Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period
Abstract
Simple Summary
Abstract
1. Introduction
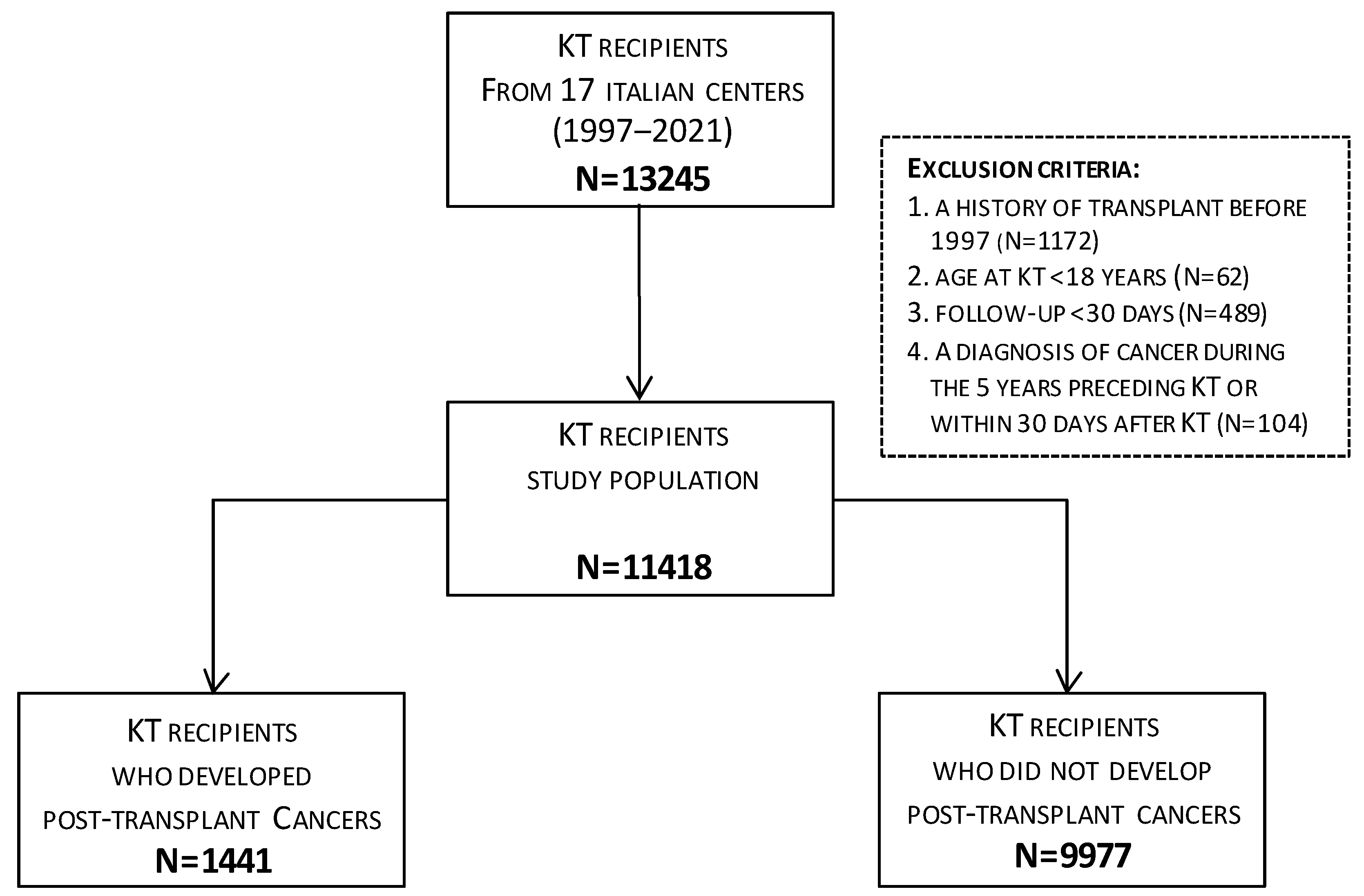
2. Materials and Methods
Statistical Analysis
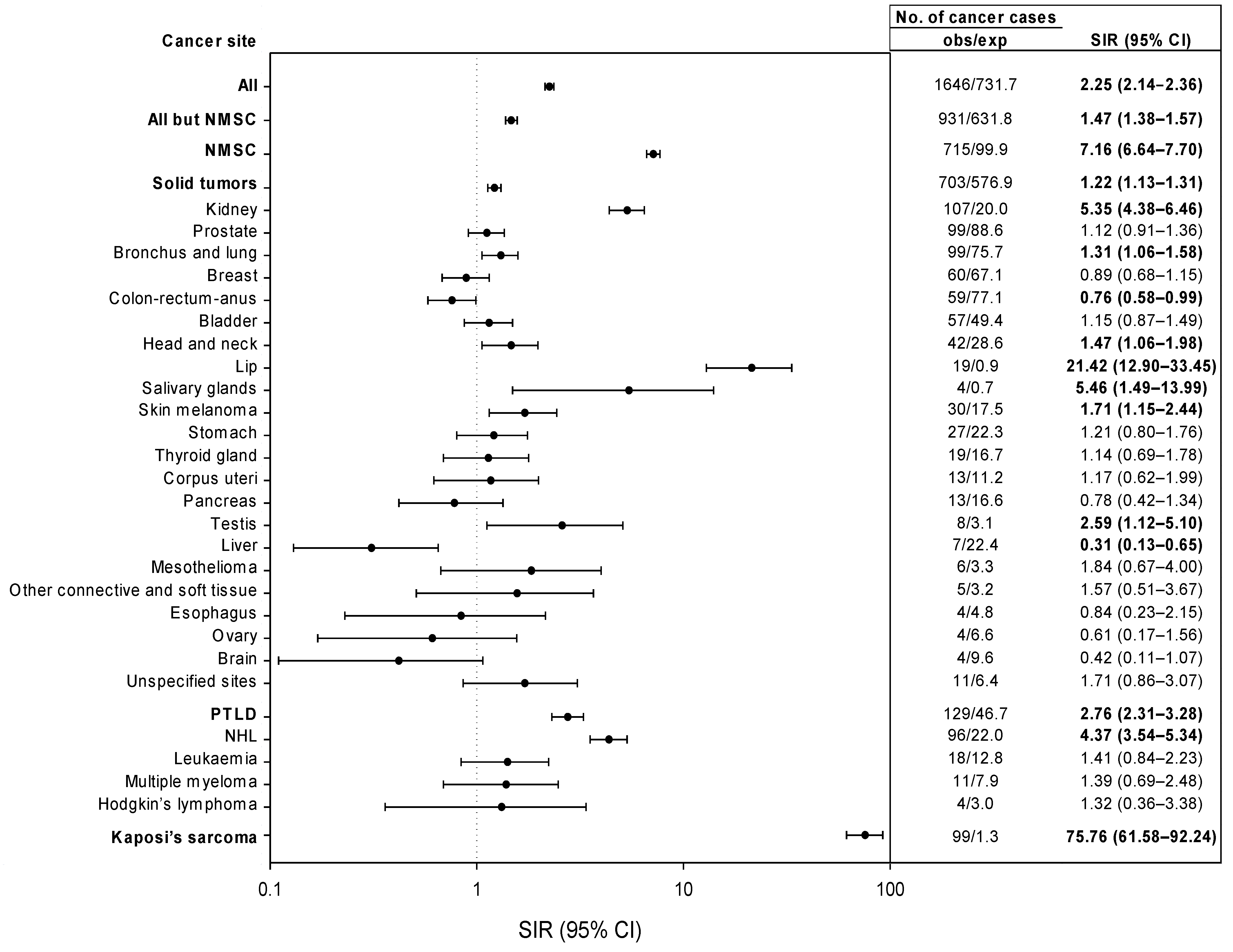
3. Results
3.1. Internal Comparison
3.2. External Comparison
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Friman, T.K.; Jäämaa-Holmberg, S.; Åberg, F.; Helanterä, I.; Halme, M.; Pentikäinen, M.O.; Nordin, A.; Lemström, K.B.; Jahnukainen, T.; Räty, R.; et al. Cancer risk and mortality after solid organ transplantation: A population-based 30-year cohort study in Finland. Int. J. Cancer 2022, 150, 1779–1791. [Google Scholar] [CrossRef]
- Acuna, S.A. Etiology of increased cancer incidence after solid organ transplantation. Transplant. Rev. 2018, 32, 218–224. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of Cancer Risk Among US Solid Organ Transplant Recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Au, E.; Wong, G.; Chapman, J.R. Cancer in kidney transplant recipients. Nat. Rev. Nephrol. 2018, 14, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Piselli, P.; Serraino, D.; Segoloni, G.P.; Sandrini, S.; Piredda, G.B.; Scolari, M.P.; Rigotti, P.; Busnach, G.; Messa, P.; Donati, D.; et al. Risk of de novo cancers after transplantation: Results from a cohort of 7217 kidney transplant recipients, Italy 1997–2009. Eur. J. Cancer 2013, 49, 336–344. [Google Scholar] [CrossRef]
