Discordance of HER2-Low between Primary Tumors and Matched Distant Metastases in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
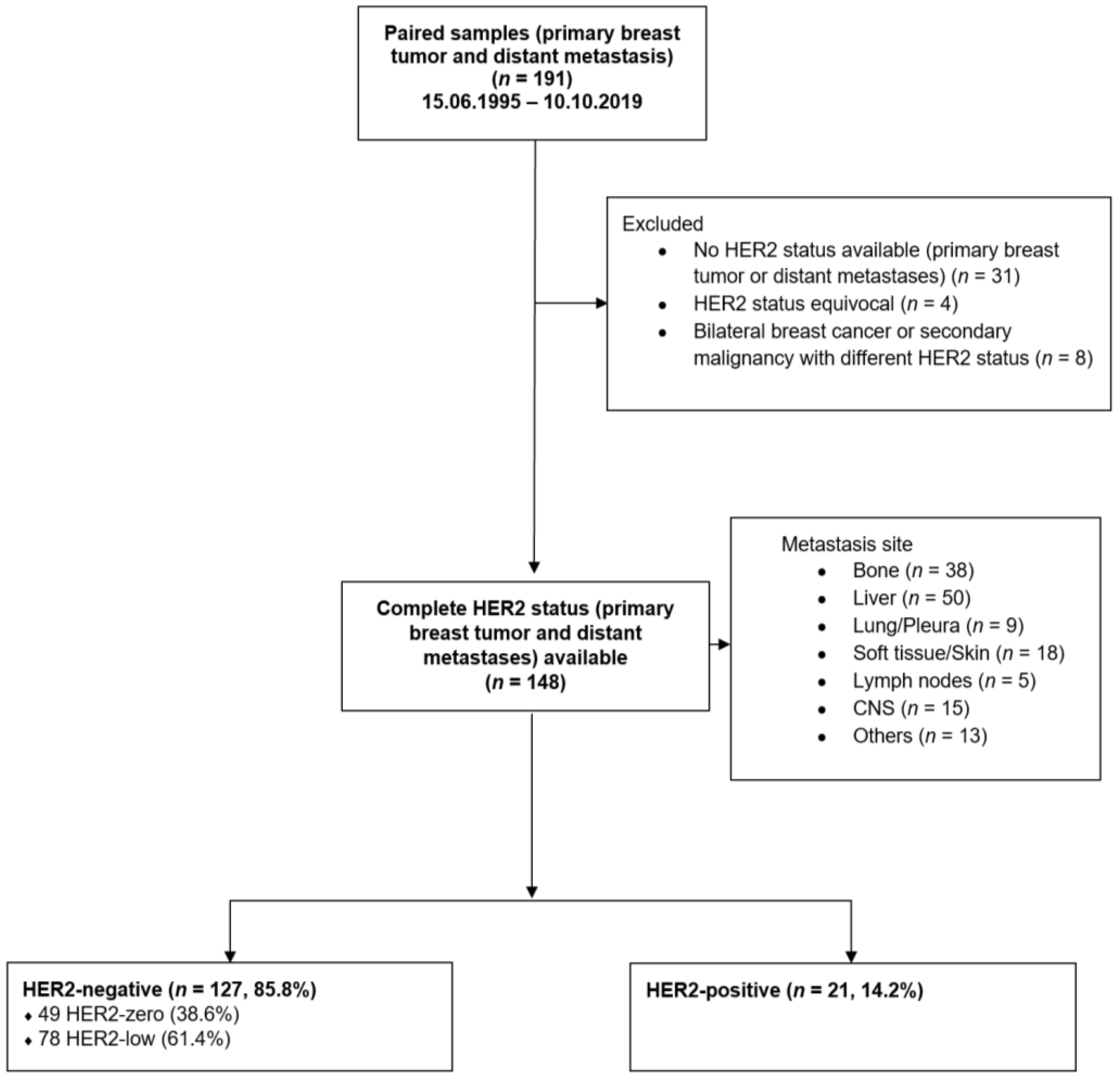
2.1. Study Cohort
2.2. Immunohistochemistry (IHC) and In Situ Hybridization (ISH)
2.3. Statistical Analysis
3. Results
3.1. Patient Population
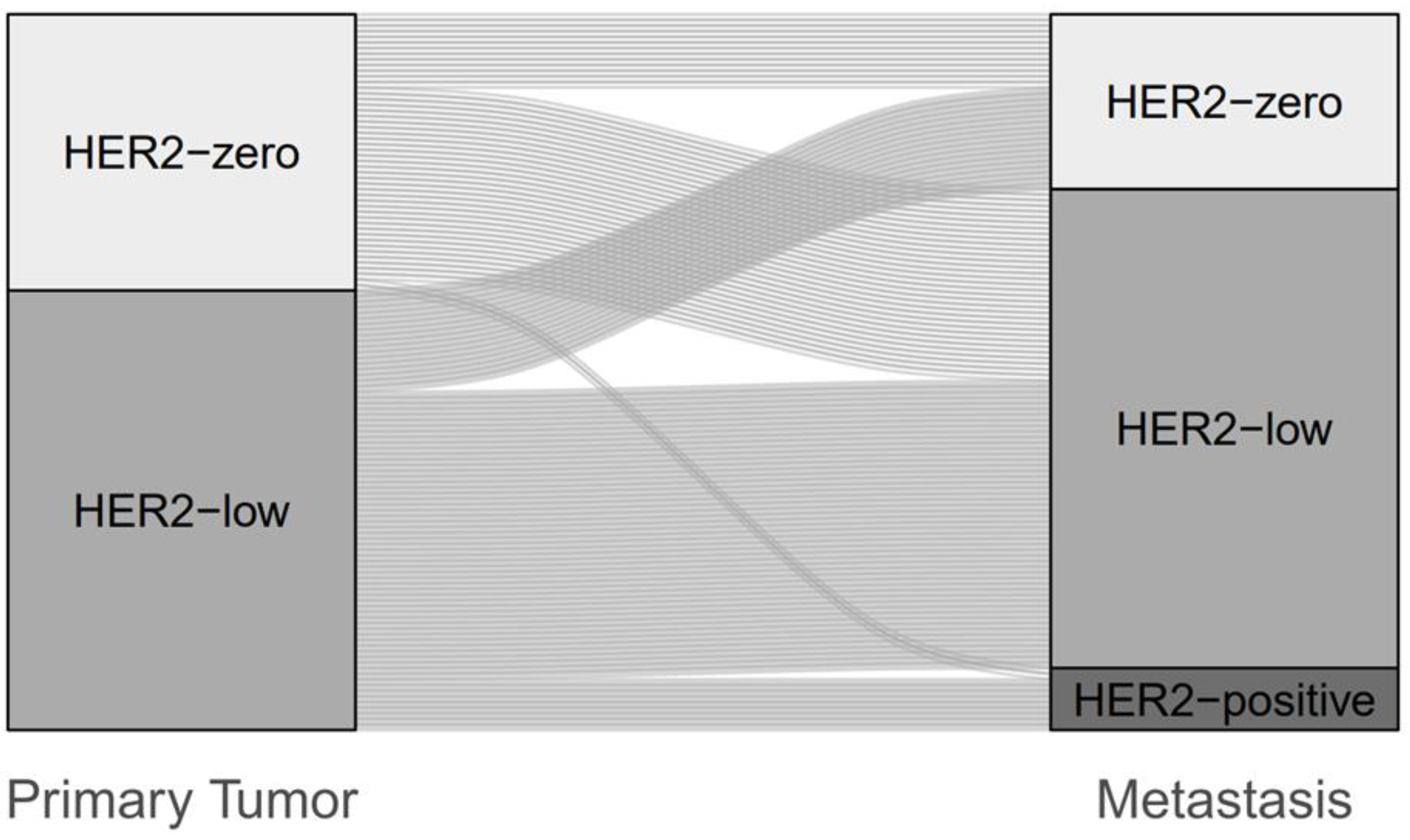
3.2. Change of HER2 Status between Primary Breast Cancer and Metastasis
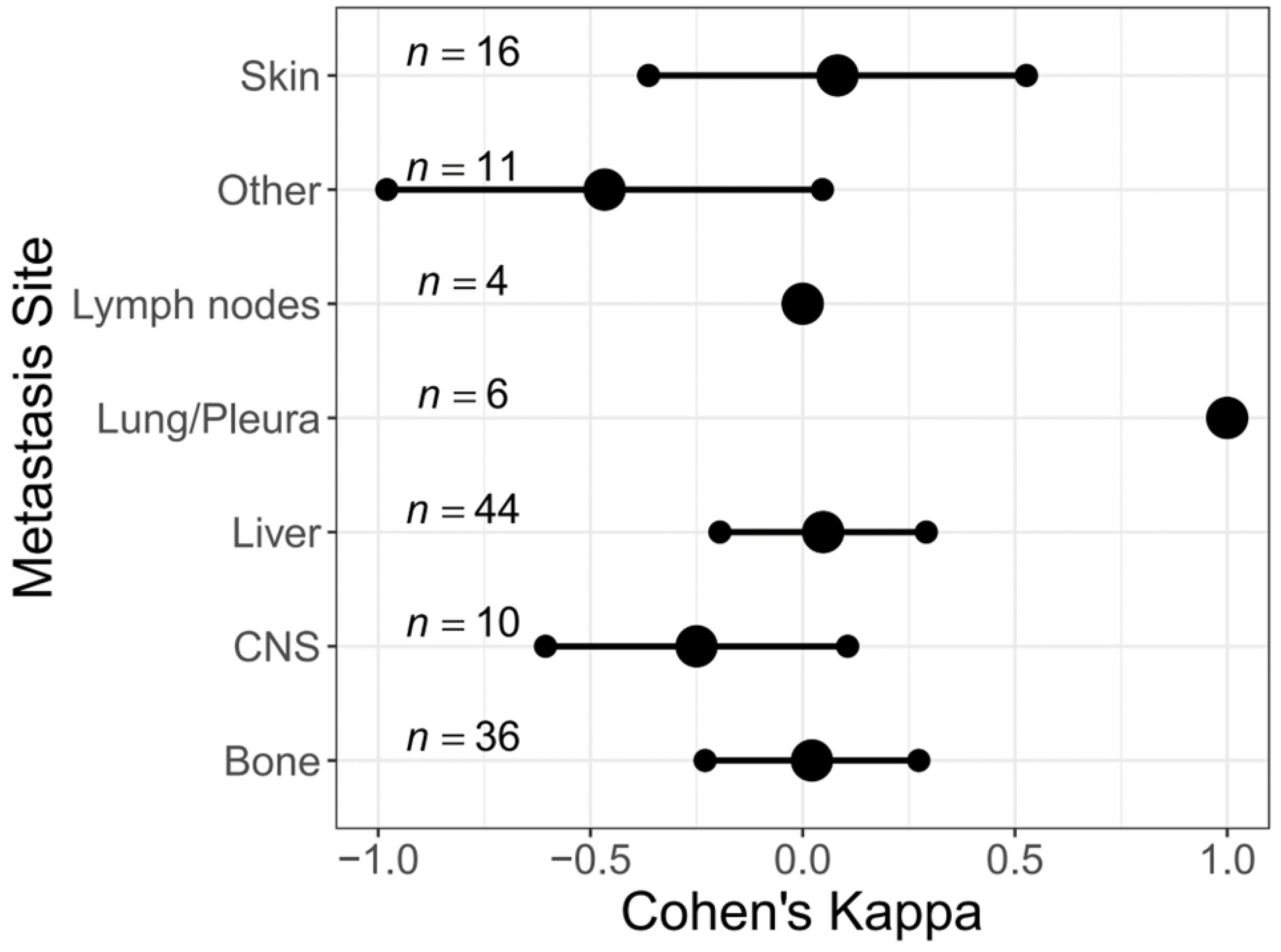
3.3. Change of HER2 Status in Different Metastatic Sites
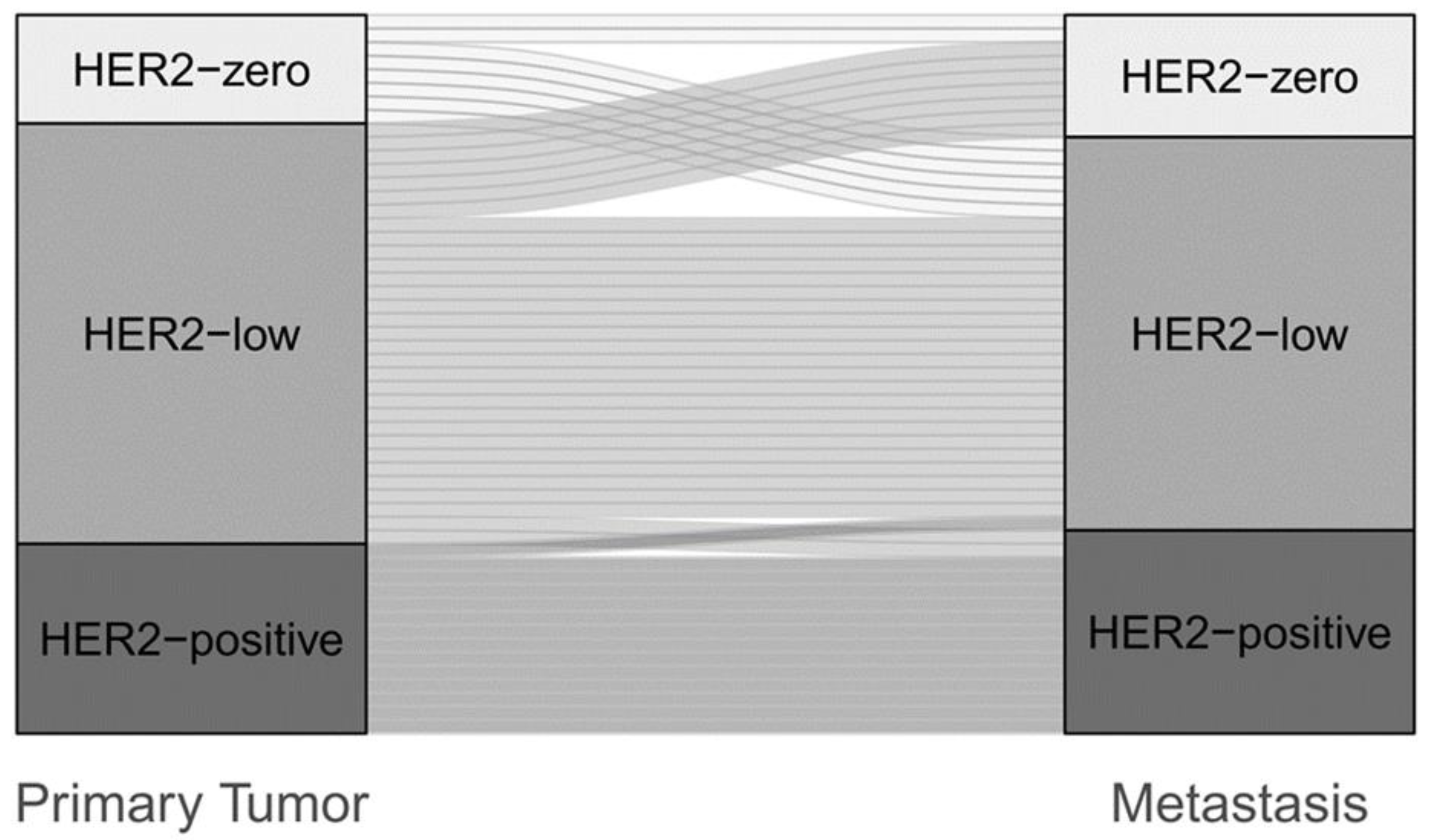
3.4. Change of HER2 Status in Different Molecular Subtypes
3.5. Change of HER2 Status in Primary vs. Secondary Metastatic Breast Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021, 22, 1139–1150. [CrossRef]
- Paik, S.; Kim, C.; Wolmark, N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N. Engl. J. Med. 2008, 358, 1409–1411. [Google Scholar] [CrossRef]
- Perez, E.A.; Reinholz, M.M.; Hillman, D.W.; Tenner, K.S.; Schroeder, M.J.; Davidson, N.E.; Martino, S.; Sledge, G.W.; Harris, L.N.; Gralow, J.R.; et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J. Clin. Oncol. 2010, 28, 4307–4315. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E.; Rastogi, P.J.R.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.-F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; de La Haba-Rodriguez, J.