A Phase 2 Trial of Ibrutinib and Nivolumab in Patients with Relapsed or Refractory Classical Hodgkin’s Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Treatments
2.3. End Points and Assessments
2.4. Immune Phenotyping by CyTOF
2.5. Statistics
3. Results
3.1. Patient Baseline Characteristics
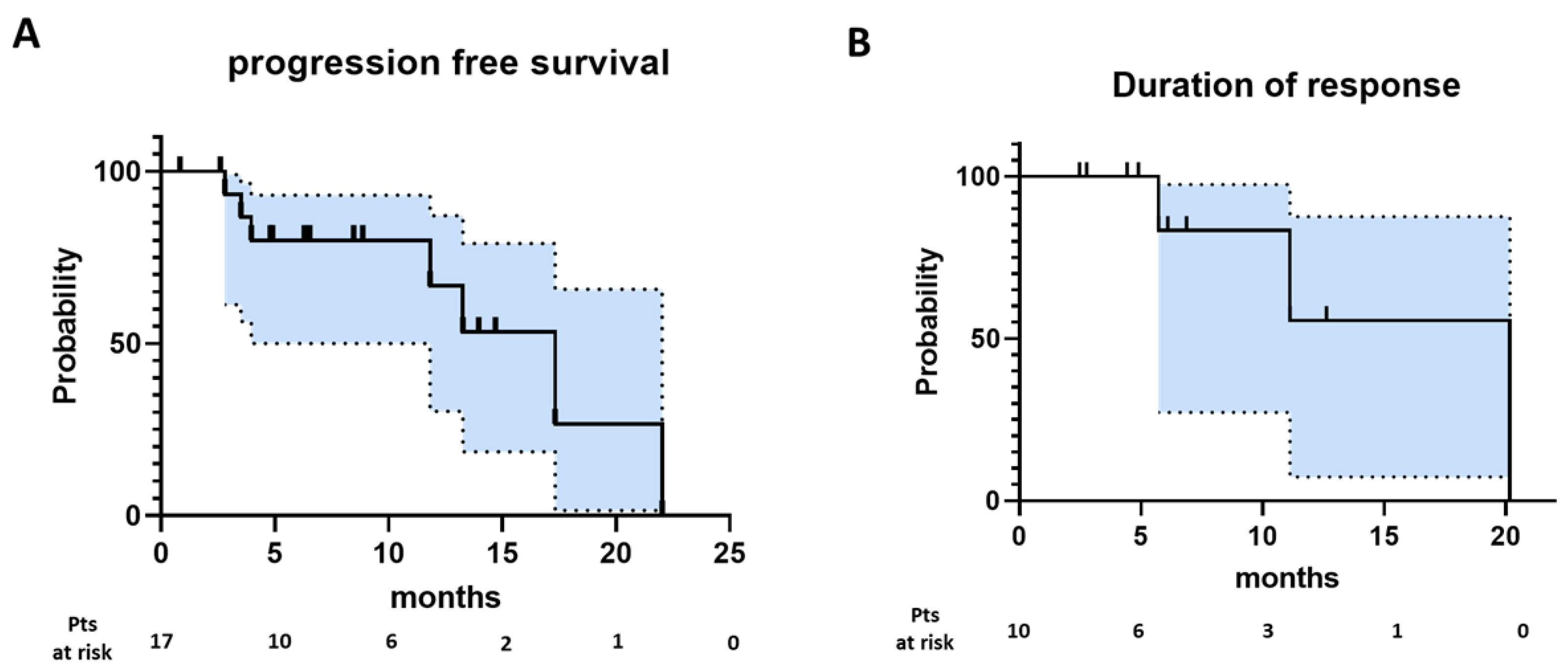
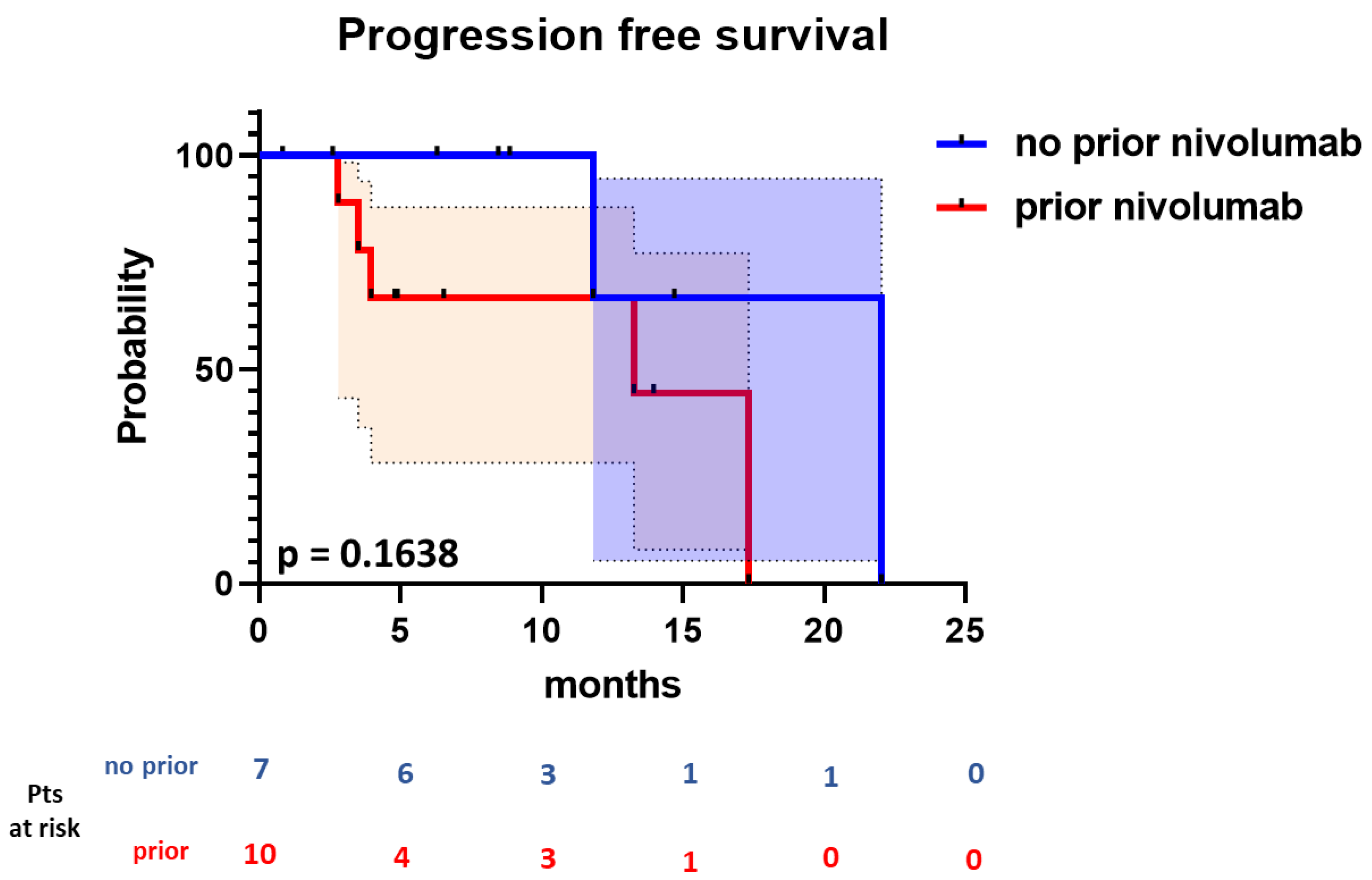
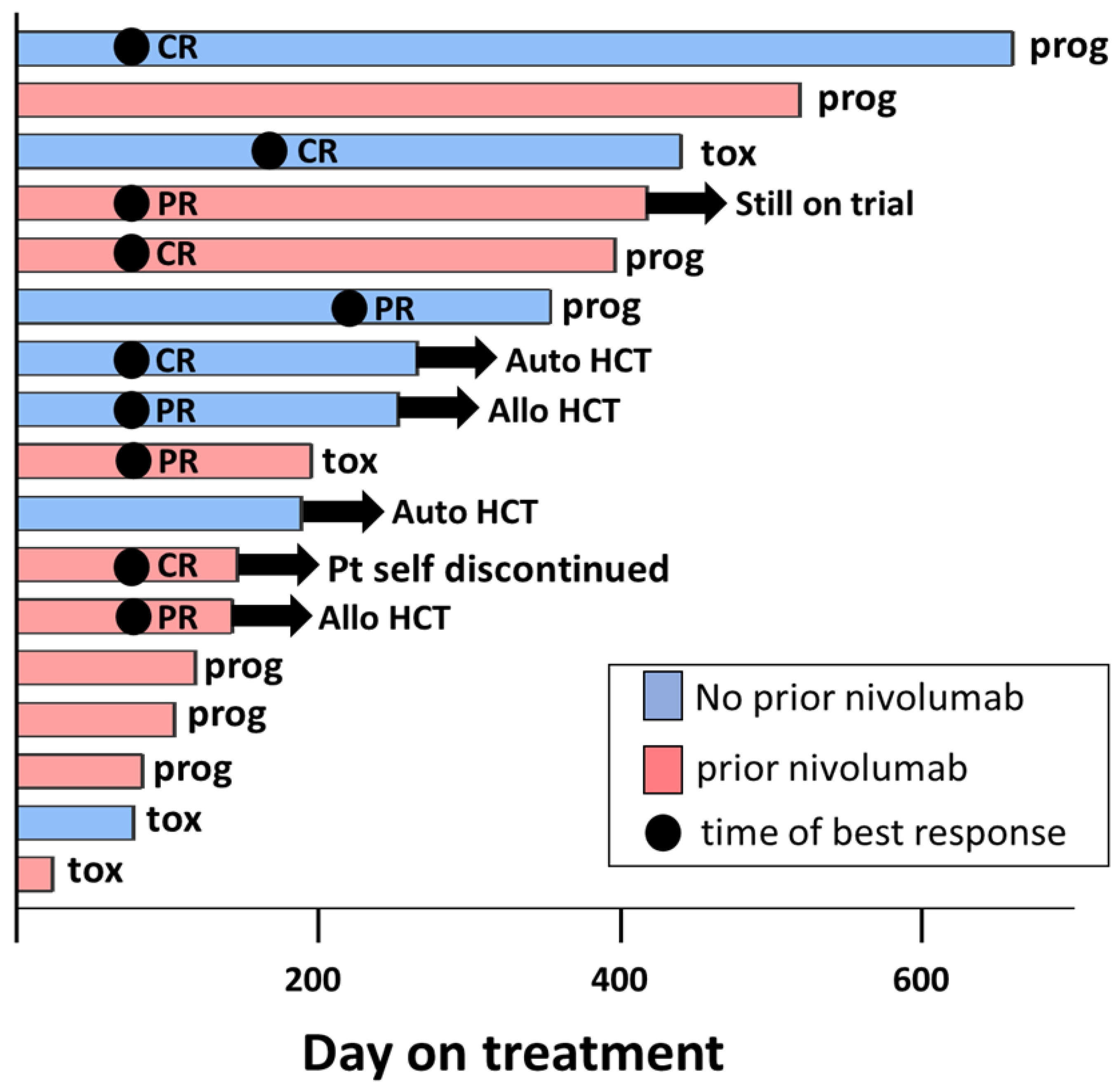
3.2. Efficacy
3.3. Safety
3.4. Exploratory Immune Phenotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Epperla, N.; Hamadani, M. Double-refractory Hodgkin lymphoma: Tackling relapse after brentuximab vedotin and checkpoint inhibitors. Hematology 2021, 2021, 247–253. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Herrera, A.F.; Beaven, A.W. Relapsed and Refractory Classical Hodgkin Lymphoma: Keeping Pace With Novel Agents and New Options for Salvage Therapy. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Zinzani, P.L. The role of transplantation in Hodgkin lymphoma. Br. J. Haematol. 2019, 184, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Connors, J.M.; Engert, A.; Larsen, E.K.; Huebner, D.; et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood 2016, 128, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zinzani, P.L.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019, 134, 1144–1153. [Google Scholar] [CrossRef]
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma after Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J. Clin. Oncol. 2018, 36, 1428–1439. [Google Scholar] [CrossRef]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar] [CrossRef]
- Alinari, L.; Quinion, C.; Blum, K.A. Bruton’s tyrosine kinase inhibitors in B-cell non-Hodgkin’s lymphomas. Clin. Pharmacol. Ther. 2015, 97, 469–477. [Google Scholar] [CrossRef]
