to NACT; this allows the selection of patients who can safely avoid AL, with a significant positive effect on their quality of life [133].
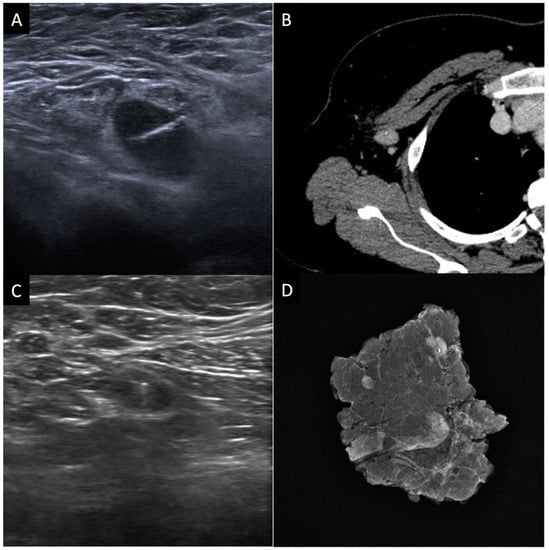
Figure 3.
Selective axillary dissection technique (SAD): (A) ultrasound image of a clip marker placed in a histologically confirmed metastatic axillary lymph node before the beginning of NACT; (B) axial contrast enhanced CT image shows the clipped lymph node (CL); (C) ultrasound evaluation and identification of the CL before surgery; (D) surgical specimen radiograph to ensure that the CL has been removed along with the other lymph nodes.
5.3. Surgery Omission after NACT
Due to the constant progress in neoadjuvant therapies and imaging techniques, the possibility of omitting surgery in breast cancers is now being considered as a viable alternative.
Approximately 19% of patients treated with neoadjuvant chemotherapy achieve a pCR, even if this probability varies between the different subtypes: for hormone-positive tumors it is 8.3%, for HER2+/hormone-positive tumors it is 18.7%, for TN it is 31.1% and for HER2+/hormone negative it is 38.9% [134].
In patients with an excellent response to neoadjuvant chemotherapy, it has long been proposed to avoid surgery in favor of a radiation therapy alone, with disappointing results in terms of loco-regional recurrence (21–47%) [135,136,137,138,139]. However, these studies had suboptimal methodologies: selection of patients based on clinical response alone, lack of selection based on tumor subtypes, and no use of a radiological guidance biopsy to document the pathological response. In particular, a major obstacle to the possibility of omitting surgery is related to the suboptimal specificity of the imaging methods in predicting sufficiently accurately the absence of residual disease after neoadjuvant chemotherapy, and therefore today surgery remains indispensable for verifying the response to the NACT in the surgical specimen [53,139,140,141].
Multiple clinical feasibility trials were performed or are currently ongoing all around the world to solve this problem: their goal is to identify key elements to guarantee the accuracy and safety for patients selected for non-operative management [142,143,144,145,146,147,148]. These include the possibility of sampling residual abnormalities thanks to image-guided percutaneous biopsy, which helps to evaluate tumoral response after-treatment [149]. The main question remains which is the best technique or combination of techniques to combine with vacuum-assisted core biopsy (VACB), to reduce false-negative rates and to augment negative predictive values. If these studies lead to the expected results, they will probably induce a drastic change in the way we manage breast cancer after NACT, both from a therapeutic and diagnostic point of view.
6. New Perspectives: Ultrafast Breast MRI, Contrast-Enhancement Mammography, Radiomics, and Machine Learning
Ultrafast MRI is an emerging technique that is increasingly used in clinical practice, as the first studies carried out on its non-inferiority are very promising. Its goal is to reveal the early wash-in of contrast material at high temporal resolution, usually less than 6–7 s. Unlike conventional kinetic curves, these new sequences allow obtaining early wash-in kinetic curves through rapid sequential imaging taken in the first 120 s after contrast injection [150].
A limit to the widespread use of ultrafast MRI is the need for specific coils and sequences that enable a high temporal resolution in parallel to a diagnostic spatial resolution. The strength of this technique is its ability to detect the early contrast enhancement that characterizes breast cancers [151].
A prospective study, published in 2022 and conducted on 50 patients that underwent neoadjuvant therapy, demonstrated that the wash-in slope at initial ultrafast DCE-MRI can be used as a predictive factor of pCR, reporting a sensitivity and specificity of 94% and 59%, respectively (WIS cut-off value equal to 1.6% per second) [152].
Moreover, a recently published Korean study investigated the association between kinetic features obtained from ultrafast MRI and pCR in 256 women with invasive breast cancer undergoing NACT and surgery, discovering an independent association between a higher volume ratio between two different time points of lesion enhancement and pCR in TN tumors [153].
Though there is still little data in the current literature, some recent studies confirmed that the diagnostic performance of contrast-enhanced mammography (CEM) in the valuation of pathological response after neoadjuvant therapies is very encouraging.
Moustafa and colleagues, in 2019, also evaluated the use of a quantitative mathematical objective tool for this purpose, in comparison to RECIST 1.1 criteria and a subjective visual analysis, on 42 women: this tool was effective for assessing dimensional changes, but also for obtaining information about the constitutional differences of tumor residua after NACT and to eliminate bias in the evaluation [154].
In a prospective study conducted in 2017, the reported specificity, sensitivity, NPV, and PPV in the prediction of the response to therapy depicted by CEM were 91%, 40%, 80%, and 62.5%, respectively, with a sensitivity and specificity for complete response of 100% and 83% [155].
Promising results were also obtained when CEM was compared with MRI; in fact, a meta-analysis conducted on 24 studies in 2020 concluded that the two imaging techniques have an equal specificity, whilst CEM has a better sensitivity than MRI [156].
Radiomics represents the future of imaging, and the focus of the scientific community on these new technologies is increasing, including on breast cancer, i.e., with several studies whose purpose is to explore their applications in differential diagnosis or prognosis [157,158,159].
Some interesting studies have also been performed to predict the tumor response after neoadjuvant treatment through imaging omics, with surprising results. For this purpose, Zhuang et al. established a nomogram able to guide therapeutic decisions, thanks to an analysis based on multiparametric MRI radiomics combined with clinico-pathological factors [160].
A multicenter study published in 2019 showed that a radiomic signature based on multiparametric MRI and combinations of sequences achieved a higher AUC (0.79) compared with a single-sequence model. It also showed good results in hormone receptor positive, HER2-negative, and triple negative tumors [161].
Cain et al. studied a multivariate model based on machine learning and able to obtain features to predict pCR after NACT on pre-treatment DCE-MRI, in patients with HER2 over-expressing and triple negative cancers [162].
Although MRI parameters have been the most studied, other techniques also show encouraging results, i.e., as demonstrated by a study by Antunovic et al. on positron emission tomography (PET)/CT-based models [163].
7. Conclusions
The ability of imaging to predict tumoral response and to assess residual burden is crucial in delivering tailored treatments to women with breast cancer undergoing neoadjuvant therapies. This entails the reduction of unnecessary treatments and associated toxicity, and the orientation towards conservative surgical treatment, to achieve the best oncological outcome. Knowledge is improving so quickly that important information on the biological characteristics of tumors can already be obtained, thanks to the current advanced imaging technologies, of which MRI is the most representative. Further new technologies, while being at the initial stages, are providing encouraging results in this field and will surely change the assessment of these patients.