- Miao, Y.; Everly, J.J.; Gross, T.G.; Tevar, A.D.; First, M.R.; Alloway, R.R.; Woodle, E.S. De Novo Cancers Arising in Organ Transplant Recipients are Associated With Adverse Outcomes Compared With the General Population. Transplantation 2009, 87, 1347–1359. [Google Scholar] [CrossRef]
- Au, E.H.; Chapman, J.R.; Craig, J.C.; Lim, W.H.; Teixeira-Pinto, A.; Ullah, S.; McDonald, S.; Wong, G. Overall and Site-Specific Cancer Mortality in Patients on Dialysis and after Kidney Transplant. J. Am. Soc. Nephrol. 2019, 30, 471–480. [Google Scholar] [CrossRef]
- Taborelli, M.; Serraino, D.; Cimaglia, C.; Furian, L.; Biancone, L.; Busnach, G.; Todeschini, P.; Bossini, N.; Iaria, M.; Campise, M.R.; et al. The impact of cancer on the risk of death with a functioning graft of Italian kidney transplant recipients. Am. J. Transplant. 2021, 22, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.F. Cancer and viral infections in immunocompromised individuals. Int. J. Cancer 2009, 125, 1755–1763. [Google Scholar] [CrossRef]
- Piselli, P.; Verdirosi, D.; Cimaglia, C.; Busnach, G.; Fratino, L.; Ettorre, G.M.; De Paoli, P.; Citterio, F.; Serraino, D. Epidemiology of de novo malignancies after solid-organ transplantation: Immunosuppression, infection and other risk factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A. Cancer in Solid Organ Transplant Recipients: There Is Still Much to Learn and Do. Am. J. Transplant. 2017, 17, 1967–1969. [Google Scholar] [CrossRef]