- Zhu, S.; Gokhale, S.; Jung, J.; Spirollari, E.; Tsai, J.; Arceo, J.; Wu, B.W.; Victor, E.; Xie, P. Multifaceted Immunomodulatory Effects of the BTK Inhibitors Ibrutinib and Acalabrutinib on Different Immune Cell Subsets—Beyond B Lymphocytes. Front. Cell Dev. Biol. 2021, 9, 727531. [Google Scholar] [CrossRef]
- Long, M.; Beckwith, K.; Do, P.; Mundy, B.L.; Gordon, A.; Lehman, A.M.; Maddocks, K.J.; Cheney, C.; Jones, J.A.; Flynn, J.M.; et al. Ibrutinib treatment improves T cell number and function in CLL patients. J. Clin. Investig. 2017, 127, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.M.; Mirajkar, N.; Cutmore, N.; Zuo, J.; Long, H.; Kwok, M.; Oldrieve, C.; Hudson, C.; Stankovic, T.; Paneesha, S.; et al. Long-Term Ibrutinib Therapy Reverses CD8(+) T Cell Exhaustion in B Cell Chronic Lymphocytic Leukaemia. Front. Immunol. 2019, 10, 2832. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.E.; Handunnetti, S.M.; Ludford-Menting, M.; Sharpe, C.; Blombery, P.; Anderson, M.A.; Roberts, A.W.; Seymour, J.F.; Tam, C.S.; Ritchie, D.S.; et al. Immune recovery in patients with mantle cell lymphoma receiving long-term ibrutinib and venetoclax combination therapy. Blood Adv. 2020, 4, 4849–4859. [Google Scholar] [CrossRef]
- Dubovsky, J.A.; Beckwith, K.A.; Natarajan, G.; Woyach, J.A.; Jaglowski, S.; Zhong, Y.; Hessler, J.D.; Liu, T.M.; Chang, B.Y.; Larkin, K.M.; et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood 2013, 122, 2539–2549. [Google Scholar] [CrossRef]
- Hassenruck, F.; Knodgen, E.; Gockeritz, E.; Midda, S.H.; Vondey, V.; Neumann, L.; Herter, S.; Klein, C.; Hallek, M.; Krause, G. Sensitive Detection of the Natural Killer Cell-Mediated Cytotoxicity of Anti-CD20 Antibodies and Its Impairment by B-Cell Receptor Pathway Inhibitors. BioMed Res. Int. 2018, 2018, 1023490. [Google Scholar] [CrossRef] [PubMed]