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Li, Y.; Abudureheiyimu, N.; Mo, H.; Guan, X.; Lin, S.; Wang, Z.; Chen, Y.; Chen, S.; Li, Q.; Cai, R.; et al. In Real Life, Low-Level HER2 Expression May Be Associated With Better Outcome in HER2-Negative Breast Cancer: A Study of the National Cancer Center, China. Front. Oncol. 2021, 11, 774577. [Google Scholar] [CrossRef]
- Mutai, R.; Barkan, T.; Moore, A.; Sarfaty, M.; Shochat, T.; Yerushalmi, R.; Stemmer, S.M.; Goldvaser, H. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast 2021, 60, 62–69. [Google Scholar] [CrossRef]
- Denkert, C.; Seither, F.; Schneeweiss, A.; Link, T.; Blohmer, J.-U.; Just, M.; Wimberger, P.; Forberger, A.; Tesch, H.; Jackisch, C.; et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021, 22, 1151–1161. [Google Scholar] [CrossRef]
- Almstedt, K.; Heimes, A.-S.; Kappenberg, F.; Battista, M.J.; Lehr, H.-A.; Krajnak, S.; Lebrecht, A.; Gehrmann, M.; Stewen, K.; Brenner, W.; et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur. J. Cancer 2022, 173, 10–19. [Google Scholar] [CrossRef]
- Rosso, C.; Voutsadakis, I.A. Characteristics, Clinical Differences and Outcomes of Breast Cancer Patients with Negative or Low HER2 Expression. Clin. Breast Cancer 2022, 22, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.S.Y.C.; Ong, W.S.; Lee, K.-H.; Lim, A.H.; Park, S.; Park, Y.H.; Lin, C.-H.; Lu, Y.