Author Contributions
Conceptualization, M.C. and F.M.; methodology, M.C. and F.M.; formal analysis, M.C. and F.M.; investigation M.C. and F.M.; resources, M.C., F.M., E.G. and G.R.; data curation, M.C. and F.M.; writing—original draft preparation, M.C. and F.M.; writing—review and editing, M.C., F.M. and P.B.; supervision, E.B., A.D., C.P., V.D.P., G.C., S.P. and P.B.; project administration, P.B. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by Fondazione Policlinico Agostino Gemelli—IRCCS.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors want to thank Fondazione Policlinico Agostino Gemelli—IRCCS for its support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the Benefits of Therapy for Early-Stage Breast Cancer: The St. Gallen International Consensus Guidelines for the Primary Therapy of Early Breast Cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef]
- Puig, C.A.; Hoskin, T.L.; Day, C.N.; Habermann, E.B.; Boughey, J.C. National Trends in the Use of Neoadjuvant Chemotherapy for Hormone Receptor-Negative Breast Cancer: A National Cancer Data Base Study. Ann. Surg. Oncol. 2017, 24, 1242–1250. [Google Scholar] [CrossRef]
- Killelea, B.K.; Yang, V.Q.; Mougalian, S.; Horowitz, N.R.; Pusztai, L.; Chagpar, A.B.; Lannin, D.R. Neoadjuvant Chemotherapy for Breast Cancer Increases the Rate of Breast Conservation: Results from the National Cancer Database. J. Am. Coll. Surg. 2015, 220, 1063. [Google Scholar] [CrossRef]
- Mougalian, S.S.; Soulos, P.R.; Killelea, B.K.; Lannin, D.R.; Abu-Khalaf, M.M.; DiGiovanna, M.P.; Sanft, T.B.; Pusztai, L.; Gross, C.P.; Chagpar, A.B. Use of Neoadjuvant Chemotherapy for Patients with Stage I to III Breast Cancer in the United States. Cancer 2015, 121, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Di Leone, A.; Natale, M.; Sanchez, M.A.; Masett, R. Conservative Surgery after Neoadjuvant Chemotherapy in Patients with Operable Breast Cancer. Ann. Ital. Chir. 2018, 89, 290. [Google Scholar] [PubMed]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Parmar, V.; Krishnamurthy, A.; Hawaldar, R.; Nadkarni, M.S.; Sarin, R.; Chinoy, R.; Nair, R.; Dinshaw, K.A.; Badwe, R.A. Breast Conservation Treatment in Women with Locally Advanced Breast Cancer—Experience from a Single Centre. Int. J. Surg. Lond. Engl. 2006, 4, 106–114. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G. Preoperative Therapy: What, When and for Whom? Ann. Oncol. 2008, 19, v113–v116. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.K.; Yi, M.; Mittendorf, E.A.; Guerrero, C.; Babiera, G.V.; Bedrosian, I.; Hwang, R.F.; Kuerer, H.M.; Ross, M.I.; Meric-Bernstam, F. Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy Is Accurate and Reduces the Need for Axillary Dissection in Breast Cancer Patients. Ann. Surg. 2009, 250, 558–564. [Google Scholar] [CrossRef]
- Cen, C.; Chun, J.; Kaplowitz, E.; Axelrod, D.; Shapiro, R.; Guth, A.; Schnabel, F. Margin Assessment and Re-Excision Rates for Patients Who Have Neoadjuvant Chemotherapy and Breast-Conserving Surgery. Ann. Surg. Oncol. 2021, 28, 5142–5148. [Google Scholar] [CrossRef]
- Margolese, R.G. Surgical Considerations in Preoperative Chemotherapy of Breast Cancer. Recent Results Cancer Res. Fortschr. Krebsforsch. Prog. Dans Rech. Sur. Cancer 1998, 152, 193–201. [Google Scholar] [CrossRef]
- Kaufmann, M.; von Minckwitz, G.; Mamounas, E.P.; Cameron, D.; Carey, L.A.; Cristofanilli, M.; Denkert, C.; Eiermann, W.; Gnant, M.; Harris, J.R.; et al. Recommendations from an International Consensus Conference on the Current Status and Future of Neoadjuvant Systemic Therapy in Primary Breast Cancer. Ann. Surg. Oncol. 2012, 19, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Cirrincione, C.T.; Sikov, W.M.; Carey, L.A.; Berry, D.A.; Overmoyer, B.; Henry, N.L.; Somlo, G.; Port, E.; Burstein, H.J.; et al. Impact of Neoadjuvant Therapy on Eligibility for and Frequency of Breast Conservation in Stage II-III HER2-Positive Breast Cancer: Surgical Results of CALGB 40601 (Alliance). Breast Cancer Res. Treat. 2016, 160, 297–304. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Newman, L.A.; Buzdar, A.U.; Hunt, K.K.; Dhingra, K.; Buchholz, T.A.; Binkley, S.M.; Ames, F.C.; Feig, B.W.; Ross, M.I.; et al. Residual Metastatic Axillary Lymph Nodes Following Neoadjuvant Chemotherapy Predict Disease-Free Survival in Patients with Locally Advanced Breast Cancer. Am. J. Surg. 1998, 176, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Suman, V.J.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Leitch, A.M.; Kuerer, H.M.; Bowling, M.; Flippo-Morton, T.S.; et al. Sentinel Lymph Node Surgery after Neoadjuvant Chemotherapy in Patients with Node-Positive Breast Cancer: The ACOSOG Z1071 (Alliance) Clinical Trial. JAMA 2013, 310, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Foy, M.; Cox, D.D.; Kuerer, H.M.; Hunt, K.K.; Cormier, J.N. Meta-Analysis of Sentinel Lymph Node Biopsy after Preoperative Chemotherapy in Patients with Breast Cancer. Br. J. Surg. 2006, 93, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Balch, G.C.; Mithani, S.K.; Richards, K.R.; Beauchamp, R.D.; Kelley, M.C. Lymphatic Mapping and Sentinel Lymphadenectomy after Preoperative Therapy for Stage II and III Breast Cancer. Ann. Surg. Oncol. 2003, 10, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Bryant, J.; Wolmark, N.; Mamounas, E.; Brown, A.; Fisher, E.R.; Wickerham, D.L.; Begovic, M.; DeCillis, A.; Robidoux, A.; et al. Effect of Preoperative Chemotherapy on the Outcome of Women with Operable Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 2672–2685. [Google Scholar] [CrossRef]
- Bonadonna, G.; Valagussa, P.; Brambilla, C.; Ferrari, L.; Moliterni, A.; Terenziani, M.; Zambetti, M. Primary Chemotherapy in Operable Breast Cancer: Eight-Year Experience at the Milan Cancer Institute. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 93–100. [Google Scholar] [CrossRef]
- Buchholz, T.A.; Hill, B.S.; Tucker, S.L.; Frye, D.K.; Kuerer, H.M.; Buzdar, A.U.; McNeese, M.D.; Singletary, S.E.; Ueno, N.T.; Pusztai, L.; et al. Factors Predictive of Outcome in Patients with Breast Cancer Refractory to Neoadjuvant Chemotherapy. Cancer J. Sudbury Mass 2001, 7, 413–420. [Google Scholar]
- Kuerer, H.M.; Newman, L.A.; Smith, T.L.; Ames, F.C.; Hunt, K.K.; Dhingra, K.; Theriault, R.L.; Singh, G.; Binkley, S.M.; Sneige, N.; et al. Clinical Course of Breast Cancer Patients with Complete Pathologic Primary Tumor and Axillary Lymph Node Response to Doxorubicin-Based Neoadjuvant Chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Symmans, W.F.; Peintinger, F.; Hatzis, C.; Rajan, R.; Kuerer, H.; Valero, V.; Assad, L.; Poniecka, A.; Hennessy, B.; Green, M.; et al. Measurement of Residual Breast Cancer Burden to Predict Survival after Neoadjuvant Chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 4414–4422. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic Accuracy of Mammography, Clinical Examination, US, and MR Imaging in Preoperative Assessment of Breast Cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef]
