Simple Summary
The importance of identifying and targeting in treating familial breast cancers is becoming increasingly recognized, in particular with increased availability of PARPi-based therapies. This review paper focuses on recognized and emerging prognostic and predictive tumour biomarkers, both recognized and emerging, specifically within familial breast cancer. In particular, these familial breast cancers appear to be different to sporadic lesions, with different interactions and clinical relevance of well-described cancer networks. More so, pre-clinical studies and in vitro models also demonstrate potentially relevant clinical biomarkers, as yet not studied in these groups. Currently, the wider literature has largely focused on prognostic and predictive factors in all breast cancers in general, or biomarkers for genetic susceptibility. This review is novel as there are none currently reviewing biomarkers only within familial breast cancers.
Abstract
Large numbers of breast cancers arise within a familial context, either with known inherited germline mutations largely within DNA repair genes, or with a strong family history of breast and/or ovarian cancer, with unknown genetic underlying mechanisms. These cancers appear to be different to sporadic cases, with earlier age of onset, increased multifocality and with association with specific breast cancer histological and phenotypic subtypes. Furthermore, tumours showing homologous recombination deficiency, due to loss of BRCA1, BRCA2, PALB2 and CHEK2 function, have been shown to be especially sensitive to platinum-based chemotherapeutics and PARP inhibition. While there is extensive research and data accrued on risk stratification and genetic predisposition, there are few data pertaining to relevant prognostic and predictive biomarkers within this breast cancer subgroup. The following is a review of such biomarkers in male and female familial breast cancer, although the data for the former are particularly sparse.
1. Introduction
Breast cancer is the most common cancer seen, affecting 1 in 8 women worldwide, and also contributing to 15% of all cancer-related deaths [1]. Within this population, approximately 5–10% are described as familial breast cancers, with a strong family history of breast or ovarian cancer, and frequently carrying pathogenic germline mutations [2,3]. Of these, the most commonly seen are in the Breast Cancer gene 1 (BRCA1), Breast Cancer gene 2 (BRCA2), Checkpoint kinase 2 (CHEK2) and partner and localizer of the BRCA2 gene (PALB2) tumour suppressor genes. These account for 50% of cases of familial breast cancer and are the most-characterised clinical and biological cohorts to date.
It is increasingly recognised that familial breast cancers show multiple differences compared with sporadic breast cancer in both men and women, and form the clinical and biological standpoints [4]. These tumours often show strong geno-phenotypic correlation, with BRCA1 carriers showing increased representation of basal phenotype cancers in women [4], and BRCA2 carriers showing increased representation of micropapillary-type cancers in men [5]. Familial breast cancers show more frequent early onset and multifocality, with an increased incidence of bilateral cancers. Specifically, in familial breast cancers, inactivation of the DNA damage repair pathways has been shown to alter prognosis, which has been exploited in some newer treatment modalities focused on targeting sensitivity to DNA-damaging therapies and immunotherapies.
Women who harbour these mutations have a 45–84% lifetime risk of breast cancer [6,7]. There is some clinical and pathological heterogeneity, with variation seen in the age of onset, hormonal status and cancer type, as well as treatment outcomes. While the majority of research has focused on breast cancer susceptibility and risk modification, there is a paucity of data on potential biomarkers and predictive factors in these cancers, and whether further tailoring of therapy may be needed in these patients. The following is a review of studies and potential prognostic and predictive biomarkers in familial breast cancer.
2. Prognostic and Predictive Biomarkers
2.1. BRCA-Associated Homologous Recombination
The use of Poly ADP Ribose Polymerase (PARP) inhibitors (PARPi) has radically improved the treatment outcomes of BRCA1/2mutation (mut) breast cancer patients. The inhibition of PARP leads to an inability to recruit the base excision pair (BER) machinery for single-strand DNA break repair, with the consequent result of a DNA double-strand break formation (Figure 1) [8]. In cells with proficient Homologous Recombination (HR), this is subsequently repaired with normal function maintained. In tumours with BRCA1/2mut and no HR function, the cells undergoes cell death or are forced to rely on highly inaccurate classical non-homologous end joining (cNHEJ), resulting in eventual genomic instability and cell death (Figure 1) [9]. Clinical trials of PARPi showed anti-cancer efficacy with the approval for Olaparib and talazoparib monotherapies for locally advanced/metastatic deleterious/suspected deleterious germline BRCAmut, Human Epidermal growth factor Receptor (HER2)-negative breast cancers [10,11].