-S.; Ono, M.; Ueno, T.; et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: An international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Niman, S.M.; Erick, T.K.; Priedigkeit, N.; Harrison, B.T.; Giordano, A.; Nakhlis, F.; Bellon, J.R.; Parker, T.; Strauss, S.; et al. HER2-low inflammatory breast cancer: Clinicopathologic features and prognostic implications. Eur. J. Cancer 2022, 174, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, W.; Liu, D.; Chen, W.; Shen, K.; Wu, J.; Zhu, L. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: Features of HER2-low breast cancer. Breast Cancer 2022, 29, 844–853. [Google Scholar] [CrossRef]
- Douganiotis, G.; Kontovinis, L.; Markopoulou, E.; Ainali, A.; Zarampoukas, T.; Natsiopoulos, I.; Papazisis, K. Prognostic Significance of Low HER2 Expression in Patients With Early Hormone Receptor Positive Breast Cancer. Cancer Diagn. Progn. 2022, 2, 316–323. [Google Scholar] [CrossRef]
- Horisawa, N.; Adachi, Y.; Takatsuka, D.; Nozawa, K.; Endo, Y.; Ozaki, Y.; Sugino, K.; Kataoka, A.; Kotani, H.; Yoshimura, A.; et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 2021, 29, 234–241. [Google Scholar] [CrossRef]
- Jacot, W.; Maran-Gonzalez, A.; Massol, O.; Sorbs, C.; Mollevi, C.; Guiu, S.; Boissière-Michot, F.; Ramos, J. Prognostic Value of HER2-Low Expression in Non-Metastatic Triple-Negative Breast Cancer and Correlation with Other Biomarkers. Cancers 2021, 13, 6059. [Google Scholar] [CrossRef]
- Agostinetto, E.; Rediti, M.; Fimereli, D.; Debien, V.; Piccart, M.; Aftimos, P.; Sotiriou, C.; de Azambuja, E. HER2-Low Breast Cancer: Molecular Characteristics and Prognosis. Cancers (Basel) 2021, 13, 2824. [Google Scholar] [CrossRef]