- Chapman, J.R.; Webster, A.; Wong, G. Cancer in the Transplant Recipient. Cold Spring Harb. Perspect. Med. 2013, 3, a015677. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Tang, S.C.W. An update on cancer after kidney transplantation. Nephrol. Dial. Transplant. 2018, 34, 914–920. [Google Scholar] [CrossRef]
- Blosser, C.D.; Haber, G.; Engels, E.A. Changes in cancer incidence and outcomes among kidney transplant recipients in the United States over a thirty-year period. Kidney Int. 2021, 99, 1430–1438. [Google Scholar] [CrossRef]
- Nordin, A.; Åberg, F.; Pukkala, E.; Pedersen, C.R.; Storm, H.H.; Rasmussen, A.; Bennet, W.; Olausson, M.; Wilczek, H.; Ericzon, B.-G.; et al. Decreasing incidence of cancer after liver transplantation—A Nordic population-based study over 3 decades. Am. J. Transplant. 2017, 18, 952–963. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Classification of Diseases and Related Health Problems (10th Revision, ICD-10-CM); WHO: Geneva, Switzerland, 1992. [Google Scholar]
- Breslow, N.E.; Day, N.E. Statistical Methods in Cancer Research, Volume II. The Design and Analysis of Cohort Studies; International Agency for Research on Cancer: Lyon, France, 1987; pp. 65–76.
