The CXCL10/CXCR3 Pathway Contributes to the Synergy of Thermal Ablation and PD-1 Blockade Therapy against Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Animals
2.2. Tumor Model, MWA Treatment, and Anti-PD-1 Therapy
2.3. Flow Cytometry
2.4. Isolation of Tumor-Infiltrating CD8+T Cells
2.5. Analysis of RNA-Seq Data
2.6. Analysis of scRNA-Seq Data
2.7. Differential Gene Expression Analysis
2.8. Gene Set Enrichment Analysis
2.9. GO Enrichment Analysis
2.10. Statistical Analysis
3. Results
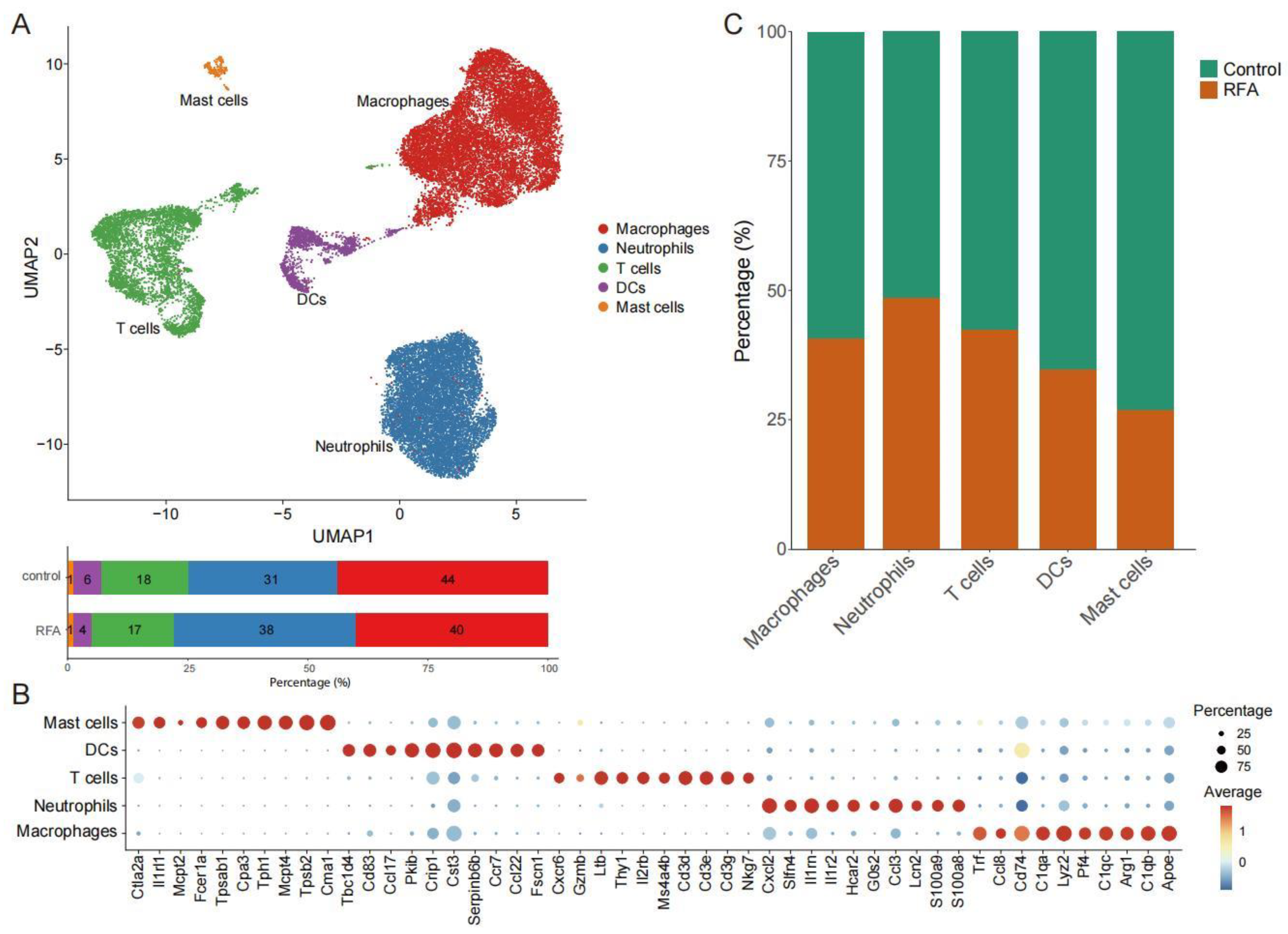
3.1. scRNA-Seq Identifies Tumor-Infiltrating Immune Cells
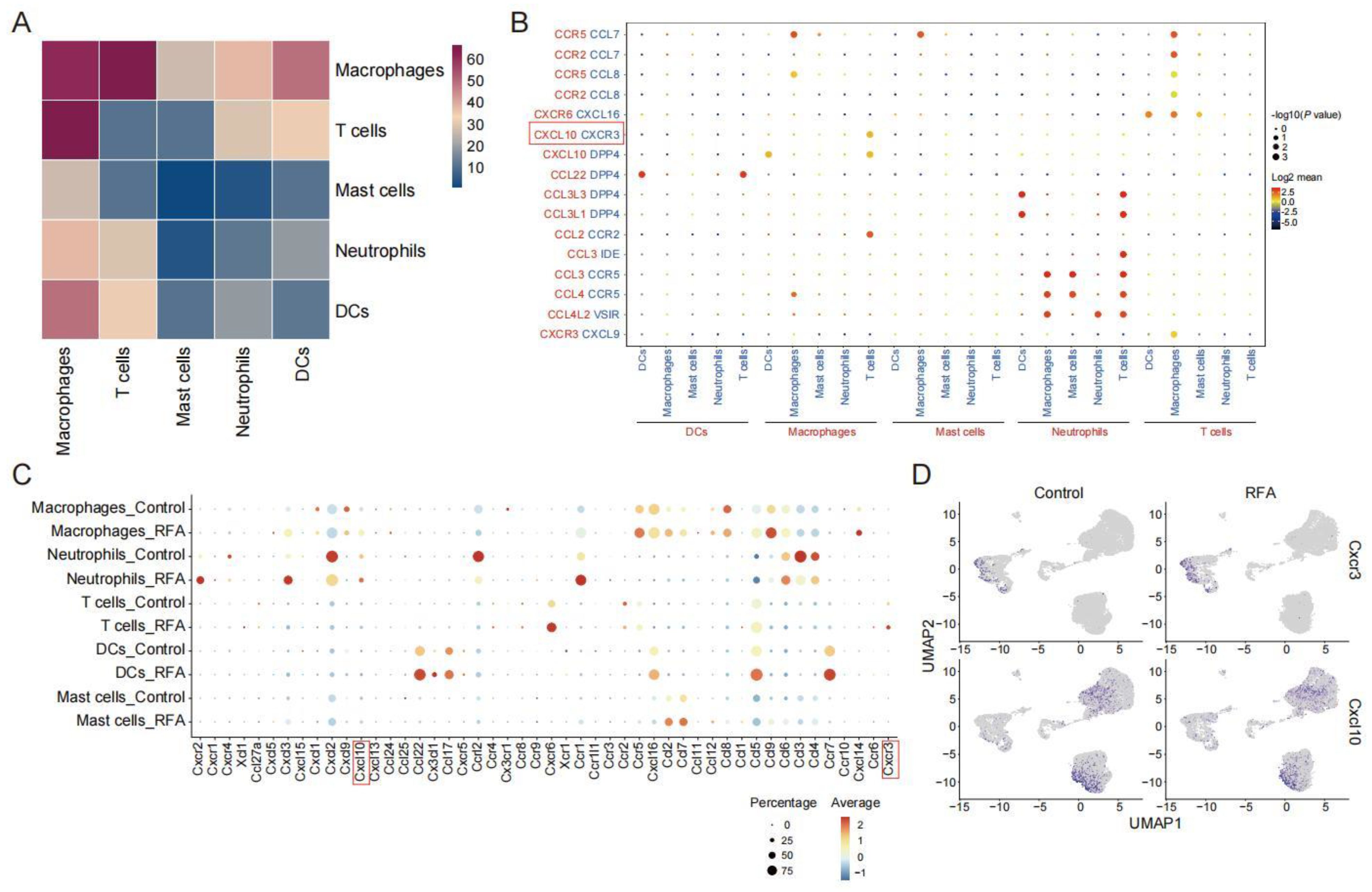
3.2. Ablation Therapy Affects the Interaction of Tumor-Infiltrating Immune Cell Subsets
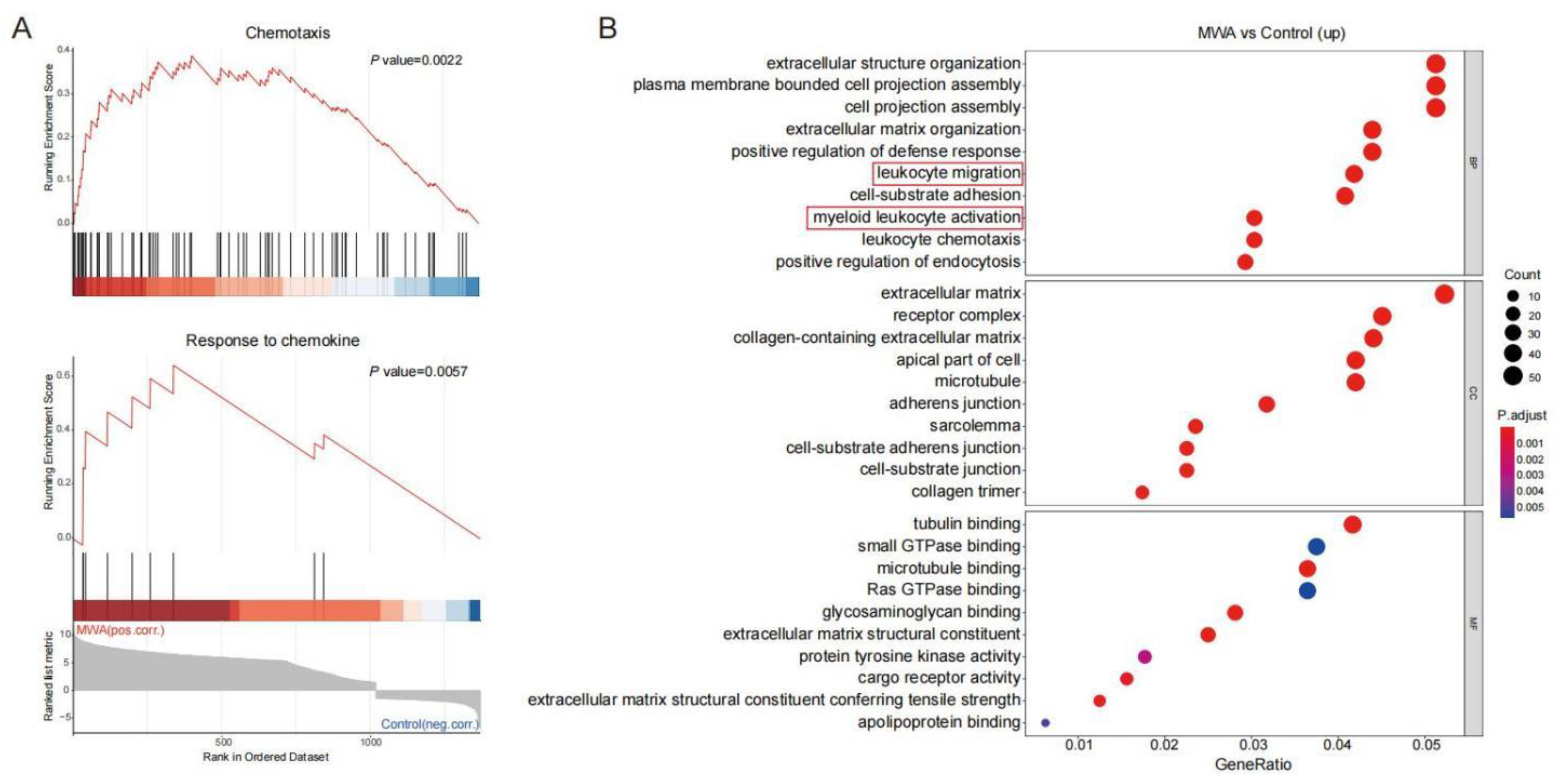
3.3. Ablation-Induced Remodeling of TILs in the TME
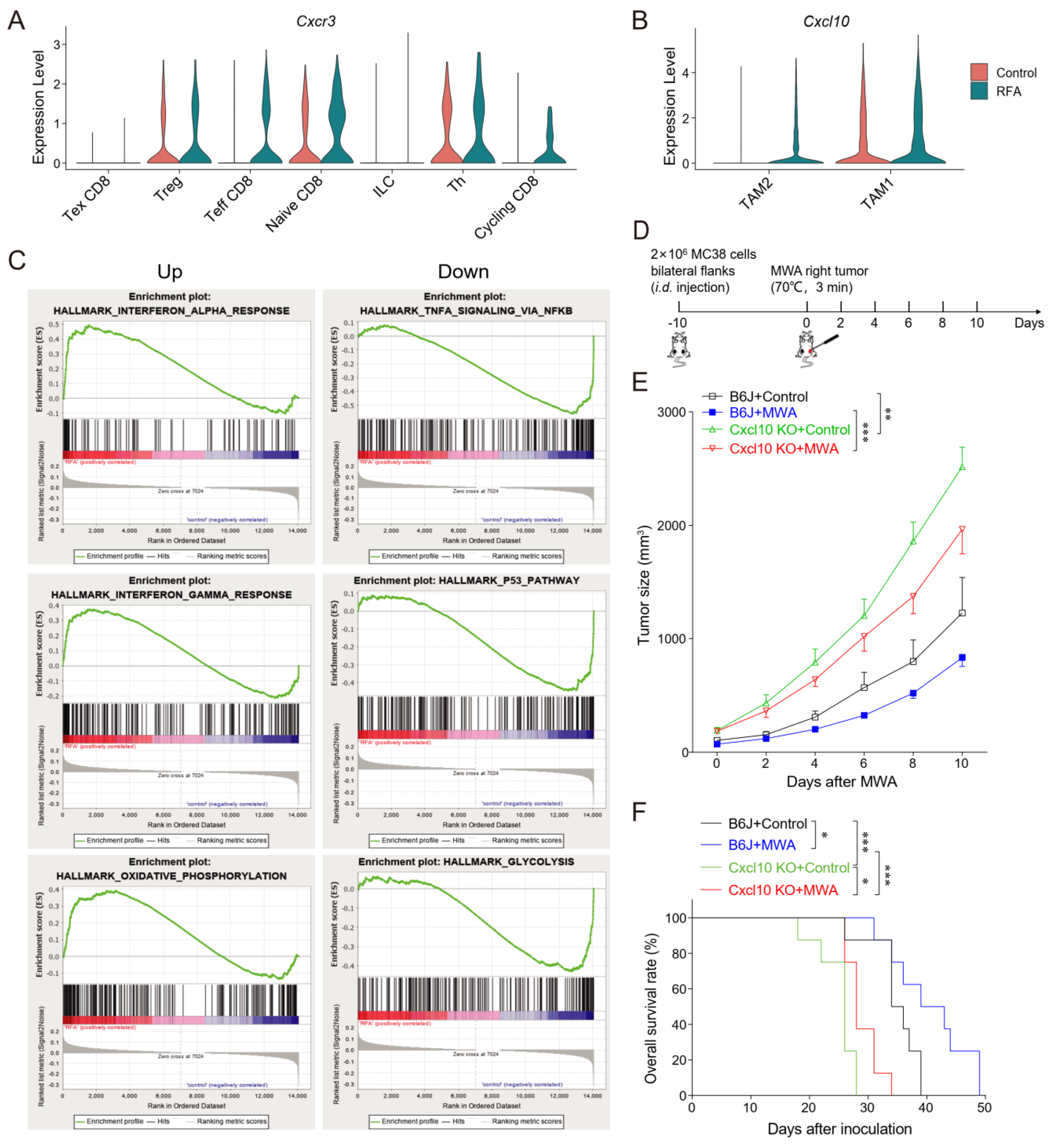
3.4. CXCL10/CXCR3 Contributes Essentially to Thermal-Ablation-Induced Anti-Tumor Effect
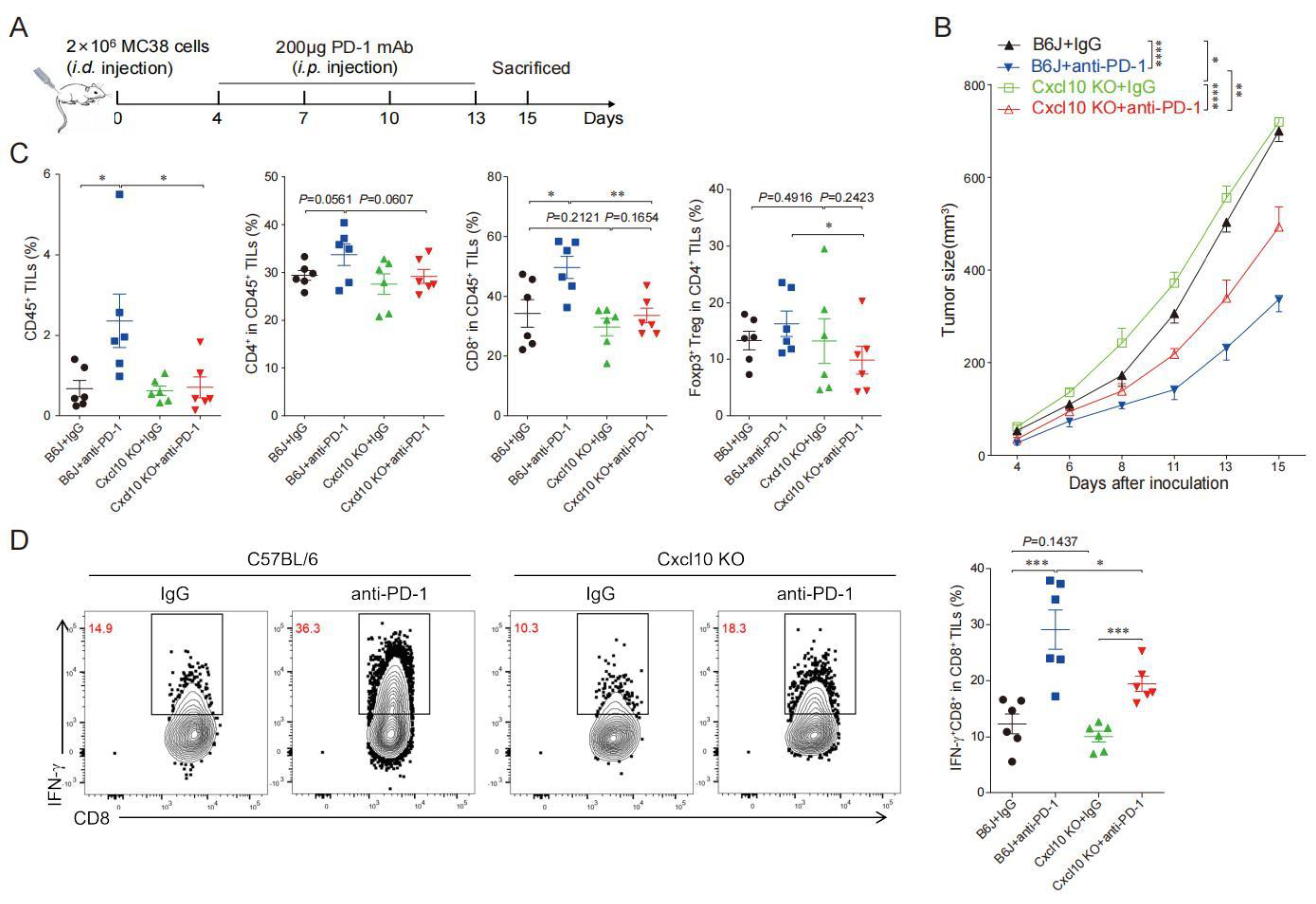
3.5. CXCL10 Is Required for Effective Response to PD-1 Blockade Therapy
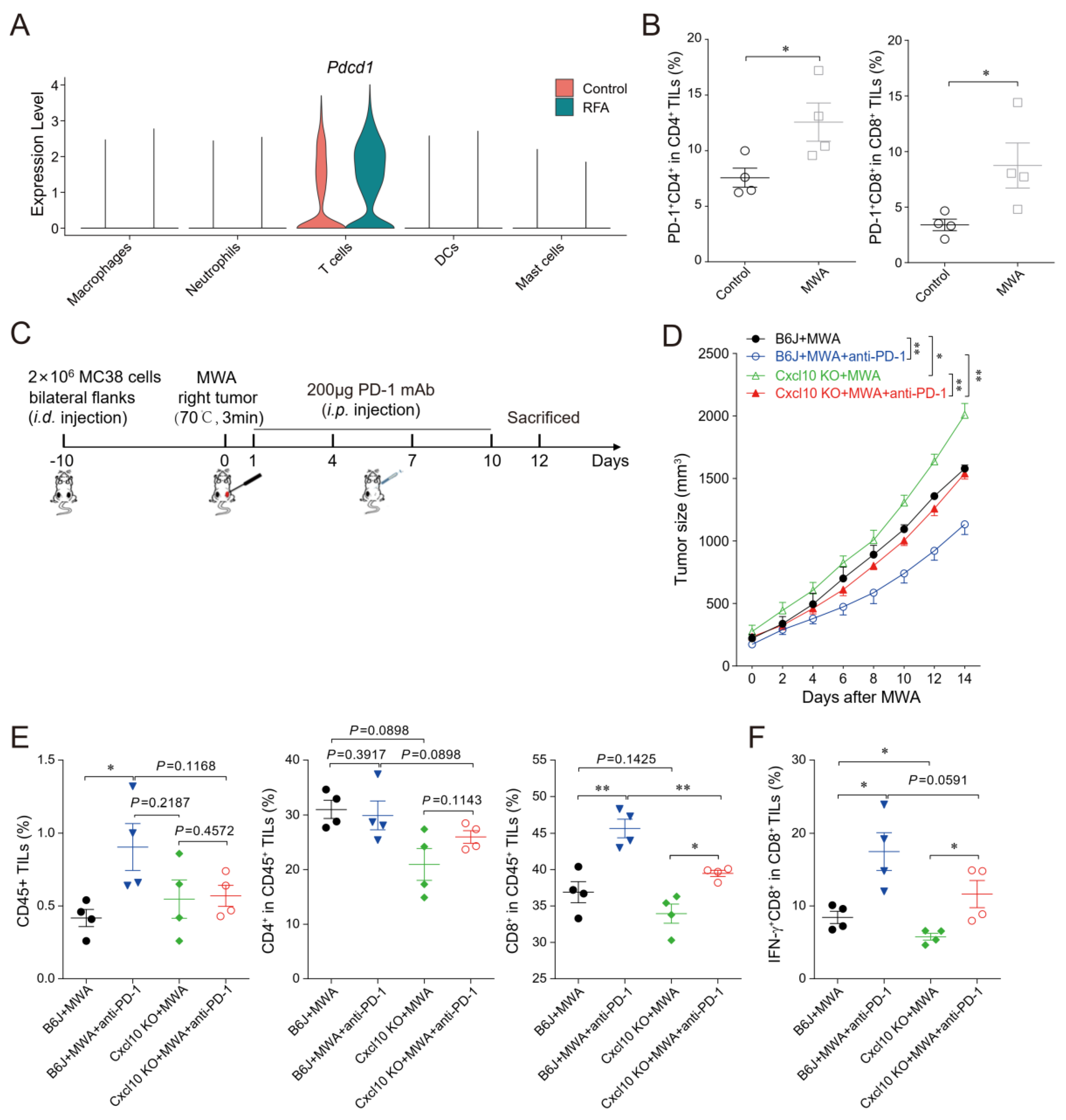
3.6. CXCL10 Enhances the Synergistic Anti-Tumor Effect of Thermal Ablation Combined with PD-1 Blockade