- Schettini, F.; Chic, N.; Brasó-Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez-Sáez, O.; Adamo, B.; Vidal, M.; Barnadas, E.; et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, Y.; Luo, Z.; Guan, H.; Zhu, F.; He, Y.; Chen, Q.; Liu, C.; Nie, B.; Liu, H. Clinical, Pathological Complete Response, and Prognosis Characteristics of HER2-Low Breast Cancer in the Neoadjuvant Chemotherapy Setting: A Retrospective Analysis. Ann. Surg. Oncol. 2022, 29, 8026–8034. [Google Scholar] [CrossRef]
- Shu, L.; Tong, Y.; Li, Z.; Chen, X.; Shen, K. Can HER2 1+ Breast Cancer Be Considered as HER2-Low Tumor? A Comparison of Clinicopathological Features, Quantitative HER2 mRNA Levels, and Prognosis among HER2-Negative Breast Cancer. Cancers 2022, 14, 4250. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Gandini, S.; Nicolò, E.; Trillo, P.; Giugliano, F.; Zagami, P.; Vivanet, G.; Bellerba, F.; Trapani, D.; Marra, A.; et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur. J. Cancer 2022, 163, 35–43. [Google Scholar] [CrossRef] [PubMed]
- van den Ende, N.S.; Smid, M.; Timmermans, A.; van Brakel, J.B.; Hansum, T.; Foekens, R.; Trapman, A.M.A.C.; Heemskerk-Gerritsen, B.A.M.; Jager, A.; Martens, J.W.M.; et al. HER2-low breast cancer shows a lower immune response compared to HER2-negative cases. Sci. Rep. 2022, 12, 12974. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Ahn, J.; Kim, Y.; Kim, J.S.; Song, J.-Y.; Kim, H.-K.; Lee, J.; Park, H.K.; Kim, Y.-S. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 2022, 24, 22. [Google Scholar] [CrossRef]