- Weatherall, P.T.; Evans, G.F.; Metzger, G.J.; Saborrian, M.H.; Leitch, A.M. MRI vs. Histologic Measurement of Breast Cancer Following Chemotherapy: Comparison with x-Ray Mammography and Palpation. J. Magn. Reson. Imaging JMRI 2001, 13, 868–875. [Google Scholar] [CrossRef]
- Keune, J.D.; Jeffe, D.B.; Schootman, M.; Hoffman, A.; Gillanders, W.E.; Aft, R.L. Accuracy of Ultrasonography and Mammography in Predicting Pathologic Response after Neoadjuvant Chemotherapy for Breast Cancer. Am. J. Surg. 2010, 199, 477–484. [Google Scholar] [CrossRef]
- Leddy, R.; Irshad, A.; Metcalfe, A.; Mabalam, P.; Abid, A.; Ackerman, S.; Lewis, M. Comparative Accuracy of Preoperative Tumor Size Assessment on Mammography, Sonography, and MRI: Is the Accuracy Affected by Breast Density or Cancer Subtype? J. Clin. Ultrasound JCU 2016, 44, 17–25. [Google Scholar] [CrossRef]
- Skarping, I.; Förnvik, D.; Heide-Jørgensen, U.; Rydén, L.; Zackrisson, S.; Borgquist, S. Neoadjuvant Breast Cancer Treatment Response; Tumor Size Evaluation through Different Conventional Imaging Modalities in the NeoDense Study. Acta Oncol. Stockh. Swed. 2020, 59, 1528–1537. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Chang, J.M.; Moon, H.-G.; Lee, J.; Shin, S.U.; Moon, W.K. Residual Mammographic Microcalcifications and Enhancing Lesions on MRI After Neoadjuvant Systemic Chemotherapy for Locally Advanced Breast Cancer: Correlation with Histopathologic Residual Tumor Size. Ann. Surg. Oncol. 2016, 23, 1135–1142. [Google Scholar] [CrossRef]
- Um, E.; Kang, J.-W.; Lee, S.; Kim, H.J.; Yoon, T.I.; Sohn, G.; Chung, I.Y.; Kim, J.; Lee, J.W.; Son, B.H.; et al. Comparing Accuracy of Mammography and Magnetic Resonance Imaging for Residual Calcified Lesions in Breast Cancer Patients Undergoing Neoadjuvant Systemic Therapy. Clin. Breast Cancer 2018, 18, e1087–e1091. [Google Scholar] [CrossRef]
- Feliciano, Y.; Mamtani, A.; Morrow, M.; Stempel, M.M.; Patil, S.; Jochelson, M.S. Do Calcifications Seen on Mammography After Neoadjuvant Chemotherapy for Breast Cancer Always Need to Be Excised? Ann. Surg. Oncol. 2017, 24, 1492–1498. [Google Scholar] [CrossRef]
- Expert Panel on Breast Imaging; Slanetz, P.J.; Moy, L.; Baron, P.; diFlorio, R.M.; Green, E.D.; Heller, S.L.; Holbrook, A.I.; Lee, S.-J.; Lewin, A.A. ACR Appropriateness Criteria® Monitoring Response to Neoadjuvant Systemic Therapy for Breast Cancer. J. Am. Coll. Radiol. JACR 2017, 14, S462–S475. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamamoto, Y.; Iwase, H. Clinical Imaging for the Prediction of Neoadjuvant Chemotherapy Response in Breast Cancer. Chin. Clin. Oncol. 2020, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Yu, Z.-G. Chinese Society of Breast Surgery Clinical Practice Guidelines for Pre-Operative Evaluation of Breast Cancer: Chinese Society of Breast Surgery (CSBrS) Practice Guidelines 2021. Chin. Med. J. 2021, 134, 2147–2149. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, L.; Balu-Maestro, C.; Leclère, J. Imaging in Evaluation of Response to Neoadjuvant Breast Cancer Treatment. Cancer Imaging 2005, 5, 27–31. [Google Scholar] [CrossRef]
- Roubidoux, M.A.; LeCarpentier, G.L.; Fowlkes, J.B.; Bartz, B.; Pai, D.; Gordon, S.P.; Schott, A.F.; Johnson, T.D.; Carson, P.L. Sonographic Evaluation of Early-Stage Breast Cancers That Undergo Neoadjuvant Chemotherapy. J. Ultrasound Med. 2005, 24, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Tardivon, A.A.; Ollivier, L.; El Khoury, C.; Thibault, F. Monitoring Therapeutic Efficacy in Breast Carcinomas. Eur. Radiol. 2006, 16, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Croshaw, R.; Shapiro-Wright, H.; Svensson, E.; Erb, K.; Julian, T. Accuracy of Clinical Examination, Digital Mammogram, Ultrasound, and MRI in Determining Postneoadjuvant Pathologic Tumor Response in Operable Breast Cancer Patients. Ann. Surg. Oncol. 2011, 18, 3160. [Google Scholar] [CrossRef] [PubMed]
- Heusinger, K.; Löhberg, C.; Lux, M.P.; Papadopoulos, T.; Imhoff, K.; Schulz-Wendtland, R.; Beckmann, M.W.; Fasching, P.A. Assessment of Breast Cancer Tumor Size Depends on Method, Histopathology and Tumor Size Itself*. Breast Cancer Res. Treat. 2005, 94, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Wang, H.; Yang, X.; Chang, L.; Li, Q. Comparison of Mammography and Ultrasonography for Tumor Size of DCIS of Breast Cancer. Curr. Med. Imaging Rev. 2019, 15, 209–213. [Google Scholar] [CrossRef]
- Evans, A.; Armstrong, S.; Whelehan, P.; Thomson, K.; Rauchhaus, P.; Purdie, C.; Jordan, L.; Jones, L.; Thompson, A.; Vinnicombe, S. Can Shear-Wave Elastography Predict Response to Neoadjuvant Chemotherapy in Women with Invasive Breast Cancer? Br. J. Cancer 2013, 109, 2798–2802. [Google Scholar] [CrossRef]
- Hayashi, M.; Yamamoto, Y.; Ibusuki, M.; Fujiwara, S.; Yamamoto, S.; Tomita, S.; Nakano, M.; Murakami, K.; Iyama, K.; Iwase, H. Evaluation of Tumor Stiffness by Elastography Is Predictive for Pathologic Complete Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer. Ann. Surg. Oncol. 2012, 19, 3042–3049. [Google Scholar] [CrossRef]
- Cao, X.; Xue, J.; Zhao, B. Potential Application Value of Contrast-Enhanced Ultrasound In Neoadjuvant Chemotherapy of Breast Cancer. Ultrasound Med. Biol. 2012, 38, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.J.; Lei, W.; Craft, P.; Cawson, J.N.; Morris, I.; Walleser, S.; Griffiths, A.; Parker, S.; Houssami, N. A Systematic Review of the Effectiveness of Magnetic Resonance Imaging (MRI) as an Addition to Mammography and Ultrasound in Screening Young Women at High Risk of Breast Cancer. Eur. J. Cancer 2007, 43, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Saslow, D.; Boetes, C.; Burke, W.; Harms, S.; Leach, M.O.; Lehman, C.D.; Morris, E.; Pisano, E.; Schnall, M.; Sener, S.; et al. American Cancer Society Guidelines for Breast Screening with MRI as an Adjunct to Mammography. CA Cancer J. Clin. 2007, 57, 75–89. [Google Scholar] [CrossRef]
- Turnbull, L.W. Dynamic Contrast-Enhanced MRI in the Diagnosis and Management of Breast Cancer. NMR Biomed. 2009, 22, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Lo, L.W.; Fung, P.Y.E.; Lai, H.Y.M.; She, H.L.H.; Ng, W.K.C.; Kwok, K.M.K.; Lee, C.M. Magnetic Resonance Imaging of Breast Augmentation: A Pictorial Review. Insights Imaging 2016, 7, 399–410. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Prevos, R.; Smidt, M.; Tjan-Heijnen, V.C.G.; van Goethem, M.; Schipper, R.; Beets-Tan, R.G.; Wildberger, J.E. The Role of Magnetic Resonance Imaging in Assessing Residual Disease and Pathologic Complete Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy: A Systematic Review. Insights Imaging 2013, 4, 163–175. [Google Scholar] [CrossRef]