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garnon, J.; Cazzato, R.L.; Caudrelier, J.; Nouri-Neuville, M.; Rao, P.; Boatta, E.; Ramamurthy, N.; Koch, G.; Gangi, A. Adjunctive Thermoprotection During Percutaneous Thermal Ablation Procedures: Review of Current Techniques. Cardiovasc. Interv. Radiol. 2019, 42, 344–357. [Google Scholar] [CrossRef]
- De Baere, T.; Tselikas, L.; Delpla, A.; Roux, C.; Varin, E.; Kobe, A.; Yevich, S.; Deschamps, F. Thermal ablation in the management of oligometastatic colorectal cancer. Int. J. Hyperth. 2022, 39, 627–632. [Google Scholar] [CrossRef]
- Jiang, A.N.; Wang, B.; Wang, S.; Zhao, K.; Wu, H.; Yan, K.; Wu, W.; Yang, W. The study of direct and indirect effects of radiofrequency ablation on tumor microenvironment in liver tumor animal model. BMC Cancer 2022, 22, 663. [Google Scholar] [CrossRef] [PubMed]
- van den Bijgaart, R.J.E.; Schuurmans, F.; Futterer, J.J.; Verheij, M.; Cornelissen, L.A.M.; Adema, G.J. Immune Modulation Plus Tumor Ablation: Adjuvants and Antibodies to Prime and Boost Anti-Tumor Immunity In Situ. Front. Immunol. 2021, 12, 617365. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, L.; Wu, C.; Zhu, Y.; Xu, B.; Zheng, X.; Sun, M.; Wen, W.; Dai, X.; Yang, M.; et al. PD-1 Blockade Boosts Radiofrequency Ablation-Elicited Adaptive Immune