- Xu, H.; Han, Y.; Wu, Y.; Wang, Y.; Li, Q.; Zhang, P.; Yuan, P.; Luo, Y.; Fan, Y.; Chen, S.; et al. Clinicopathological Characteristics and Prognosis of HER2-Low Early-Stage Breast Cancer: A Single-Institution Experience. Front. Oncol. 2022, 12, 906011. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Dezentjé, V.; Lambertini, M.; de Nonneville, A.; Marhold, M.; Le Du, F.; Cortés Salgado, A.; Alpuim Costa, D.; Vaz Batista, M.; Chic Ruché, N.; et al. Influence of HER2 expression on prognosis in metastatic triple-negative breast cancer-results from an international, multicenter analysis coordinated by the AGMT Study Group. ESMO Open 2022, 8, 100747. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Moy, B.; Rumble, R.B.; Carey, L.A. Chemotherapy and Targeted Therapy for Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer That Is Either Endocrine-Pretreated or Hormone Receptor-Negative: ASCO Guideline Rapid Recommendation Update. J. Clin. Oncol. 2022, 40, 3088–3090. [Google Scholar] [CrossRef]
- ESMO. EMA Recommends Extension of Therapeutic Indications for Trastuzumab Deruxtecan. European Society for Medical Oncology (ESMO) [Online]. 22 December 2022. Available online: https://www.esmo.org/oncology-news/ema-recommends-extension-of-therapeutic-indications-for-trastuzumab-deruxtecan2 (accessed on 4 January 2023).