- Ulm, K. A simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am. J. Epidemiol. 1990, 131, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Dahle, D.O.; Skauby, M.; Langberg, C.W.; Brabrand, K.; Wessel, N.; Midtvedt, K. Renal Cell Carcinoma and Kidney Transplantation: A Narrative Review. Transplantation 2021, 106, e52–e63. [Google Scholar] [CrossRef]
- Karami, S.; Yanik, E.L.; Moore, L.E.; Pfeiffer, R.M.; Copeland, G.; Gonsalves, L.; Hernandez, B.Y.; Lynch, C.F.; Pawlish, K.; Engels, E.A. Risk of Renal Cell Carcinoma Among Kidney Transplant Recipients in the United States. Am. J. Transplant. 2016, 16, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 384, 72–79. [Google Scholar] [CrossRef]
- Acuna, S.A.; Lam, W.; Daly, C.; Kim, S.J.; Baxter, N.N. Cancer evaluation in the assessment of solid organ transplant candidates: A systematic review of clinical practice guidelines. Transplant. Rev. 2018, 32, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.H.; Aagnes, B.; Holdaas, H.; Gude, E.; Boberg, K.M.; Bjørtuft, Ø.; Helsing, P.; Leivestad, T.; Møller, B.; Gjersvik, P. Long-term Change in the Risk of Skin Cancer After Organ Transplantation: A Population-Based Nationwide Cohort Study. JAMA Dermatol. 2017, 153, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Menzies, S.; O’Leary, E.; Callaghan, G.; Galligan, M.; Deady, S.; Gadallah, B.; Lenane, P.; Lally, A.; Houlihan, D.; Morris, P.; et al. Declining incidence of keratinocyte carcinoma in organ transplant recipients. Br. J. Dermatol. 2019, 181, 983–991. [Google Scholar] [CrossRef] [PubMed]
- García-Astudillo, L.A.; Leyva-Cobián, F. Human herpesvirus-8 infection and Kaposi’s sarcoma after liver and kidney transplantation in different geographical areas of Spain. Transpl. Immunol. 2006, 17, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Serraino, D.; Piselli, P.; Angeletti, C.; Minetti, E.; Pozzetto, A.; Civati, G.; Bellelli, S.; Farchi, F.; Citterio, F.; Rezza, G.; et al. Risk of Kaposi’s sarcoma and of other cancers in Italian renal transplant patients. Br. J. Cancer 2005, 92, 572–575. [Google Scholar] [CrossRef]
- Krynitz, B.; Edgren, G.; Lindelöf, B.; Baecklund, E.; Brattström, C.; Wilczek, H.; Smedby, K.E. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—A Swedish population-based study. Int. J. Cancer 2013, 132, 1429–1438. [Google Scholar] [CrossRef]
- Cahoon, E.K.; Linet, M.S.; Clarke, C.A.; Pawlish, K.S.; Engels, E.A.; Pfeiffer, R.M. Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int. J. Cancer 2018, 143, 2741–2748. [Google Scholar] [CrossRef]
- Piselli, P.; Taborelli, M.; Cimaglia, C.; Serraino, D.; Italian Transplant & Cancer Cohort Study. Decreased incidence of Kaposi sarcoma after kidney transplant in Italy and role of mTOR-inhibitors: 1997–2016: Risk of Kaposi sarcoma after solid organ transplantation in the United States. Int. J. Cancer 2019, 145, 597–598. [Google Scholar] [CrossRef]