Responses against Tumor. Clin. Cancer Res. 2016, 22, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Ngiow, S.F.; Ribas, A.; Teng, M.W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 2016, 13, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Wang, Y.; Chlewicki, L.K.; Zhang, Y.; Guo, J.; Liang, W.; Wang, J.; Wang, X.; Fu, Y.X. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell 2016, 29, 285–296. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; A Curley, S. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef]
- Ghosn, M.; Solomon, S.B. Current Management of Oligometastatic Lung Cancer and Future Perspectives: Results of Thermal Ablation as a Local Ablative Therapy. Cancers 2021, 13, 5202. [Google Scholar] [CrossRef]
- Zhu, F.; Rhim, H. Thermal ablation for hepatocellular carcinoma: What’s new in 2019. Chin. Clin. Oncol. 2019, 8, 58. [Google Scholar] [CrossRef]
- Takahashi, H.; Berber, E. Role of thermal ablation in the management of colorectal liver metastasis. Hepatobiliary Surg. Nutr. 2020, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Fu, Y.; Zhu, X.; Wang, L.; Ling, C. Effect of RFA and TACE combined with postoperative cytokine-induced killer cell immunotherapy in primary hepatocellular carcinoma. J. BUON 2021, 26, 235–242. [Google Scholar] [PubMed]