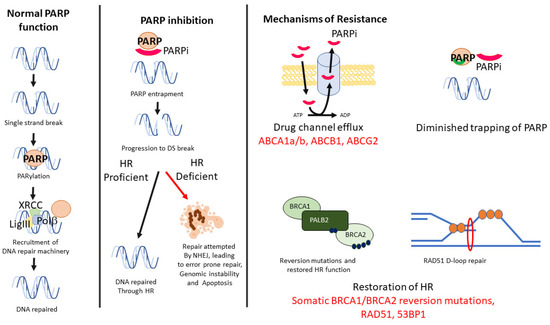
Figure 1.
Mechanism of PARP, with repair of single-strand DNA break through PARPylation and recruitment of repair proteins of the X-ray repair cross-complementing protein 1 (XRCC1) complex. Inhibition of PARP leads to double-strand breaks, which may be repaired by HRR proteins if proficient, or apoptosis in HR-deficient cells. PARPi may occur through multiple mechanisms, including efflux of the inhibitor, mutation of PAPRP affecting trapping, reversion of HRR function or through RAD51-mediated repair, with potential biomarkers highlighted (in red).
Several mechanisms of PARPi have now been described that account for the treatment resistance and tumour growth observed both in vitro and in vivo. Similar to other therapies, decreasing availability of intracellular PARPi may occur through up-regulation of drug efflux transporter genes, such as ATP Binding Cassette Subfamily (ABC)-A1a/b and ABCG2 [12]. While shown in mouse models [13], as yet, there are no studies describing ABCB1 levels in PARPi-treated BRCA1/2mut patients and, therefore, it is unclear whether this may be a potential biomarker of treatment resistance, and whether coadministration of Multi-Drug Resistance 1(MDR1) inhibitors with PARPi treatment might be a possible future strategy in these patients.
Alterations to the binding of PARPi have also been observed, with numerous mutations in PARP1 resulting in a loss or decreased trapping ability of PARPi [14]. In some instances of BRCA1/2mut, where there is still some functional HR, PARP1 mutations, which reduce PARP1 binding affinity to DNA or disrupt PARylation via disruption of PAR removal, may also lead to acquired PARP inhibitor resistance [14,15].
Cancer cells may also abrogate PARP loss by restoring HR repair or by re-establishing replication fork stability [16,17,18,19]. HR restitution may occur with reversion mutations in HR genes, resulting in functioning HR proteins, which has been observed in tumour samples from patients with acquired resistance to PARPi. This can even occur with expression of hypomorphic mutant proteins that are still capable of RAD51-mediated DNA end resection, formation of D-loop and subsequent repair [20]. The reliance of cyclin-dependent kinases (CDKs) on DNA end resection also restores HR, through ATR activation and phosphorylation of CtIP [21] and the Mre11/RAD50/Nbs1(MRN) complex [22]. In vitro studies have showed reversal of PARPi resistance in BRCAmut models, with a case study showing improved efficacy of PARPi combined with a CDK4/6 inhibitor (palbociclib) compared with PARPi alone in treating BRCAmut, ER-positive breast cancers [10]. In addition, defects in CDK accessory factors, such as 53BP1, Shieldin complex and rvesionless 7-like (REV7), activated by Ataxia-Telangiectasia (ATM), appear to mediate p53-binding protein 1 (53BP1)-dependent DNA repair in PAPRi resistance [23,24]. Limited studies have been performed on these biomarkers in breast cancers, with uncertain prognostic potential, and most likely suggestive of different functionality between tumours that are HR-proficient versus -deficient tumours [25,26].