- Chagpar, A.B.; Middleton, L.P.; Sahin, A.A.; Dempsey, P.; Buzdar, A.U.; Mirza, A.N.; Ames, F.C.; Babiera, G.V.; Feig, B.W.; Hunt, K.K.; et al. Accuracy of Physical Examination, Ultrasonography, and Mammography in Predicting Residual Pathologic Tumor Size in Patients Treated With Neoadjuvant Chemotherapy. Ann. Surg. 2006, 243, 257–264. [Google Scholar] [CrossRef]
- Belli, P.; Costantini, M.; Malaspina, C.; Magistrelli, A.; LaTorre, G.; Bonomo, L. MRI Accuracy in Residual Disease Evaluation in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Clin. Radiol. 2006, 61, 946–953. [Google Scholar] [CrossRef]
- Hollingsworth, A.B.; Stough, R.G.; O’Dell, C.A.; Brekke, C.E. Breast Magnetic Resonance Imaging for Preoperative Locoregional Staging. Am. J. Surg. 2008, 196, 389–397. [Google Scholar] [CrossRef]
- Semiglazov, V. RECIST for Response (Clinical and Imaging) in Neoadjuvant Clinical Trials in Operable Breast Cancer. JNCI Monogr. 2015, 2015, 21–23. [Google Scholar] [CrossRef]
- Marinovich, M.L.; Macaskill, P.; Irwig, L.; Sardanelli, F.; Mamounas, E.; von Minckwitz, G.; Guarneri, V.; Partridge, S.C.; Wright, F.C.; Choi, J.H.; et al. Agreement between MRI and Pathologic Breast Tumor Size after Neoadjuvant Chemotherapy, and Comparison with Alternative Tests: Individual Patient Data Meta-Analysis. BMC Cancer 2015, 15, 662. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.; Slanetz, P.; Kopans, D.B.; Rafferty, E.; Georgian-Smith, D.; Moy, L.; Halpern, E.; Moore, R.; Kuter, I.; Taghian, A. Prospective Comparison of Mammography, Sonography, and MRI in Patients Undergoing Neoadjuvant Chemotherapy for Palpable Breast Cancer. AJR Am. J. Roentgenol. 2005, 184, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Sobrido, C. Neoadjuvant Approach in Patients with Early Breast Cancer: Patient Assessment, Staging, and Planning. Breast Edinb. Scotl. 2022, 62 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- van la Parra, R.F.D.; Clough, K.B.; Thygesen, H.H.; Levy, E.; Poulet, B.; Sarfati, I.; Nos, C. Oncological Safety of Oncoplastic Level II Mammoplasties After Neoadjuvant Chemotherapy for Large Breast Cancers: A Matched-Cohort Analysis. Ann. Surg. Oncol. 2021, 28, 5920–5928. [Google Scholar] [CrossRef]
- Sardanelli, F.; Boetes, C.; Borisch, B.; Decker, T.; Federico, M.; Gilbert, F.J.; Helbich, T.; Heywang-Köbrunner, S.H.; Kaiser, W.A.; Kerin, M.J.; et al. Magnetic Resonance Imaging of the Breast: Recommendations from the EUSOMA Working Group. Eur. J. Cancer 2010, 46, 1296–1316. [Google Scholar] [CrossRef]
- Partridge, S.C.; Gibbs, J.E.; Lu, Y.; Esserman, L.J.; Sudilovsky, D.; Hylton, N.M. Accuracy of MR Imaging for Revealing Residual Breast Cancer in Patients Who Have Undergone Neoadjuvant Chemotherapy. AJR Am. J. Roentgenol. 2002, 179, 1193–1199. [Google Scholar] [CrossRef]
- Rosen, E.L.; Blackwell, K.L.; Baker, J.A.; Soo, M.S.; Bentley, R.C.; Yu, D.; Samulski, T.V.; Dewhirst, M.W. Accuracy of MRI in the Detection of Residual Breast Cancer after Neoadjuvant Chemotherapy. AJR Am. J. Roentgenol. 2003, 181, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Straver, M.E.; Loo, C.E.; Rutgers, E.J.T.; Oldenburg, H.S.A.; Wesseling, J.; Vrancken Peeters, M.-J.T.F.D.; Gilhuijs, K.G.A. MRI-Model to Guide the Surgical Treatment in Breast Cancer Patients after Neoadjuvant Chemotherapy. Ann. Surg. 2010, 251, 701–707. [Google Scholar] [CrossRef]
- Vriens, B.E.P.J.; de Vries, B.; Lobbes, M.B.I.; van Gastel, S.M.; van den Berkmortel, F.W.P.J.; Smilde, T.J.; van Warmerdam, L.J.C.; de Boer, M.; van Spronsen, D.J.; Smidt, M.L.; et al. Ultrasound Is at Least as Good as Magnetic Resonance Imaging in Predicting Tumour Size Post-Neoadjuvant Chemotherapy in Breast Cancer. Eur. J. Cancer 2016, 52, 67–76. [Google Scholar] [CrossRef]
- Chen, J.-H.; Bahri, S.; Mehta, R.S.; Carpenter, P.M.; McLaren, C.E.; Chen, W.-P.; Fwu, P.T.; Hsiang, D.J.B.; Lane, K.T.; Butler, J.A.; et al. Impact of Factors Affecting the Residual Tumor Size Diagnosed by MRI Following Neoadjuvant Chemotherapy in Comparison to Pathology. J. Surg. Oncol. 2014, 109, 158–167. [Google Scholar] [CrossRef]
- De Los Santos, J.F.; Cantor, A.; Amos, K.D.; Forero, A.; Golshan, M.; Horton, J.K.; Hudis, C.A.; Hylton, N.M.; McGuire, K.; Meric-Bernstam, F.; et al. Magnetic Resonance Imaging as a Predictor of Pathologic Response in Patients Treated with Neoadjuvant Systemic Treatment for Operable Breast Cancer. Cancer 2013, 119, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Londero, V.; Bazzocchi, M.; Del Frate, C.; Puglisi, F.; Di Loreto, C.; Francescutti, G.; Zuiani, C. Locally Advanced Breast Cancer: Comparison of Mammography, Sonography and MR Imaging in Evaluation of Residual Disease in Women Receiving Neoadjuvant Chemotherapy. Eur. Radiol. 2004, 14, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M. The Effectiveness of MR Imaging in the Assessment of Invasive Lobular Carcinoma of the Breast. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 259–276. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.P.; Toro-Burguete, J.; Dang, H.; Young, J.; Soran, A.; Zuley, M.; Bhargava, R.; Bonaventura, M.; Johnson, R.; Ahrendt, G. MRI Staging After Neoadjuvant Chemotherapy for Breast Cancer: Does Tumor Biology Affect Accuracy? Ann. Surg. Oncol. 2011, 18, 3149. [Google Scholar] [CrossRef] [PubMed]
- Michishita, S.; Kim, S.J.; Shimazu, K.; Sota, Y.; Naoi, Y.; Maruyama, N.; Kagara, N.; Shimoda, M.; Shimomura, A.; Noguchi, S. Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy by Magnetic Resonance Imaging in Breast Cancer Patients. Breast 2015, 24, 159–165. [Google Scholar] [CrossRef]
- Richard, R.; Thomassin, I.; Chapellier, M.; Scemama, A.; de Cremoux, P.; Varna, M.; Giacchetti, S.; Espié, M.; de Kerviler, E.; de Bazelaire, C. Diffusion-Weighted MRI in Pretreatment Prediction of Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer. Eur. Radiol. 2013, 23, 2420–2431. [Google Scholar] [CrossRef]
- Schelfout, K.; Van Goethem, M.; Kersschot, E.; Verslegers, I.; Biltjes, I.; Leyman, P.; Colpaert, C.; Thienpont, L.; Van den Haute, J.; Gillardin, J.P.; et al. Preoperative Breast MRI in Patients with Invasive Lobular Breast Cancer. Eur. Radiol. 2004, 14, 1209–1216. [Google Scholar] [CrossRef]
- Yeh, E.D.; Slanetz, P.J.; Edmister, W.B.; Talele, A.; Monticciolo, D.; Kopans, D.B. Invasive Lobular Carcinoma: Spectrum of Enhancement and Morphology on Magnetic Resonance Imaging. Breast J. 2003, 9, 13–18. [Google Scholar] [CrossRef]