- Keisari, Y. Tumor abolition and anti-tumor immunostimulation by physico-chemical tumor ablation. Front. Biosci. 2017, 22, 310–347. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Ribas, A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef]
- Abril-Rodriguez, G.; Ribas, A. SnapShot: Immune Checkpoint Inhibitors. Cancer Cell 2017, 31, 848–848.e1. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- de Miguel, M.; Calvo, E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell 2020, 38, 326–333. [Google Scholar] [CrossRef]
- Kiefer, F.; Siekmann, A.F. The role of chemokines and their receptors in angiogenesis. Cell. Mol. Life Sci. CMLS 2011, 68, 2811–2830. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Kikuchi, N.; Ye, J.; Hirakawa, J.; Kawashima, H. Forced Expression of CXCL10 Prevents Liver Metastasis of Colon Carcinoma Cells by the Recruitment of Natural Killer Cells. Biol. Pharm. Bull. 2019, 42, 57–65. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Ragusa, F.; Ruffilli, I.; Elia, G.; Paparo, S.R.; Antonelli, A. Th1 Chemokines in Autoimmune Endocrine Disorders. J. Clin. Endocrinol. Metab. 2020, 105, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget 2015, 6, 2981–2991. [Google Scholar] [CrossRef] [PubMed]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Pan, Y.; Lin, W.; Zhou, Y.; Yu, X.; Hou, Z.; Yu, X.; Lin, X.; Lin, R.; Lu, F.; et al. High-dimensional single-cell analysis delineates radiofrequency ablation induced immune microenvironmental remodeling in pancreatic cancer. Cell Death Dis. 2020, 11, 589. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, H.; Li, Y.; Xiao, W.; Liu, Y.; Chen, R.; Zhu, Y.; Zheng, X.; Wu, C.; Chen, L. TIGIT Blockade Exerts Synergistic Effects on Microwave Ablation Against Cancer. Front. Immunol. 2022, 13, 832230. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e429. [Google Scholar] [CrossRef]
- Efremova, M.; Vento-Tormo, M.; Teichmann, S.A.; Vento-Tormo, R. CellPhoneDB: Inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 2020, 15, 1484–1506. [Google Scholar] [CrossRef]
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.e622. [Google Scholar] [CrossRef]
- Browaeys, R.; Saelens, W.; Saeys, Y. NicheNet: Modeling intercellular communication by linking ligands to target genes. Nat. Methods 2020, 17, 159–162. [Google Scholar] [CrossRef]