In a recent study of 308 BRCA1/2mut tumours, interrogation of non-BRCA DNA Homologous Recombination Repair (HRR) genes showed no obvious effect on talazoparib efficacy of BRCA Loss of Heterozygosity (LOH), effect of HRR gene mutational burden and tumour homologous recombination deficiency, as assessed by genomic LOH (gLOH)] [27]. Several study limitations were noted, however, and although there was high concordance between germline and tumour BRCA1/2mut, with 82.6% LOH of BRCA1/2, there was unknown methylation status of BRCA1/2 wild-type (wt) and HRR genes, and a lack of power due to small patient numbers in some subgroups.
DNA repair due to increased loading of RAD51 appears to be a critical step in the HR pathway. Indeed, using RAD51 as a biomarker for PARPi has been shown to be biologically relevant, with an immunofluorescence-based RAD51 assay showing capability to identify HR proficiency in both pre-clinical and clinical samples [28,29]. Practical limitations of some assays, however, were present, as the methodology utilized irradiated live tumour cells [30], which are often not available in clinical specimens. Nevertheless, transfer of the RAD51 assay on formalin-fixed samples without fresh tissue have shown some promise, with an ability to identify HR-deficient tumour cells that were sensitive to PARPi therapy [28,29,31].
With the promise of increased tumour efficacy of PARPi in combination with immunotherapies and other molecular targets, including the PI3K/AKT/mTOR pathway and other HR mechanisms seen in pre-clinical models, there are likely to be further biomarkers identified to aid more specific therapies.
2.2. Non-BRCA-Associated Homologous Recombination
As mentioned, there is a paucity of studies evaluating biomarkers, specifically in familial breast cancers, even in BRCA-associated tumours. For non-BRCA-associated tumours, the data are even more sparse. A single study examined RAD21, a key protein in the cohesin complex required for chromosome segregation and high-fidelity DNA repair by homologous recombination. The study of 94 familial breast cancers [32] demonstrated RAD21 overexpression in 43%, 44% and 33% of BRCA1, BRCA2 and BRCAX tumours, respectively, and expression was associated with shortened cancer-specific overall survival (Hazards Ratio 1.66, p = 0.003). In particular, when stratified for RAD21 expression, there were significantly shorter survival outcomes in chemotherapy-treated patients (p = 0.036) but not those treated with hormone therapy (p = 0.881). Expression was prognostic in BRCA2mut (p = 0.006) and BRCAX (p = 0.008) subsets but not in BRCA1mut cancers (0.71). This suggests a mechanism of chemotherapy resistance in BRCA2 and BRCAX cancers, which may be abrogated in BRCA1mut tumours, most likely due to inherent inactivation of RAD21, as BRCA1wt has been shown to be required to activate RAD21 function [33].
Similarly, a study was performed on HuR, an mRNA binding Hu family protein that enhances the regulation translation of target transcripts through enhancing their stability [34]. In many cancers, including pancreatic exocrine adenocarcinoma, ovarian carcinoma, non-small cell lung carcinoma, colon carcinoma and upper genito-urinary carcinomas [35], HuR levels are increased and oncogenic, primarily activated by mitogen-activated protein kinases (MAP-Ks). In a study of 623 familial breast cancers [36], HuR protein expression was found to be prognostic in BRCAX patients (n = 525). Cytoplasmic HuR expression was higher in BRCAX patients (39%) when compared with sporadic tumours (39%) and was associated with ER and PR negativity, p53 immunopositivity, high tumour grade and ductal histology. Notably, HuR levels were even higher in BRCA1 (63%) and BRCA2 (62%) tumours and associated with a non-significant reduction in survival in BRCA2 mutation carriers. There was no prognostic effect in the BRCA1 group. Based on these findings, HuR appears to be involved in the oncogenic pathways of familial breast cancer, probably through activation of the MAP kinase pathway, and it is a prognostic factor in at least some subsets of familial breast cancer.