- Fröhlich, F.A.; Halleck, F.; Lehner, L.; Schrezenmeier, E.V.; Naik, M.; Schmidt, D.; Khadzhynov, D.; Kast, K.; Budde, K.; Staeck, O. De-novo malignancies after kidney transplantation: A long-term observational study. PLoS ONE 2020, 15, e0242805. [Google Scholar] [CrossRef]
- O’Neill, J.P.; Sexton, D.J.; O’Leary, E.; O’Kelly, P.; Murray, S.; Deady, S.; Daly, F.; Williams, Y.; Dean, B.; Fitzgerald, C.; et al. Post-transplant malignancy in solid organ transplant recipients in Ireland, The Irish Transplant Cancer Group. Clin. Transplant. 2019, 33, e13669. [Google Scholar] [CrossRef]
- Wojciechowski, D.; Wiseman, A. Long-Term Immunosuppression Management: Opportunities and Uncertainties. Clin. J. Am. Soc. Nephrol. 2021, 16, 1264–1271. [Google Scholar] [CrossRef]
- Krisl, J.C.; Doan, V.P. Chemotherapy and Transplantation: The Role of Immunosuppression in Malignancy and a Review of Antineoplastic Agents in Solid Organ Transplant Recipients. Am. J. Transplant. 2017, 17, 1974–1991. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, M.; Montico, B.; Faè, D.A.; Steffan, A.; Dolcetti, R. Dissecting the Multiplicity of Immune Effects of Immunosuppressive Drugs to Better Predict the Risk of de novo Malignancies in Solid Organ Transplant Patients. Front. Oncol. 2019, 9, 160. [Google Scholar] [CrossRef]
- De Fijter, J.W. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation 2017, 101, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Adra, D.; Al-Qaoud, T.; Fowler, K.; Wong, G. De Novo Malignancies after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2022, 17, 434–443. [Google Scholar] [CrossRef]
- Huang, B.; Huang, M.; Zhang, C.; Yu, Z.; Hou, Y.; Miao, Y.; Chen, Z. Individual dynamic prediction and prognostic analysis for long-term allograft survival after kidney transplantation. BMC Nephrol. 2022, 23, 359. [Google Scholar] [CrossRef] [PubMed]
- Turshudzhyan, A. Post-renal transplant malignancies: Opportunities for prevention and early screening. Cancer Treat. Res. Commun. 2021, 26, 100283. [Google Scholar] [CrossRef]
| All Patients | ||
|---|---|---|
| N | % | |
| Sex | ||
| Male | 7286 | 63.8 |
| Female | 4132 | 36.2 |
| Age at transplant (years) | ||
| 18–39 | 2907 | 25.5 |
| 40–49 | 2682 | 23.5 |
| 50–59 | 3439 | 30.1 |
| ≥60 | 2390 | 20.9 |
| Median (IQR) | 50 (39–58) | |
| Calendar year at transplant | ||
| 1997–2004 | 5737 | 50.3 |
| 2005–2012 | 3893 | 34.1 |
| 2013–2021 | 1788 | 15.7 |
| Area of residence | ||
| Northern Italy | 6471 | 56.7 |
| Central Italy | 1405 | 12.3 |
| Southern Italy | 3489 | 30.5 |
| Abroad | 53 | 0.5 |
| Status of the donor | ||
| Alive | 1079 | 9.4 |
| Deceased | 10,339 | 90.6 |
| Primary cause of kidney failure | ||
| Glomerulonephritis | 4212 | 36.9 |
| Polycystic kidney disease | 1936 | 17.0 |
| Pyelonephritis/Interstitial nephritis | 1116 | 9.8 |
| Hypertensive nephropathy/vascular disease | 691 | 6.0 |
| Diabetes | 604 | 5.3 |
| Congenital | 394 | 3.4 |