- Ko, E.S.; Han, B.-K.; Kim, R.B.; Ko, E.Y.; Shin, J.H.; Hahn, S.Y.; Nam, S.J.; Lee, J.E.; Lee, S.K.; Im, Y.-H.; et al. Analysis of Factors That Influence the Accuracy of Magnetic Resonance Imaging for Predicting Response after Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer. Ann. Surg. Oncol. 2013, 20, 2562–2568. [Google Scholar] [CrossRef]
- Bahri, S.; Chen, J.-H.; Mehta, R.S.; Carpenter, P.M.; Nie, K.; Kwon, S.-Y.; Yu, H.J.; Nalcioglu, O.; Su, M.-Y. Residual Breast Cancer Diagnosed by MRI in Patients Receiving Neoadjuvant Chemotherapy with and Without Bevacizumab. Ann. Surg. Oncol. 2009, 16, 1619–1628. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, Y.-H.; Han, B.-K.; Choi, N.; Lee, J.; Kim, J.H.; Choi, Y.-L.; Ahn, J.-S.; Nam, S.-J.; Park, Y.S.; et al. Accuracy of MRI for Estimating Residual Tumor Size after Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer: Relation to Response Patterns on MRI. Acta Oncol. Stockh. Swed. 2007, 46, 996–1003. [Google Scholar] [CrossRef]
- Chen, J.H.; Feig, B.; Agrawal, G.; Yu, H.; Carpenter, P.M.; Mehta, R.S.; Nalcioglu, O.; Su, M.Y. MRI Evaluation of Pathologically Complete Response and Residual Tumors in Breast Cancer after Neoadjuvant Chemotherapy. Cancer 2008, 112, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Denis, F.; Desbiez-Bourcier, A.V.; Chapiron, C.; Arbion, F.; Body, G.; Brunereau, L. Contrast Enhanced Magnetic Resonance Imaging Underestimates Residual Disease Following Neoadjuvant Docetaxel Based Chemotherapy for Breast Cancer. Eur. J. Surg. Oncol. EJSO 2004, 30, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Scheel, J.R.; Kim, E.; Partridge, S.C.; Lehman, C.D.; Rosen, M.A.; Bernreuter, W.K.; Pisano, E.D.; Marques, H.S.; Morris, E.A.; Weatherall, P.T.; et al. MRI, Clinical Examination, and Mammography for Preoperative Assessment of Residual Disease and Pathologic Complete Response After Neoadjuvant Chemotherapy for Breast Cancer: ACRIN 6657 Trial. AJR Am. J. Roentgenol. 2018, 210, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Ecanow, J.S.; Abe, H.; Newstead, G.M.; Ecanow, D.B.; Jeske, J.M. Axillary Staging of Breast Cancer: What the Radiologist Should Know. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc 2013, 33, 1589–1612. [Google Scholar] [CrossRef]
- Amin, M.; Edge, S.; Greene, F. AJCC (American Joint Committee on Cancer) Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2018. [Google Scholar]
- Maaskant-Braat, A.J.; van de Poll-Franse, L.V.; Voogd, A.C.; Coebergh, J.W.W.; Roumen, R.M.; Nolthenius-Puylaert, M.C.T.; Nieuwenhuijzen, G.A. Sentinel Node Micrometastases in Breast Cancer Do Not Affect Prognosis: A Population-Based Study. Breast Cancer Res. Treat. 2011, 127, 195–203. [Google Scholar] [CrossRef]
- Canavese, G.; Tinterri, C.; Carli, F.; Garrone, E.; Spinaci, S.; Della Valle, A.; Barbieri, E.; Marrazzo, E.; Bruzzi, P.; Dozin, B. Correlation between Outcome and Extent of Residual Disease in the Sentinel Node after Neoadjuvant Chemotherapy in Clinically Fine-Needle Proven Node-Positive Breast Cancer Patients. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2021, 47, 1920–1927. [Google Scholar] [CrossRef]
- Alvarez, S.; Añorbe, E.; Alcorta, P.; López, F.; Alonso, I.; Cortés, J. Role of Sonography in the Diagnosis of Axillary Lymph Node Metastases in Breast Cancer: A Systematic Review. AJR Am. J. Roentgenol. 2006, 186, 1342–1348. [Google Scholar] [CrossRef]
- Kim, W.H.; Kim, H.J.; Lee, S.M.; Cho, S.H.; Shin, K.M.; Lee, S.Y.; Lim, J.K. Prediction of High Nodal Burden with Ultrasound and Magnetic Resonance Imaging in Clinically Node-Negative Breast Cancer Patients. Cancer Imaging 2019, 19, 4. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.H. Breast Magnetic Resonance Imaging for Assessment of Internal Mammary Lymph Node Status in Breast Cancer. J. Breast Cancer 2016, 19, 191–198. [Google Scholar] [CrossRef]
- Baltzer, P.A.T.; Dietzel, M.; Burmeister, H.P.; Zoubi, R.; Gajda, M.; Camara, O.; Kaiser, W.A. Application of MR Mammography beyond Local Staging: Is There a Potential to Accurately Assess Axillary Lymph Nodes? Evaluation of an Extended Protocol in an Initial Prospective Study. AJR Am. J. Roentgenol. 2011, 196, W641–W647. [Google Scholar] [CrossRef]
- Hieken, T.J.; Boughey, J.C.; Jones, K.N.; Shah, S.S.; Glazebrook, K.N. Imaging Response and Residual Metastatic Axillary Lymph Node Disease after Neoadjuvant Chemotherapy for Primary Breast Cancer. Ann. Surg. Oncol. 2013, 20, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, R.; Yi, M.; Le-Petross, H.; Gilcrease, M.; Mittendorf, E.A.; Bedrosian, I.; Hwang, R.F.; Caudle, A.S.; Babiera, G.V.; Akins, J.S.; et al. The Role for Sentinel Lymph Node Dissection after Neoadjuvant Chemotherapy in Patients Who Present with Node-Positive Breast Cancer. Ann. Surg. Oncol. 2012, 19, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-X.; Lin, S.-Y.; Ou, Y.; Shi, C.-G.; Zhong, Y.; Wei, M.-J.; Pei, X.-Q. Combining Conventional Ultrasound and Sonoelastography to Predict Axillary Status after Neoadjuvant Chemotherapy for Breast Cancer. Eur. Radiol. 2022, 32, 5986–5996. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.K.; Greenwood, H.; Kelil, T.; Guo, R.; Brabham, C.; Hylton, N.; Wong, J.; Alvarado, M.; Ewing, C.; Esserman, L.J.; et al. Accuracy of Breast MRI in Evaluating Nodal Status after Neoadjuvant Therapy in Invasive Lobular Carcinoma. NPJ Breast Cancer 2021, 7, 25. [Google Scholar] [CrossRef]
- Samiei, S.; de Mooij, C.M.; Lobbes, M.B.I.; Keymeulen, K.B.M.I.; van Nijnatten, T.J.A.; Smidt, M.L. Diagnostic Performance of Noninvasive Imaging for Assessment of Axillary Response After Neoadjuvant Systemic Therapy in Clinically Node-Positive Breast Cancer: A Systematic Review and Meta-Analysis. Ann. Surg. 2021, 273, 694–700. [Google Scholar] [CrossRef]
- Javid, S.; Segara, D.; Lotfi, P.; Raza, S.; Golshan, M. Can Breast MRI Predict Axillary Lymph Node Metastasis in Women Undergoing Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2010, 17, 1841–1846. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- van Dongen, J.A.; Voogd, A.C.; Fentiman, I.S.; Legrand, C.; Sylvester, R.J.; Tong, D.; van der Schueren, E.; Helle, P.A.; van Zijl, K.; Bartelink, H. Long-Term Results of a Randomized Trial Comparing Breast-Conserving Therapy with Mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef]
- Poggi, M.M.; Danforth, D.N.; Sciuto, L.C.; Smith, S.L.; Steinberg, S.M.; Liewehr, D.J.; Menard, C.; Lippman, M.E.; Lichter, A.S.; Altemus, R.M. Eighteen-Year Results in the Treatment of Early Breast Carcinoma with Mastectomy versus Breast Conservation Therapy: The National Cancer Institute Randomized Trial. Cancer 2003, 98, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Blichert-Toft, M.; Rose, C.; Andersen, J.A.; Overgaard, M.; Axelsson, C.K.; Andersen, K.W.; Mouridsen, H.T. Danish Randomized Trial Comparing Breast Conservation Therapy with Mastectomy: Six Years of Life-Table Analysis. Danish Breast Cancer Cooperative Group. J. Natl. Cancer Inst. Monogr. 1992, 11, 19–25. [Google Scholar]