- House, I.G.; Savas, P.; Lai, J.; Chen, A.X.; Oliver, A.J.; Teo, Z.L.; Todd, K.L.; Henderson, M.A.; Giuffrida, L.; Petley, E.V.; et al. Macrophage-Derived CXCL9 and CXCL10 Are Required for anti-tumor Immune Responses Following Immune Checkpoint Blockade. Clin. Cancer Res. 2020, 26, 487–504. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, S.; Ni, H.; Zhao, P.; Chen, G.; Xu, B.; Yuan, L. The role of CXCR3 and its ligands in cancer. Front. Oncol. 2022, 12, 1022688. [Google Scholar] [CrossRef] [PubMed]
- Groom, J.R.; Luster, A.D. CXCR3 in T cell function. Exp. Cell Res. 2011, 317, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Leuchte, K.; Staib, E.; Thelen, M.; Gödel, P.; Lechner, A.; Zentis, P.; Garcia-Marquez, M.; Waldschmidt, D.; Datta, R.R.; Wahba, R.; et al. Microwave ablation enhances tumor-specific immune response in patients with hepatocellular carcinoma. Cancer Immunol. Immunother. 2021, 70, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef]
- Pandey, V.; Fleming-Martinez, A.; Bastea, L.; Doeppler, H.R.; Eisenhauer, J.; Le, T.; Edenfield, B.; Storz, P. CXCL10/CXCR3 signaling contributes to an inflammatory microenvironment and its blockade enhances progression of murine pancreatic precancerous lesions. eLife 2021, 10, e60646. [Google Scholar] [CrossRef]