| Uncertain | 1781 | 15.6 |
| Other | 684 | 6.0 |
| Follow-up (years) | ||
| Median (IQR) | 7.1 (3.9–10.6) | |
| Total person-years | 85,208.7 | |
| 1997–2004 | 2005–2012 | 2013–2021 | 2005–2012 | 2013–2021 | 2005–2012 | 2013–2021 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Site | No. | IR (SE) | No. | IR (SE) | No. | IR (SE) | Unadjusted IRR (95% CI) a | Ptrend | Adjusted IRR (95% CI) a,b | Ptrend | ||
| All | 289 | 15.5 (0.9) | 771 | 19.9 (0.7) | 586 | 21.0 (0.9) | 1.29 (1.13–1.48) | 1.36 (1.18–1.57) | <0.01 | 1.05 (0.92-1.21) | 0.92 (0.80-1.06) | 0.11 |
| All but NMSC | 194 | 10.4 (0.7) | 427 | 11.0 (0.5) | 310 | 11.1 (0.6) | 1.06 (0.90–1.26) | 1.07 (0.90–1.28) | 0.48 | 0.90 (0.76–1.07) | 0.72 (0.60–0.87) | <0.01 |
| NMSC | 95 | 5.1 (0.5) | 344 | 8.9 (0.5) | 276 | 9.9 (0.6) | 1.75 (1.40–2.20) | 1.95 (1.54–2.46) | <0.01 | 1.36 (1.08–1.71) | 1.30 (1.02–1.65) | 0.11 |
| Solid tumors | 114 | 6.1 (0.6) | 332 | 8.6 (0.5) | 257 | 9.2 (0.6) | 1.41 (1.14–1.74) | 1.51 (1.21–1.88) | <0.01 | 1.17 (0.95–1.46) | 1.00 (0.80–1.26) | 0.62 |
| Kidney | 17 | 0.9 (0.2) | 46 | 1.2 (0.2) | 44 | 1.6 (0.2) | 1.31 (0.75–2.28) | 1.74 (0.99–3.04) | 0.04 | 1.30 (0.74–2.29) | 1.50 (0.84–2.67) | 0.17 |
| Prostate | 13 | 0.7 (0.2) | 53 | 1.4 (0.2) | 33 | 1.2 (0.2) | 1.98 (1.08–3.63) | 1.71 (0.90–3.26) | 0.19 | 1.45 (0.78–2.67) | 0.97 (0.50–1.86) | 0.49 |
| Bronchus and lung | 14 | 0.7 (0.2) | 44 | 1.1 (0.2) | 41 | 1.5 (0.2) | 1.52 (0.83–2.78) | 1.97 (1.07–3.61) | 0.03 | 1.14 (0.62–2.09) | 1.01 (0.55–1.89) | 0.88 |
| Breast | 9 | 0.5 (0.2) | 33 | 0.9 (0.1) | 18 | 0.6 (0.2) | 1.78 (0.85–3.71) | 1.34 (0.60–2.99) | 0.65 | 1.62 (0.76–3.42) | 1.06 (0.46–2.41) | 0.81 |
| Colon-rectum-anus | 7 | 0.4 (0.1) | 29 | 0.8 (0.1) | 23 | 0.8 (0.2) | 2.00 (0.88–4.57) | 2.21 (0.95–5.15) | 0.09 | 1.54 (0.67–3.54) | 1.39 (0.59–3.29) | 0.63 |
| Bladder | 12 | 0.6 (0.2) | 30 | 0.8 (0.1) | 15 | 0.5 (0.1) | 1.21 (0.62–2.36) | 0.84 (0.39–1.80) | 0.58 | 0.97 (0.49–1.92) | 0.63 (0.29–1.37) | 0.20 |
| Head and neck | 6 | 0.3 (0.1) | 19 | 0.5 (0.1) | 17 | 0.6 (0.1) | 1.53 (0.61–3.83) | 1.91 (0.75–4.84) | 0.17 | 1.29 (0.51–3.26) | 1.32 (0.51–3.43) | 0.62 |
| Skin melanoma | 7 | 0.4 (0.1) | 13 | 0.3 (0.1) | 10 | 0.4 (0.1) | 0.90 (0.36–2.25) | 0.96 (0.37–2.52) | 0.96 | 0.92 (0.36–2.34) | 0.99 (0.36–2.70) | 0.99 |
| Stomach | 5 | 0.3 (0.1) | 14 | 0.4 (0.1) | 8 | 0.3 (0.1) | 1.35 (0.49–3.76) | 1.07 (0.35–3.28) | 0.98 | 1.01 (0.36–2.84) | 0.49 (0.16–1.52) | 0.13 |
| PTLD | 34 | 1.8 (0.3) | 52 | 1.3 (0.2) | 43 | 1.5 (0.2) | 0.74 (0.48–1.14) | 0.85 (0.54–1.33) | 0.56 | 0.69 (0.44–1.07) | 0.71 (0.44–1.13) | 0.19 |
| NHL | 26 | 1.4 (0.3) | 39 | 1.0 (0.2) | 31 | 1.1 (0.2) | 0.73 (0.44–1.19) | 0.80 (0.48–1.35) | 0.47 | 0.69 (0.41–1.14) | 0.68 (0.39–1.17) | 0.19 |
| Kaposi’s sarcoma | 46 | 2.5 (0.4) | 43 | 1.1 (0.2) | 10 | 0.4 (0.1) | 0.45 (0.30–0.69) | 0.15 (0.07–0.29) | <0.01 | 0.37 (0.24–0.57) | 0.09 (0.04–0.18) | <0.01 |