- Da Roit, F.; Engelberts, P.J.; Taylor, R.P.; Breij, E.C.; Gritti, G.; Rambaldi, A.; Introna, M.; Parren, P.W.; Beurskens, F.J.; Golay, J. Ibrutinib interferes with the cell-mediated anti-tumor activities of therapeutic CD20 antibodies: Implications for combination therapy. Haematologica 2015, 100, 77–86. [Google Scholar] [CrossRef]
- Feng, M.; Chen, J.Y.; Weissman-Tsukamoto, R.; Volkmer, J.P.; Ho, P.Y.; McKenna, K.M.; Cheshier, S.; Zhang, M.; Guo, N.; Gip, P.; et al. Macrophages eat cancer cells using their own calreticulin as a guide: Roles of TLR and Btk. Proc. Natl. Acad. Sci. USA 2015, 112, 2145–2150. [Google Scholar] [CrossRef]
- Fernandez-Vega, I.; Quiros, L.M.; Santos-Juanes, J.; Pane-Foix, M.; Marafioti, T. Bruton’s tyrosine kinase (Btk) is a useful marker for Hodgkin and B cell non-Hodgkin lymphoma. Virchows Arch. 2015, 466, 229–235. [Google Scholar] [CrossRef]
- Martin, P.; Salas, C.; Provencio, M.; Abraira, V.; Bellas, C. Heterogeneous expression of Src tyrosine kinases Lyn, Fyn and Syk in classical Hodgkin lymphoma: Prognostic implications. Leuk. Lymphoma 2011, 52, 2162–2168. [Google Scholar] [CrossRef]
- Hamadani, M.; Balasubramanian, S.; Hari, P.N. Ibrutinib in Refractory Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 373, 1381–1382. [Google Scholar] [CrossRef]
- Badar, T.; Astle, J.; Kakar, I.K.; Zellner, K.; Hari, P.N.; Hamadani, M. Clinical activity of ibrutinib in classical Hodgkin lymphoma relapsing after allogeneic stem cell transplantation is independent of tumor BTK expression. Br. J. Haematol. 2020, 190, e98–e101. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, A.L.; Lymphoma, G.; Eastern Cooperative Oncology, G.; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Osa, A.; Uenami, T.; Koyama, S.; Fujimoto, K.; Okuzaki, D.; Takimoto, T.; Hirata, H.; Yano, Y.; Yokota, S.; Kinehara, Y.; et al. Clinical implications of monitoring nivolumab immunokinetics in non-small cell lung cancer patients. JCI Insight 2018, 3, e59125. [Google Scholar] [CrossRef] [PubMed]
- Advani, R.H.; Moskowitz, A.J.; Bartlett, N.L.; Vose, J.M.; Ramchandren, R.; Feldman, T.A.; LaCasce, A.S.; Christian, B.A.; Ansell, S.M.; Moskowitz, C.H.; et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood 2021, 138, 427–438. [Google Scholar] [CrossRef]
- Herrera, A.F.; Chen, R.W.; Palmer, J.; Tsai, N.-C.; Mei, M.; Popplewell, L.L.; Nademanee, A.P.; Nikolaenko, L.; McBride, K.; Ortega, R.; et al. PET-Adapted Nivolumab or Nivolumab Plus ICE As First Salvage Therapy in Relapsed or Refractory Hodgkin Lymphoma. Blood 2019, 134 (Suppl. S1), 239. [Google Scholar] [CrossRef]
- Armand, P.; Lesokhin, A.; Borrello, I.; Timmerman, J.; Gutierrez, M.; Zhu, L.; Popa McKiver, M.; Ansell, S.M. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/refractory lymphoid malignancies. Leukemia 2021, 35, 777–786. [Google Scholar] [CrossRef]
- Diefenbach, C.S.; Hong, F.; Ambinder, R.F.; Cohen, J.B.; Robertson, M.J.; David, K.A.; Advani, R.H.; Fenske, T.S.; Barta, S.K.; Palmisiano, N.D.; et al. Ipilimumab, nivolumab, and brentuximab vedotin combination therapies in patients with relapsed or refractory Hodgkin lymphoma: Phase 1 results of an open-label, multicentre, phase 1/2 trial. Lancet Haematol. 2020, 7, e660–e670. [Google Scholar] [CrossRef]
- Younes, A.; Brody, J.; Carpio, C.; Lopez-Guillermo, A.; Ben-Yehuda, D.; Ferhanoglu, B.; Nagler, A.; Ozcan, M.; Avivi, I.; Bosch, F.; et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: A phase 1/2a study. Lancet Haematol. 2019, 6, e67–e78. [Google Scholar] [CrossRef]