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Grassini, D.; Cascardi, E.; Sarotto, I.; Annaratone, L.; Sapino, A.; Berrino, E.; Marchiò, C. Unusual Patterns of HER2 Expression in Breast Cancer: Insights and Perspectives. Pathobiology 2022, 89, 278–296. [Google Scholar] [CrossRef]
- Denkert, C.; Lebeau, A.; Schildhaus, H.U.; Jackisch, C.; Rüschoff, J. New treatment options for metastatic HER2-low breast cancer: Consequences for histopathological diagnosis. Pathologie (Heidelb) 2022. [Google Scholar] [CrossRef]
- Schrijver, W.A.M.E.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qarmali, M.; Siegal, G.P.; Wei, S. Receptor conversion in metastatic breast cancer: Analysis of 390 cases from a single institution. Mod. Pathol. 2020, 33, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Aurilio, G.; Disalvatore, D.; Pruneri, G.; Bagnardi, V.; Viale, G.; Curigliano, G.; Adamoli, L.; Munzone, E.; Sciandivasci, A.; de Vita, F.; et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 2014, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Dieci, M.V.; Barbieri, E.; Piacentini, F.; Omarini, C.; Ficarra, G.; Bettelli, S.; Conte, P.F. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann. Oncol. 2013, 24, 2990–2994. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Wu, Y.; Scaltriti, M.; Meric-Bernstam, F.; Hunt, K.K.; Dawood, S.; Esteva, F.J.; Buzdar, A.U.; Chen, H.; Eksambi, S.; et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009, 15, 7381–7388. [Google Scholar] [CrossRef] [PubMed]
- Tural, D.; Karaca, M.; Zirtiloglu, A.; Hacioglu, B.M.; Sendur, M.A.; Ozet, A. Receptor discordances after neoadjuvant chemotherapy and their effects on survival. J. BUON 2019, 24, 20–25. [Google Scholar]
- Wang, R.-X.; Chen, S.; Jin, X.; Chen, C.-M.; Shao, Z.-M. Weekly paclitaxel plus carboplatin with or without trastuzumab as neoadjuvant chemotherapy for HER2-positive breast cancer: Loss of HER2 amplification and its impact on response and prognosis. Breast Cancer Res. Treat. 2017, 161, 259–267. [Google Scholar] [CrossRef]
- Ignatov, T.; Gorbunow, F.; Eggemann, H.; Ortmann, O.; Ignatov, A. Loss of HER2 after HER2-targeted treatment. Breast Cancer Res. Treat. 2019, 175, 401–408. [Google Scholar] [CrossRef]
- Branco, F.P.; Machado, D.; Silva, F.F.; André, S.; Catarino, A.; Madureira, R.; Pinto, J.M.; Godinho, J.P.; Simões, P.D.; Brito, M.; et al. Loss of HER2 and disease prognosis after neoadjuvant treatment of HER2+ breast cancer. Am. J. Transl. Res. 2019, 11, 6110–6116. [Google Scholar]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; de Bartolo, D.; Vernaci, G.; et al. Evolution of HER2-low expression from primary to recurrent breast cancer. NPJ Breast Cancer 2021, 7, 137. [Google Scholar] [CrossRef]
- Andre, F.; Slimane, K.; Bachelot, T.; Dunant, A.; Namer, M.; Barrelier, A.; Kabbaj, O.; Spano, J.P.; Marsiglia, H.; Rouzier, R.; et al. Breast cancer with synchronous metastases: Trends in survival during a 14-year period. J. Clin. Oncol. 2004, 22, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Barinoff, J.; Schmidt, M.; Schneeweiss, A.; Schoenegg, W.; Thill, M.; Keitel, S.; Lattrich, C.R.; Hinke, A.; Kutscheidt, A.; Jackisch, C. Primary metastatic breast cancer in the era of targeted therapy—Prognostic impact and the role of breast tumour surgery. Eur. J. Cancer 2017, 83, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 5 October 2022).
- Prat, A.; Bardia, A.; Curigliano, G.; Hammond, M.E.H.; Loibl, S.; Tolaney, S.M.; Viale, G. An Overview of Clinical Development of Agents for Metastatic or Advanced Breast Cancer Without ERBB2 Amplification (HER2-Low). JAMA Oncol. 2022, 8, 1676–1687. [Google Scholar] [CrossRef]
- de Calbiac, O.; Lusque, A.; Mailliez, A.; Bachelot, T.; Uwer, L.; Mouret-Reynier, M.-A.; Emile, G.; Jouannaud, C.; Gonçalves, A.; Patsouris, A.; et al. Comparison of Management and Outcomes in ERBB2 -Low vs ERBB2 -Zero Metastatic Breast Cancer in France. JAMA Netw. Open 2022, 5, e2231170. [Google Scholar] [CrossRef]