| 1997–2004 | 2005–2012 | 2013–2021 | Ptrend | ||
|---|---|---|---|---|---|
| Cancer Site | SIR (95% CI) | SIR (95% CI) | SIR (95% CI) | Unadjusted | Adjusted a |
| All | 2.54 (2.26–2.85) | 2.38 (2.21–2.55) | 1.99 (1.83–2.16) | <0.01 | <0.01 |
| All but NMSC | 1.96 (1.70–2.26) | 1.52 (1.38–1.67) | 1.23 (1.10–1.38) | <0.01 | <0.01 |
| NMSC | 6.40 (5.18–7.83) | 8.05 (7.23–8.95) | 6.52 (5.77–7.33) | 0.40 | 0.42 |
| Solid tumors | 1.27 (1.04–1.52) | 1.29 (1.16–1.44) | 1.12 (0.99–1.26) | 0.14 | 0.17 |
| Kidney | 5.33 (3.10–8.53) | 5.17 (3.78–6.89) | 5.56 (4.04–7.46) | 0.81 | 0.38 |
| Prostate | 1.28 (0.68–2.19) | 1.34 (1.00–1.75) | 0.85 (0.59–1.20) | 0.07 | 0.09 |
| Bronchus and lung | 1.16 (0.64–1.95) | 1.33 (0.96–1.78) | 1.35 (0.97–1.83) | 0.68 | 0.86 |
| Breast | 0.73 (0.33–1.38) | 1.08 (0.74–1.52) | 0.74 (0.44–1.17) | 0.78 | 0.71 |
| Colon-rectum-anus | 0.61 (0.24–1.25) | 0.85 (0.57–1.22) | 0.73 (0.47–1.10) | 0.87 | 0.83 |
| Bladder | 1.66 (0.86–2.90) | 1.38 (0.93–1.97) | 0.73 (0.41–1.21) | 0.02 | 0.12 |
| Head and neck | 1.08 (0.40–2.36) | 1.49 (0.90–2.33) | 1.64 (0.96–2.63) | 0.40 | 0.45 |
| Skin melanoma | 2.42 (0.97–5.00) | 1.66 (0.89–2.84) | 1.47 (0.70–2.70) | 0.34 | 0.58 |
| Stomach | 1.34 (0.44–3.13) | 1.44 (0.79–2.42) | 0.90 (0.39–1.77) | 0.38 | 0.15 |
| PTLD | 4.34 (3.01–6.07) | 2.51 (1.87–3.29) | 2.37 (1.72–3.20) | 0.02 | 0.11 |
| NHL | 6.77 (4.42–9.92) | 3.96 (2.82–5.42) | 3.74 (2.54–5.31) | 0.04 | 0.17 |
| Kaposi’s sarcoma | 189.16 (138.49–252.32) | 75.66 (54.76–101.92) | 20.19 (9.68–37.14) | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piselli, P.; Serraino, D.; Cimaglia, C.; Furian, L.; Biancone, L.; Busnach, G.; Bossini, N.; Todeschini, P.; Iaria, M.; Citterio, F.; et al. Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period. Cancers 2023, 15, 1347. https://doi.org/10.3390/cancers15041347
Piselli P, Serraino D, Cimaglia C, Furian L, Biancone L, Busnach G, Bossini N, Todeschini P, Iaria M, Citterio F, et al. Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period. Cancers. 2023; 15(4):1347. https://doi.org/10.3390/cancers15041347
Chicago/Turabian StylePiselli, Pierluca, Diego Serraino, Claudia Cimaglia, Lucrezia Furian, Luigi Biancone, Ghil Busnach, Nicola Bossini, Paola Todeschini, Maurizio Iaria, Franco Citterio, and et al. 2023. "Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period" Cancers 15, no. 4: 1347. https://doi.org/10.3390/cancers15041347
APA StylePiselli, P., Serraino, D., Cimaglia, C., Furian, L., Biancone, L., Busnach, G., Bossini, N., Todeschini, P., Iaria, M., Citterio, F., Campise, M., Veroux, M., Tisone, G., Cantaluppi, V., Mangino, M., Simone, S., Argiolas, D., Ambrosini, A., Pisani, F., ... Taborelli, M., on behalf of the Italian Transplant and Cancer Cohort Study. (2023). Variation in Post-Transplant Cancer Incidence among Italian Kidney Transplant Recipients over a 25-Year Period. Cancers, 15(4), 1347. https://doi.org/10.3390/cancers15041347