- Jain, N.; Senapati, J.; Thakral, B.; Ferrajoli, A.; Thompson, P.A.; Burger, J.A.; Basu, S.; Kadia, T.M.; Daver, N.G.; Borthakur, G.; et al. A Phase 2 Study of Nivolumab Combined with Ibrutinib in Patients with Diffuse Large B-cell Richter Transformation of CLL. Blood Adv. 2022. [Google Scholar] [CrossRef]
- Manson, G.; Brice, P.; Herbaux, C.; Bouabdallah, K.; Antier, C.; Poizeau, F.; Dercle, L.; Houot, R. Efficacy of anti-PD1 re-treatment in patients with Hodgkin lymphoma who relapsed after anti-PD1 discontinuation. Haematologica 2020, 105, 2664–2666. [Google Scholar] [CrossRef]
- Fedorova, L.V.; Lepik, K.V.; Mikhailova, N.B.; Kondakova, E.V.; Zalyalov, Y.R.; Baykov, V.V.; Babenko, E.V.; Kozlov, A.V.; Moiseev, I.S.; Afanasyev, B.V. Nivolumab discontinuation and retreatment in patients with relapsed or refractory Hodgkin lymphoma. Ann. Hematol. 2021, 100, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Schreck, S.; Friebel, D.; Buettner, M.; Distel, L.; Grabenbauer, G.; Young, L.S.; Niedobitek, G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol. Oncol. 2009, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Berglof, A.; Hamasy, A.; Meinke, S.; Palma, M.; Krstic, A.; Mansson, R.; Kimby, E.; Osterborg, A.; Smith, C.I. Targets for Ibrutinib Beyond B Cell Malignancies. Scand. J. Immunol. 2015, 82, 208–217. [Google Scholar] [CrossRef] [PubMed]
| Demographics | N = 17 |
|---|---|
| Age, years, median (range) | 40 (20–84) |
| Sex, no (%) | |
| Male | 8 (47.1%) |
| Female | 9 (52.9%) |
| Race, no (%) | |
| White | 16 (94.1%) |
| Black or African American | 1 (5.9%) |
| Diagnosis | |
| Classical Hodgkin’s lymphoma | 17 (100%) |
| Prior lines of treatment, median (range) | 5 (1–8) |