- Morris, A.D.; Morris, R.D.; Wilson, J.F.; White, J.; Steinberg, S.; Okunieff, P.; Arriagada, R.; Lê, M.G.; Blichert-Toft, M.; van Dongen, J.A. Breast-Conserving Therapy vs Mastectomy in Early-Stage Breast Cancer: A Meta-Analysis of 10-Year Survival. Cancer J. Sci. Am. 1997, 3, 6–12. [Google Scholar] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: An Overview of the Randomised Trials. Lancet Lond. Engl. 2005, 366, 2087–2106. [Google Scholar] [CrossRef]
- Morrow, M.; Strom, E.A.; Bassett, L.W.; Dershaw, D.D.; Fowble, B.; Giuliano, A.; Harris, J.R.; O’Malley, F.; Schnitt, S.J.; Singletary, S.E.; et al. Standard for Breast Conservation Therapy in the Management of Invasive Breast Carcinoma. CA. Cancer J. Clin. 2002, 52, 277–300. [Google Scholar] [CrossRef]
- Newman, L.A.; Washington, T.A. New Trends in Breast Conservation Therapy. Surg. Clin. N. Am. 2003, 83, 841–883. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y. Local Recurrence after Breast-Conserving Surgery and Mastectomy Following Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer—A Meta-Analysis. Breast Care Basel Switz. 2016, 11, 345–351. [Google Scholar] [CrossRef]
- Peterson, M.E.; Schultz, D.J.; Reynolds, C.; Solin, L.J. Outcomes in Breast Cancer Patients Relative to Margin Status after Treatment with Breast-Conserving Surgery and Radiation Therapy: The University of Pennsylvania Experience. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 1029–1035. [Google Scholar] [CrossRef]
- Smitt, M.C.; Nowels, K.W.; Zdeblick, M.J.; Jeffrey, S.; Carlson, R.W.; Stockdale, F.E.; Goffinet, D.R. The Importance of the Lumpectomy Surgical Margin Status in Long-Term Results of Breast Conservation. Cancer 1995, 76, 259–267. [Google Scholar] [CrossRef]
- Mirza, N.Q.; Vlastos, G.; Meric, F.; Buchholz, T.A.; Esnaola, N.; Singletary, S.E.; Kuerer, H.M.; Newman, L.A.; Ames, F.C.; Ross, M.I.; et al. Predictors of Locoregional Recurrence among Patients with Early-Stage Breast Cancer Treated with Breast-Conserving Therapy. Ann. Surg. Oncol. 2002, 9, 256–265. [Google Scholar] [CrossRef]
- Singletary, S.E. Surgical Margins in Patients with Early-Stage Breast Cancer Treated with Breast Conservation Therapy. Am. J. Surg. 2002, 184, 383–393. [Google Scholar] [CrossRef]
- Newman, L.A.; Kuerer, H.M. Advances in Breast Conservation Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 1685–1697. [Google Scholar] [CrossRef]
- Kreike, B.; Hart, A.A.M.; van de Velde, T.; Borger, J.; Peterse, H.; Rutgers, E.; Bartelink, H.; van de Vijver, M.J. Continuing Risk of Ipsilateral Breast Relapse after Breast-Conserving Therapy at Long-Term Follow-Up. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Magno, S.; Fabbri, C.; Chiesa, F.; Di Leone, A.; Moschella, F.; Scafetta, I.; Scaldaferri, A.; Fragomeni, S.; Adesi Barone, L.; et al. Conservative and Radical Oncoplastic Approches in the Surgical Treatment of Breast Cancer. Eur. Rev. Med. Pharmacol. Sci. 2008, 12, 387–396. [Google Scholar] [PubMed]
- Sanchez, A.M.; Franceschini, G.; D’Archi, S.; De Lauretis, F.; Scardina, L.; Di Giorgio, D.; Accetta, C.; Masetti, R. Results Obtained with Level II Oncoplastic Surgery Spanning 20 Years of Breast Cancer Treatment: Do We Really Need Further Demonstration of Reliability? Breast J. 2020, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, G.; Scardina, L.; Di Leone, A.; Terribile, D.A.; Sanchez, A.M.; Magno, S.; D’Archi, S.; Franco, A.; Mason, E.J.; Carnassale, B.; et al. Immediate Prosthetic Breast Reconstruction after Nipple-Sparing Mastectomy: Traditional Subpectoral Technique versus Direct-to-Implant Prepectoral Reconstruction without Acellular Dermal Matrix. J. Pers. Med. 2021, 11, 153. [Google Scholar] [CrossRef]
- Hershko, D. Surgical Management of the Breast and Axilla after Neoadjuvant Therapy. Chir. Buchar. Rom. 1990 2021, 116, 143–149. [Google Scholar] [CrossRef]
- Kuhl, C.; Kuhn, W.; Braun, M.; Schild, H. Pre-Operative Staging of Breast Cancer with Breast MRI: One Step Forward, Two Steps Back? Breast Edinb. Scotl. 2007, 16 (Suppl. 2), S34–S44. [Google Scholar] [CrossRef]
- Steinhof-Radwańska, K.; Lorek, A.; Holecki, M.; Barczyk-Gutkowska, A.; Grażyńska, A.; Szczudło-Chraścina, J.; Bożek, O.; Habas, J.; Szyluk, K.; Niemiec, P.; et al. Multifocality and Multicentrality in Breast Cancer: Comparison of the Efficiency of Mammography, Contrast-Enhanced Spectral Mammography, and Magnetic Resonance Imaging in a Group of Patients with Primarily Operable Breast Cancer. Curr. Oncol. 2021, 28, 4016–4030. [Google Scholar] [CrossRef]
- Jatoi, I.; Benson, J.R. The Case against Routine Preoperative Breast MRI. Future Oncol. Lond. Engl. 2013, 9, 347–353. [Google Scholar] [CrossRef]
- Sardanelli, F.; Trimboli, R.M.; Houssami, N.; Gilbert, F.J.; Helbich, T.H.; Álvarez Benito, M.; Balleyguier, C.; Bazzocchi, M.; Bult, P.; Calabrese, M.; et al. Magnetic Resonance Imaging before Breast Cancer Surgery: Results of an Observational Multicenter International Prospective Analysis (MIPA). Eur. Radiol. 2022, 32, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Q.; Qian, C.; Lin, H. Impact of Preoperative Magnetic Resonance Imaging on Surgical Outcomes in Women with Invasive Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 6440952. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazal, S.K.; Fallowfield, L.; Blamey, R.W. Comparison of Psychological Aspects and Patient Satisfaction Following Breast Conserving Surgery, Simple Mastectomy and Breast Reconstruction. Eur. J. Cancer 2000, 36, 1938–1943. [Google Scholar] [CrossRef]
- Schain, W.S.; d’Angelo, T.M.; Dunn, M.E.; Lichter, A.S.; Pierce, L.J. Mastectomy versus Conservative Surgery and Radiation Therapy. Psychosocial Consequences. Cancer 1994, 73, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Kiebert, G.M.; de Haes, J.C.; van de Velde, C.J. The Impact of Breast-Conserving Treatment and Mastectomy on the Quality of Life of Early-Stage Breast Cancer Patients: A Review. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1991, 9, 1059–1070. [Google Scholar] [CrossRef]
- Dominici, L.; Golshan, M. Can Axillary Lymph Node Dissection Be Omitted in Patients with Breast Cancer and Positive Sentinel Nodes? Minerva Chir. 2010, 65, 547–554. [Google Scholar]
- Gimbergues, P.; Abrial, C.; Durando, X.; Le Bouedec, G.; Cachin, F.; Penault-Llorca, F.; Mouret-Reynier, M.A.; Kwiatkowski, F.; Maublant, J.; Tchirkov, A.; et al. Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy Is Accurate in Breast Cancer Patients with a Clinically Negative Axillary Nodal Status at Presentation. Ann. Surg. Oncol. 2008, 15, 1316–1321. [Google Scholar] [CrossRef]