- Marchiò, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2-low carcinomas and beyond. Semin. Cancer Biol. 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Shah, S.P.; Morin, R.D.; Khattra, J.; Prentice, L.; Pugh, T.; Burleigh, A.; Delaney, A.; Gelmon, K.; Guliany, R.; Senz, J.; et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 2009, 461, 809–813. [Google Scholar] [CrossRef]
- Kan, S.; Koido, S.; Okamoto, M.; Hayashi, K.; Ito, M.; Kamata, Y.; Komita, H.; Ishidao, T.; Nagasaki, E.; Homma, S. Gemcitabine treatment enhances HER2 expression in low HER2-expressing breast cancer cells and enhances the antitumor effects of trastuzumab emtansine. Oncol. Rep. 2015, 34, 504–510. [Google Scholar] [CrossRef]
- Knowlden, J.M.; Hutcheson, I.R.; Jones, H.E.; Madden, T.; Gee, J.M.W.; Harper, M.E.; Barrow, D.; Wakeling, A.E.; Nicholson, R.I. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 2003, 144, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Li, S.; Wang, Z.; Ahmed, K.M.; Degnan, M.E.; Fan, M.; Dynlacht, J.R.; Li, J.J. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat. Res. 2009, 171, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, M.; Verma, C.; Guzman, M.; Jimenez, J.; Parra, J.L.; Pedersen, K.; Smith, D.J.; Landolfi, S.; Ramon y Cajal, S.; Arribas, J.; et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009, 28, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Jin, Y.; Lv, H.; Hu, X.; Zhang, J. Incidence and prognostic significance of receptor discordance between primary breast cancer and paired bone metastases. Int. J. Cancer 2022, 152, 1476–1489. [Google Scholar] [CrossRef]
- Denkert, C.; Nekljudova, V.; Loibl, S. HER2-low-positive breast cancer from four neoadjuvant clinical trials—Authors’ reply. Lancet Oncol. 2021, 22, e427. [Google Scholar] [CrossRef]
- Miglietta, F.; Griguolo, G.; Bottosso, M.; Giarratano, T.; Lo Mele, M.; Fassan, M.; Cacciatore, M.; Genovesi, E.; de Bartolo, D.; Vernaci, G.; et al. HER2-low-positive breast cancer: Evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer 2022, 8, 66. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.M.; Kuba, M.G.; Ly, A.; et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol. 2022, 8, 607–610. [Google Scholar] [CrossRef]
| Total Number of Patients (n = 148) | HER2-Negative (n = 127) | HER2-Positive (n = 21) | p-Value | |||
|---|---|---|---|---|---|---|
| HER2-Zero (n = 49) | HER2-Low (n = 78) | |||||
| Age at primary breast surgery | 0.087 | |||||
| Median [years] | 53 | 48 | 54 | 52 | ||
| <50 years | 66 (44.6%) | 28 (57.1%) | 29 (37.2%) | 9 (42.9%) | ||
| >50 years | 82 (55.4%) | 21 (42.9%) | 49 (62.8%) | 12 (57.1%) | ||
| Histological subtype | 0.221 | |||||
| Invasive carcinoma of no special type (NST) | 115 (77.7%) | 33 (67.3%) | 63 (80.8%) | 19 (90.5%) | ||
| Invasive lobular carcinoma | 24 (16.2%) | 12 (24.5%) | 11 (14.1%) | 1 (4.8%) | ||
| other | 9 (6.1%) | 4 (8.2%) | 4 (5.1%) | 1 (4.8%) | ||
| Tumor size | 0.032 | |||||
| pT1 | 32 (22.2%) | 11 (22.9%) | 18 (24.0%) | 3 (14.3%) | ||
| pT2 | 67 (46.5%) | 30 (62.5%) | 30 (40.0%) | 7 (33.3%) | ||
| pT3/4 | 45 (31.3%) | 7 (14.6%) | 27 (36.0%) | 11 (52.3%) | ||
| missing | 4 (2.7%) | |||||
| Nodal status | 0.710 | |||||
| Negative | 44 (29.7%) | 13 (26.5%) | 25 (33.3%) | 6 (28.6%) | ||
| positive | 101 (68.2%) | 36 (73.5%) | 50 (66.7%) | 15 (71.4%) | ||
| missing | 3 (2.0%) | |||||
| Histological grade | 0.049 | |||||
| G1 | 11 (7.4%) | 6 (12.2%) | 5 (6.7%) | 0 | ||
| G2 | 72 (48.6%) | 19 (38.8%) | 45 (60.0%) | 8 (40.0%) | ||
| G3 | 61 (41.2%) | 24 (49.0%) | 25 (33.3%) | 12 (60.0%) | ||
| missing | 4 (2.7%) | |||||
| Hormone receptor status | 0.252 | |||||
| negative | 23 (15.5%) | 11 (22.4%) | 9 (11.5%) | 3 (14.3%) | ||
| positive | 125 (84.5%) | 38 (77.6%) | 69 (88.5%) | 18 (85.7%) | ||
| HER2 status | ||||||
| Negative | 127 (85.8%) | 49 (100.0%) | 78 (100.0%) | |||
| Positive | 21 (14.2%) | 21 (14.2%) | ||||
| 0 | 49 (33.1%) | 49 (100.0%) | ||||
| 1+ | 59 (39.9%) | 59 (75.6%) | ||||
| 2+ | 22 (14.9%) | 19 (24.4%) | 3 (14.3%) | |||