| Prior combination chemotherapy N (%) ABVD AVD + BV AVD other | 15 (88.2%) 12 (70.6%) 1 (5.9%) 1 (5.9%) 1 (5.9%) |
| Prior autologous stem cell transplant, N (%) | 8 (47.1%) |
| Prior brentuximab, N (%) | 13 (76.5%) |
| Prior nivolumab, N (%) | 10 (58.8%) |
| Best Response | N = 17 |
| Overall response (95% CI) | 52.9 (31.0–73.8) |
| Complete response (95% CI) | 29.4 (12.9–53.4) |
| Partial response (95% CI) | 23.5 (9.0–47.7) |
| Stable disease (95% CI) | 23.5 (9.0–47.5) |
| Progressive disease (95% CI) | 5.9 (0–28.9) |
| Not evaluable due to toxicity (95% CI) | 11.7 (2.0–35.6) |
| Off-treatment Reason | N = 16 |
| Disease progression (percent) | 7 (43.8%) |
| Adverse event (percent) | 4 (25.0%) |
| Auto-HCT transplant (percent) | 2 (12.5%) |
| Allo-HCT transplant (percent) | 2 (12.5%) |
| Patient withdrawal | 1 (6.3%) |
| Progression-free survival | |
| Number of events | 7 |
| Number censored | 10 |
| Median | 17.3 |
| Median follow-up (months) | 8.9 |
| Duration of response | |
| Number of events | 3 |
| Number censored | 7 |
| Median | 20.2 |
| Median follow-up (months) | 6.1 |
| Any Grade | Grade ≥ 3 | |
|---|---|---|
| Anemia | 6 (35) | 0 (0) |
| Lymphopenia | 6 (35) | 2 (12) |
| Fatigue | 5 (29) | 0 (0) |
| Thrombocytopenia | 5 (29) | 0 (0) |
| Myalgia | 5 (29) | 0 (0) |
| Rash | 4 (24) | 3 (18) |
| Hypertension | 4 (24) | 0 (0) |
| Blood and lymphatic system disorders | 3 (18) | 0 (0) |
| Gastroesophageal reflux | 3 (18) | 0 (0) |
| Fever | 3 (18) | 0 (0) |
| Dysgeusia | 3 (18) | 0 (0) |
| Diarrhea | 2 (12) | 0 (0) |
| Dyspepsia | 2 (12) | 0 (0) |
| Dysphagia | 2 (12) | 0 (0) |
| Nausea | 2 (12) | 0 (0) |
| Emesis | 2 (12) | 0 (0) |
| Urinary tract infection | 2 (12) | 1 (6) |
| Ecchymosis | 2 (12) | 0 (0) |