- Kinoshita, T.; Takasugi, M.; Iwamoto, E.; Akashi-Tanaka, S.; Fukutomi, T.; Terui, S. Sentinel Lymph Node Biopsy Examination for Breast Cancer Patients with Clinically Negative Axillary Lymph Nodes after Neoadjuvant Chemotherapy. Am. J. Surg. 2006, 191, 225–229. [Google Scholar] [CrossRef]
- Shimazu, K.; Tamaki, Y.; Taguchi, T.; Akazawa, K.; Inoue, T.; Noguchi, S. Sentinel Lymph Node Biopsy Using Periareolar Injection of Radiocolloid for Patients with Neoadjuvant Chemotherapy-Treated Breast Carcinoma. Cancer 2004, 100, 2555–2561. [Google Scholar] [CrossRef]
- Henry-Tillman, R.; Glover-Collins, K.; Preston, M.; Gallagher, K.; Tummel, E.; Robertson, Y.V.; Ochoa, D.; Korourian, S.; Westbrook, K.; Klimberg, V.S. The SAVE Review: Sonographic Analysis versus Excision for Axillary Staging in Breast Cancer. J. Am. Coll. Surg. 2015, 220, 560–567. [Google Scholar] [CrossRef]
- Khan, A.; Sabel, M.S.; Nees, A.; Diehl, K.M.; Cimmino, V.M.; Kleer, C.G.; Schott, A.F.; Hayes, D.F.; Chang, A.E.; Newman, L.A. Comprehensive Axillary Evaluation in Neoadjuvant Chemotherapy Patients with Ultrasonography and Sentinel Lymph Node Biopsy. Ann. Surg. Oncol. 2005, 12, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Lucci, A.; McCall, L.M.; Beitsch, P.D.; Whitworth, P.W.; Reintgen, D.S.; Blumencranz, P.W.; Leitch, A.M.; Saha, S.; Hunt, K.K.; Giuliano, A.E.; et al. Surgical Complications Associated with Sentinel Lymph Node Dissection (SLND) plus Axillary Lymph Node Dissection Compared with SLND Alone in the American College of Surgeons Oncology Group Trial Z0011. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 3657–3663. [Google Scholar] [CrossRef]
- Kuehn, T.; Bauerfeind, I.; Fehm, T.; Fleige, B.; Hausschild, M.; Helms, G.; Lebeau, A.; Liedtke, C.; von Minckwitz, G.; Nekljudova, V.; et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (SENTINA): A Prospective, Multicentre Cohort Study. Lancet Oncol. 2013, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Boileau, J.-F.; Poirier, B.; Basik, M.; Holloway, C.M.B.; Gaboury, L.; Sideris, L.; Meterissian, S.; Arnaout, A.; Brackstone, M.; McCready, D.R.; et al. Sentinel Node Biopsy after Neoadjuvant Chemotherapy in Biopsy-Proven Node-Positive Breast Cancer: The SN FNAC Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Newcombe, R.G.; Chhabra, A.; Mansel, R.E. ALMANAC Trialists Group Factors Affecting Failed Localisation and False-Negative Rates of Sentinel Node Biopsy in Breast Cancer—Results of the ALMANAC Validation Phase. Breast Cancer Res. Treat. 2006, 99, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Boughey, J.C.; Ballman, K.V.; Le-Petross, H.T.; McCall, L.M.; Mittendorf, E.A.; Ahrendt, G.M.; Wilke, L.G.; Taback, B.; Feliberti, E.C.; Hunt, K.K. Identification and Resection of Clipped Node Decreases the False-Negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-Positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann. Surg. 2016, 263, 802–807. [Google Scholar] [CrossRef]
- Donker, M.; Straver, M.E.; Wesseling, J.; Loo, C.E.; Schot, M.; Drukker, C.A.; van Tinteren, H.; Sonke, G.S.; Rutgers, E.J.T.; Vrancken Peeters, M.-J.T.F.D. Marking Axillary Lymph Nodes with Radioactive Iodine Seeds for Axillary Staging after Neoadjuvant Systemic Treatment in Breast Cancer Patients: The MARI Procedure. Ann. Surg. 2015, 261, 378–382. [Google Scholar] [CrossRef]
- Caudle, A.S.; Yang, W.T.; Krishnamurthy, S.; Mittendorf, E.A.; Black, D.M.; Gilcrease, M.Z.; Bedrosian, I.; Hobbs, B.P.; DeSnyder, S.M.; Hwang, R.F.; et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 1072–1078. [Google Scholar] [CrossRef]
- Shin, K.; Caudle, A.S.; Kuerer, H.M.; Santiago, L.; Candelaria, R.P.; Dogan, B.; Leung, J.; Krishnamurthy, S.; Yang, W. Radiologic Mapping for Targeted Axillary Dissection: Needle Biopsy to Excision. AJR Am. J. Roentgenol. 2016, 207, 1372–1379. [Google Scholar] [CrossRef]
- van Nijnatten, T.J.A.; Schipper, R.J.; Lobbes, M.B.I.; Nelemans, P.J.; Beets-Tan, R.G.H.; Smidt, M.L. The Diagnostic Performance of Sentinel Lymph Node Biopsy in Pathologically Confirmed Node Positive Breast Cancer Patients after Neoadjuvant Systemic Therapy: A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 1278–1287. [Google Scholar] [CrossRef]
- Houssami, N.; Macaskill, P.; von Minckwitz, G.; Marinovich, M.L.; Mamounas, E. Meta-Analysis of the Association of Breast Cancer Subtype and Pathologic Complete Response to Neoadjuvant Chemotherapy. Eur. J. Cancer 2012, 48, 3342–3354. [Google Scholar] [CrossRef] [PubMed]
- Daveau, C.; Savignoni, A.; Abrous-Anane, S.; Pierga, J.-Y.; Reyal, F.; Gautier, C.; Kirova, Y.M.; Dendale, R.; Campana, F.; Fourquet, A.; et al. Is Radiotherapy an Option for Early Breast Cancers with Complete Clinical Response after Neoadjuvant Chemotherapy? Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1452–1459. [Google Scholar] [CrossRef]
- Ring, A.; Webb, A.; Ashley, S.; Allum, W.H.; Ebbs, S.; Gui, G.; Sacks, N.P.; Walsh, G.; Smith, I.E. Is Surgery Necessary after Complete Clinical Remission Following Neoadjuvant Chemotherapy for Early Breast Cancer? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2003, 21, 4540–4545. [Google Scholar] [CrossRef]
- Clouth, B.; Chandrasekharan, S.; Inwang, R.; Smith, S.; Davidson, N.; Sauven, P. The Surgical Management of Patients Who Achieve a Complete Pathological Response after Primary Chemotherapy for Locally Advanced Breast Cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2007, 33, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Mauriac, L.; MacGrogan, G.; Avril, A.; Durand, M.; Floquet, A.; Debled, M.; Dilhuydy, J.M.; Bonichon, F. Neoadjuvant Chemotherapy for Operable Breast Carcinoma Larger than 3 Cm: A Unicentre Randomized Trial with a 124-Month Median Follow-up. Institut Bergonié Bordeaux Groupe Sein (IBBGS). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1999, 10, 47–52. [Google Scholar] [CrossRef]
- Schaefgen, B.; Mati, M.; Sinn, H.P.; Golatta, M.; Stieber, A.; Rauch, G.; Hennigs, A.; Richter, H.; Domschke, C.; Schuetz, F.; et al. Can Routine Imaging After Neoadjuvant Chemotherapy in Breast Cancer Predict Pathologic Complete Response? Ann. Surg. Oncol. 2016, 23, 789–795. [Google Scholar] [CrossRef]
- van Ramshorst, M.S.; Loo, C.E.; Groen, E.J.; Winter-Warnars, G.H.; Wesseling, J.; van Duijnhoven, F.; Peeters, M.-J.T.V.; Sonke, G.S. MRI Predicts Pathologic Complete Response in HER2-Positive Breast Cancer after Neoadjuvant Chemotherapy. Breast Cancer Res. Treat. 2017, 164, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Dialani, V.; Chadashvili, T.; Slanetz, P.J. Role of Imaging in Neoadjuvant Therapy for Breast Cancer. Ann. Surg. Oncol. 2015, 22, 1416–1424. [Google Scholar] [CrossRef]