| 2+/ISH negative | 19 (12.8%) | |||||
| 2+/ISH positive | 3 (2.0%) | |||||
| 3+ | 18 (12.2%) | 18 (85.7%) | ||||
| Ki-67 | 0.022 | |||||
| <20% | 19 (12.8%) | 10 (35.7%) | 9 (17.3%) | 0 | ||
| >20% | 74 (50.0%) | 18 (64.3%) | 43 (82.7%) | 13 (100.0%) | ||
| Missing | 55 (37.2%) | |||||
| Molecular subtype | <0.001 | |||||
| Luminal-like | 107 (72.3%) | 38 (77.6%) | 69 (88.5%) | 0 | ||
| Luminal-A-like | 18 (12.2%) | 9 | 9 | 0 | ||
| Lumina-B-like | 46 (31.1%) | 10 | 36 | 0 | ||
| Missing Ki-67 | 43 (29.1%) | |||||
| HER2 positive | 21 (14.2%) | 21 (100.0%) | ||||
| Triple-negative | 20 (13.5%) | 11 (22.4%) | 9 (11.5%) | 0 | ||
| Metastatic site | 0.349 | |||||
| Liver | 50 (33.8%) | 16 (32.7%) | 28 (35.9%) | 6 (28.6%) | ||
| Bone | 38 (25.7%) | 13 (26.5%) | 23 (29.5%) | 2 (9.5%) | ||
| Skin/Soft tissue | 18 (12.2%) | 6 (12.2%) | 10 (12.8%) | 2 (9.5%) | ||
| Central nervous system | 15 (10.1%) | 6 (12.2%) | 4 (5.1%) | 5 (23.8%) | ||
| others | 13 (8.8%) | 5 (10.2%) | 6 (7.7%) | 2 (9.5%) | ||
| Lung/Pleura | 9 (6.1%) | 1 (2.0%) | 5 (6.4%) | 3 (14.3%) | ||
| Lymph node | 5 (3.4%) | 2 (4.1%) | 2 (2.6%) | 1 (4.8%) | ||
| Additional metastatic biopsy | ||||||
| Yes | 19 (12.8%) | 5 (10.2%) | 11 (14.1%) | 3 (14.3%) | 0.797 | |
| HER2 concordance with previous biopsy | 8 (42.1%) | 1 (20.0%) | 6 (54.5%) | 1 (33.3%) | ||
| HER2 discordance with previous biopsy | 11 (57.9%) | 4 (80.0%) | 5 (45.4%) | 2 (66.7%) | ||
| No | 129 (87.2%) | 44 (89.8%) | 67 (85.9%) | 18 (85.7%) | ||
| Treatment for early breast cancer | ||||||
| Neo-/Adjuvant chemotherapy | 72 (48.6%) | 35 (71.4%) | 30 (38.5%) | 7 (33.3%) | <0.001 | |
| Neo-/Adjuvant Anti-HER2-therapy | 9 (6.1%) | 0 | 0 | 9 (42.9%) | <0.001 | |
| Adjuvant endocrine therapy | 79 (53.4%) | 31 (63.3%) | 42 (56.0%) | 6 (28.6%) | 0.083 | |
| Treatment for metastatic breast cancer | ||||||
| chemotherapy | 45 (30.4%) | 8 (16.3%) | 26 (34.2%) | 11 (52.4%) | 0.007 | |
| Anti-HER2-therapy | 13 (8.8%) | 2 (4.1) | 0 | 11 (52.4%) | <0.001 | |
| Endocrine therapy | 52 (35.1%) | 18 (36.7%) | 29 (38.2%) | 5 (23.8%) | 0.468 | |
| Tumor progression | ||||||
| Time to metastasis, Median [month] | 25 (0–150) | 44 (0–150) | 14 (0–121) | 0 (0–111) | ||
| Time to metastasis biopsy, Median [month] | 39 (0–165) | 51 (0–150) | 36 (0–165) | 17 (0–37) | ||
| Time from metastasis diagnosis to metastasis biopsy, Median [month] | 1 (0–131) | 0 (0–55) | 1 (0–131) | 0 (0–94) | ||
| Primary metastatic breast cancer (PMBC) | Yes | 53 (35.8%) | 8 (16.3%) | 31 (39.7%) | 14 (66.7%) | |
| No | 95 (64.9%) | 41 (83.7%) | 47 (60.3%) | 7 (33.3%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almstedt, K.; Krauthauser, L.; Kappenberg, F.; Wagner, D.-C.; Heimes, A.-S.; Battista, M.J.; Anic, K.; Krajnak, S.; Lebrecht, A.; Schwab, R.; et al. Discordance of HER2-Low between Primary Tumors and Matched Distant Metastases in Breast Cancer. Cancers 2023, 15, 1413. https://doi.org/10.3390/cancers15051413
Almstedt K, Krauthauser L, Kappenberg F, Wagner D-C, Heimes A-S, Battista MJ, Anic K, Krajnak S, Lebrecht A, Schwab R, et al. Discordance of HER2-Low between Primary Tumors and Matched Distant Metastases in Breast Cancer. Cancers. 2023; 15(5):1413. https://doi.org/10.3390/cancers15051413
Chicago/Turabian StyleAlmstedt, Katrin, Lisa Krauthauser, Franziska Kappenberg, Daniel-Christoph Wagner, Anne-Sophie Heimes, Marco J. Battista, Katharina Anic, Slavomir Krajnak, Antje Lebrecht, Roxana Schwab, and et al. 2023. "Discordance of HER2-Low between Primary Tumors and Matched Distant Metastases in Breast Cancer" Cancers 15, no. 5: 1413. https://doi.org/10.3390/cancers15051413
APA StyleAlmstedt, K., Krauthauser, L., Kappenberg, F., Wagner, D.-C., Heimes, A.-S., Battista, M. J., Anic, K., Krajnak, S., Lebrecht, A., Schwab, R., Brenner, W., Weikel, W., Rahnenführer, J., Hengstler, J. G., Roth, W., Hasenburg, A., Stewen, K., & Schmidt, M. (2023). Discordance of HER2-Low between Primary Tumors and Matched Distant Metastases in Breast Cancer. Cancers, 15(5), 1413. https://doi.org/10.3390/cancers15051413