- Chen, M.; Tan, Y.; Hu, J.; Jiang, Y.; Wang, Z.; Liu, Z.; Chen, Q. Injectable Immunotherapeutic Thermogel for Enhanced Immunotherapy Post Tumor Radiofrequency Ablation. Small 2021, 17, e2104773. [Google Scholar] [CrossRef]
- Bangs, D.J.; Tsitsiklis, A.; Steier, Z.; Chan, S.W.; Kaminski, J.; Streets, A.; Yosef, N.; Robey, E.A. CXCR3 regulates stem and proliferative CD8+ T cells during chronic infection by promoting interactions with DCs in splenic bridging channels. Cell Rep. 2022, 38, 110266. [Google Scholar] [CrossRef]
- Xiao, Q.; Nobre, A.; Pineiro, P.; Berciano-Guerrero, M.A.; Alba, E.; Cobo, M.; Lauschke, V.M.; Barragan, I. Genetic and Epigenetic Biomarkers of Immune Checkpoint Blockade Response. J. Clin. Med. 2020, 9, 286. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Xu, C.; Lou, Y.; Chen, J.; Yang, Y.; Yagita, H.; Overwijk, W.W.; Lizée, G.; Radvanyi, L.; et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012, 72, 5209–5218. [Google Scholar] [CrossRef]
- Kamada, T.; Togashi, Y.; Tay, C.; Ha, D.; Sasaki, A.; Nakamura, Y.; Sato, E.; Fukuoka, S.; Tada, Y.; Tanaka, A.; et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 9999–10008. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Chen, Y.; Huang, H.; Liu, Y.; Chen, J.; Zhu, D.; Zheng, X.; Chen, L.; Jiang, J. LAG3 blockade coordinates with microwave ablation to promote CD8(+) T cell-mediated anti-tumor immunity. J. Transl. Med. 2022, 20, 433. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Huang, H.; Zheng, P.; Liu, Y.; Chen, Y.; Chen, J.; Zheng, X.; Chen, L.; Jiang, J. The CXCL10/CXCR3 Pathway Contributes to the Synergy of Thermal Ablation and PD-1 Blockade Therapy against Tumors. Cancers 2023, 15, 1427. https://doi.org/10.3390/cancers15051427
Xiao W, Huang H, Zheng P, Liu Y, Chen Y, Chen J, Zheng X, Chen L, Jiang J. The CXCL10/CXCR3 Pathway Contributes to the Synergy of Thermal Ablation and PD-1 Blockade Therapy against Tumors. Cancers. 2023; 15(5):1427. https://doi.org/10.3390/cancers15051427
Chicago/Turabian StyleXiao, Wenlu, Hao Huang, Panpan Zheng, Yingting Liu, Yaping Chen, Junjun Chen, Xiao Zheng, Lujun Chen, and Jingting Jiang. 2023. "The CXCL10/CXCR3 Pathway Contributes to the Synergy of Thermal Ablation and PD-1 Blockade Therapy against Tumors" Cancers 15, no. 5: 1427. https://doi.org/10.3390/cancers15051427
APA StyleXiao, W., Huang, H., Zheng, P., Liu, Y., Chen, Y., Chen, J., Zheng, X., Chen, L., & Jiang, J. (2023). The CXCL10/CXCR3 Pathway Contributes to the Synergy of Thermal Ablation and PD-1 Blockade Therapy against Tumors. Cancers, 15(5), 1427. https://doi.org/10.3390/cancers15051427