- Francis, A.; Herring, K.; Molyneux, R.; Jafri, M.; Trivedi, S.; Shaaban, A.; Rea, D. Abstract P5-16-14: NOSTRA PRELIM: A Non Randomised Pilot Study Designed to Assess the Ability of Image Guided Core Biopsies to Detect Residual Disease in Patients with Early Breast Cancer Who Have Received Neoadjuvant Chemotherapy to Inform the Design of a Planned Trial. Cancer Res. 2017, 77, P5–P16. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Yang, W.T.; Krishnamurthy, S. Comment on ‘Diagnosis of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer by Minimal Invasive Biopsy Techniques’. Br. J. Cancer 2016, 114, e3. [Google Scholar] [CrossRef]
- Heil, J.; Schaefgen, B.; Sinn, P.; Richter, H.; Harcos, A.; Gomez, C.; Stieber, A.; Hennigs, A.; Rauch, G.; Schuetz, F.; et al. Can a Pathological Complete Response of Breast Cancer after Neoadjuvant Chemotherapy Be Diagnosed by Minimal Invasive Biopsy? Eur. J. Cancer Oxf. Engl. 1990 2016, 69, 142–150. [Google Scholar] [CrossRef]
- Heil, J.; Kümmel, S.; Schaefgen, B.; Paepke, S.; Thomssen, C.; Rauch, G.; Ataseven, B.; Große, R.; Dreesmann, V.; Kühn, T.; et al. Diagnosis of Pathological Complete Response to Neoadjuvant Chemotherapy in Breast Cancer by Minimal Invasive Biopsy Techniques. Br. J. Cancer 2015, 113, 1565–1570. [Google Scholar] [CrossRef]
- Kuerer, H.M.; Smith, B.D.; Krishnamurthy, S.; Yang, W.T.; Valero, V.; Shen, Y.; Lin, H.; Lucci, A.; Boughey, J.C.; White, R.L.; et al. Eliminating Breast Surgery for Invasive Breast Cancer in Exceptional Responders to Neoadjuvant Systemic Therapy: A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 1517–1524. [Google Scholar] [CrossRef]
- Heil, J.; Sinn, P.; Richter, H.; Pfob, A.; Schaefgen, B.; Hennigs, A.; Riedel, F.; Thomas, B.; Thill, M.; Hahn, M.; et al. RESPONDER—Diagnosis of Pathological Complete Response by Vacuum-Assisted Biopsy after Neoadjuvant Chemotherapy in Breast Cancer—A Multicenter, Confirmative, One-Armed, Intra-Individually-Controlled, Open, Diagnostic Trial. BMC Cancer 2018, 18, 851. [Google Scholar] [CrossRef]
- NRG Oncology. A Phase II Trial Assessing the Accuracy of Tumor Bed Biopsies in Predicting Pathologic Response in Patients With Clinical/Radiologic Complete Response After Neoadjuvant Chemotherapy in Order to Explore the Feasibility of Breast Conserving Treatment Without Surgery. 2022. Available online: https://clinicaltrials.gov/ (accessed on 9 January 2023).
- Kuerer, H.M.; Vrancken Peeters, M.-J.T.F.D.; Rea, D.W.; Basik, M.; De Los Santos, J.; Heil, J. Nonoperative Management for Invasive Breast Cancer After Neoadjuvant Systemic Therapy: Conceptual Basis and Fundamental International Feasibility Clinical Trials. Ann. Surg. Oncol. 2017, 24, 2855–2862. [Google Scholar] [CrossRef]
- Gao, Y.; Heller, S.L. Abbreviated and Ultrafast Breast MRI in Clinical Practice. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2020, 40, 1507–1527. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Semple, J.P.; Welch, W.R.; Folkman, J. Tumor Angiogenesis and Metastasis—Correlation in Invasive Breast Carcinoma. N. Engl. J. Med. 1991, 324, 46–52. [Google Scholar] [CrossRef]
- Ramtohul, T.; Tescher, C.; Vaflard, P.; Cyrta, J.; Girard, N.; Malhaire, C.; Tardivon, A. Prospective Evaluation of Ultrafast Breast MRI for Predicting Pathologic Response after Neoadjuvant Therapies. Radiology 2022, 305, 3. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, V.Y.; Shin, H.J.; Kim, M.J.; Yoon, J.H. Ultrafast Dynamic Contrast-Enhanced Breast MRI: Association with Pathologic Complete Response in Neoadjuvant Treatment of Breast Cancer. Eur. Radiol. 2022, 32, 4823–4833. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.F.I.; Kamal, R.M.; Gomaa, M.M.M.; Mostafa, S.; Mubarak, R.; El-Adawy, M. Quantitative Mathematical Objective Evaluation of Contrast-Enhanced Spectral Mammogram in the Assessment of Response to Neoadjuvant Chemotherapy and Prediction of Residual Disease in Breast Cancer. Egypt. J. Radiol. Nucl. Med. 2019, 50, 44. [Google Scholar] [CrossRef]
- ElSaid, N.A.E.; Mahmoud, H.G.M.; Salama, A.; Nabil, M.; ElDesouky, E.D. Role of Contrast Enhanced Spectral Mammography in Predicting Pathological Response of Locally Advanced Breast Cancer Post Neo-Adjuvant Chemotherapy. Egypt. J. Radiol. Nucl. Med. 2017, 48, 519–527. [Google Scholar] [CrossRef]
- Tang, S.; Xiang, C.; Yang, Q. The Diagnostic Performance of CESM and CE-MRI in Evaluating the Pathological Response to Neoadjuvant Therapy in Breast Cancer: A Systematic Review and Meta-Analysis. Br. J. Radiol. 2020, 93, 20200301. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.-Q.; Huang, Q.-X.; Huang, X.-W.; Hu, H.-T.; Zeng, F.-Q.; Wang, W. Predicting Breast Cancer in Breast Imaging Reporting and Data System (BI-RADS) Ultrasound Category 4 or 5 Lesions: A Nomogram Combining Radiomics and BI-RADS. Sci. Rep. 2019, 9, 11921. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Burnside, E.S.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Whitman, G.J.; Sutton, E.J.; Net, J.M.; et al. MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology 2016, 281, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Quiaoit, K.; DiCenzo, D.; Fatima, K.; Bhardwaj, D.; Sannachi, L.; Gangeh, M.; Sadeghi-Naini, A.; Dasgupta, A.; Kolios, M.C.; Trudeau, M.; et al. Quantitative Ultrasound Radiomics for Therapy Response Monitoring in Patients with Locally Advanced Breast Cancer: Multi-Institutional Study Results. PLoS ONE 2020, 15, e0236182. [Google Scholar] [CrossRef]
- Zhuang, X.; Chen, C.; Liu, Z.; Zhang, L.; Zhou, X.; Cheng, M.; Ji, F.; Zhu, T.; Lei, C.; Zhang, J.; et al. Multiparametric MRI-Based Radiomics Analysis for the Prediction of Breast Tumor Regression Patterns after Neoadjuvant Chemotherapy. Transl. Oncol. 2020, 13, 100831. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Qu, J.; Zhang, R.; Zhou, X.; Li, L.; Sun, K.; Tang, Z.; Jiang, H.; Li, H.; et al. Radiomics of Multiparametric MRI for Pretreatment Prediction of Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer: A Multicenter Study. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3538–3547. [Google Scholar] [CrossRef]
- Cain, E.H.; Saha, A.; Harowicz, M.R.; Marks, J.R.; Marcom, P.K.; Mazurowski, M.A. Multivariate Machine Learning Models for Prediction of Pathologic Response to Neoadjuvant Therapy in Breast Cancer Using MRI Features: A Study Using an Independent Validation Set. Breast Cancer Res. Treat. 2019, 173, 455–463. [Google Scholar] [CrossRef]
- Antunovic, L.; De Sanctis, R.; Cozzi, L.; Kirienko, M.; Sagona, A.; Torrisi, R.; Tinterri, C.; Santoro, A.; Chiti, A.; Zelic, R.; et al. PET/CT Radiomics in Breast Cancer: Promising Tool for Prediction of Pathological Response to Neoadjuvant Chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1468–1477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).