| AST increased | 2 (12) | 0 (0) |
| Neutropenia | 2 (12) | 0 (0) |
| Weight gain | 2 (12) | 0 (0) |
| Leukopenia | 2 (12) | 0 (0) |
| Hematuria | 2 (12) | 1 (6) |
| Pruritis | 2 (12) | 0 (0) |
| Skin and subcutaneous disorders | 2 (12) | 1 (6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanel, W.; Shindiapina, P.; Bond, D.A.; Sawalha, Y.; Epperla, N.; Voorhees, T.; Welkie, R.L.; Huang, Y.; Behbehani, G.K.; Zhang, X.; et al. A Phase 2 Trial of Ibrutinib and Nivolumab in Patients with Relapsed or Refractory Classical Hodgkin’s Lymphoma. Cancers 2023, 15, 1437. https://doi.org/10.3390/cancers15051437
Hanel W, Shindiapina P, Bond DA, Sawalha Y, Epperla N, Voorhees T, Welkie RL, Huang Y, Behbehani GK, Zhang X, et al. A Phase 2 Trial of Ibrutinib and Nivolumab in Patients with Relapsed or Refractory Classical Hodgkin’s Lymphoma. Cancers. 2023; 15(5):1437. https://doi.org/10.3390/cancers15051437
Chicago/Turabian StyleHanel, Walter, Polina Shindiapina, David A. Bond, Yazeed Sawalha, Narendranath Epperla, Timothy Voorhees, Rina Li Welkie, Ying Huang, Gregory K. Behbehani, Xiaoli Zhang, and et al. 2023. "A Phase 2 Trial of Ibrutinib and Nivolumab in Patients with Relapsed or Refractory Classical Hodgkin’s Lymphoma" Cancers 15, no. 5: 1437. https://doi.org/10.3390/cancers15051437
APA StyleHanel, W., Shindiapina, P., Bond, D. A., Sawalha, Y., Epperla, N., Voorhees, T., Welkie, R. L., Huang, Y., Behbehani, G. K., Zhang, X., McLaughlin, E., Chan, W. K., Brammer, J. E., Jaglowski, S., Reneau, J. C., Christian, B. A., William, B. M., Cohen, J. B., Baiocchi, R. A., ... Alinari, L. (2023). A Phase 2 Trial of Ibrutinib and Nivolumab in Patients with Relapsed or Refractory Classical Hodgkin’s Lymphoma. Cancers, 15(5), 1437. https://doi.org/10.3390/cancers15051437