While not specifically looking at familial cancers, a study by Takada et al. [37] examined breast tumours with BRCA1 or BRCA2 alterations (methylation and/or loss or uniparental disomy) and showed significant associations with higher numbers of genomic aberrations and higher percentage of TP53 alterations than BRCA1/2wt tumours. Although there was no difference in overall survival between patients with or without BRCA1/2 alterations, when stratifying by PALB2, PAGR1, RAD51B, FANCM, MLL4 or ERCC1/2a mutations, a small number of BRCA1- patients (n = 27) [37], with an additional defect in HR genes, had a significantly shorter survival. The combination of these HR defects was hypothesised to result in chemoresistance and, thus, the poorer outcomes in patients with BRCA1-altered breast cancer, and may show a similar correlation in familial breast cancers.
2.3. HER2
Within breast cancer, the identification of HER2 amplification as both a marker for intrinsic phenotypes as well as a target for specific therapies has stratified treatment protocols as well as patient outcomes [38,39]. As part of the ERBB/HER/EGFR family, amplification of this oncogene is seen in ~15% of all female breast cancers and ~5–9% of male breast cancers. HER2 amplification is associated with a more aggressive phenotype and shorter disease-free survival (DFS) and overall survival (OS) [39]. Multiple lines of therapy, including monoclonal antibodies, tyrosine kinase receptor inhibitors (TKI), signal transduction inhibitors and antibody drug conjugates, have been demonstrated in multiple trials, including at all stages, to be effective in breast cancer, albeit with inferior outcomes overall to HER2-negative tumours [40].
The characterisation of an HER2-positive tumour in familial breast cancer is still poorly described, with few large studies performed. Nevertheless, there is a reduced incidence of HER2-positive breast cancers in BRCA1- and BRCA2-associated tumours, with a recent pooled analysis of a total of 21,083 BRCA1 and BRCA2mut cancers from 73 studies [41], showing mean HER2-positive rates of 8.3% and 10.3%, respectively (combined 9.1%, 95% CI interval 7.3–11.2%). Notably, even fewer studies have characterised HER2, variably determined by either FISH or immunohistochemistry within non-BRCA1/2 (so-called BRCAX) familial breast cancers, with nine small studies showing a wide range of HER2 positivity (9–60%) [42].
The significance of the HER2 oncogenic drive in familial breast cancer is most likely heterogenous. While there are yet no studies determining the oncogenic drive of simultaneous mutations in BRCA1/2 and HER2, there are likely tumours that either show combined BRCA1/2 and HER2 mutation, or HER2 amplification with an intact BRCA1/2 pathway. Perhaps evidence of this is seen in some larger studies [43] that have shown that HER2-positive tumours within the BRCA population are more frequently associated with oestrogen positivity (75% vs. 46%, p < 0.0026), with a trend towards an older age of onset (mean 48 years vs. 42 years). Notably, there is also a tendency in BRCA1/2 mutation carriers who have had multiple tumours to show higher rates of HER2 positivity in later-onset lesions (78%), with a case report in a BRCA1mut carrier showing loss of the BRCA1mut gene, without inactivation of the BRCA1wt gene presenting with an HER2-amplified cancer [44].
A recent case-matched analysis of 700 breast cancers [45], stratified by HER2 tumour positivity and BRCA mutation status, showed significant clinical and pathological difference between HER2-positive BRCA1/2mut and HER2-negative BRCA1/2mut tumours, with a higher proliferative index (Ki67) and grade in the HER2-positive cancers. There was no difference in the HER-positive BRCAwt and HER2-positive BRCAmut groups. The HER2-positive BRCAmut subgroup showed overall worse survival compared with HER2-negative and BRCAwt tumours (Hazards Ratio = 3.4, 95% 1.3–16.7) but appeared not to show a significant difference to the HER2-positive BRCAwt or HER2-negative BRCAmut subgroups.
As of yet, while there is a theoretical advantage in combined pharmacological therapy in HER2-positive tumours occurring in BRCA1/2 mutation carriers, there is no definitive guidelines or data pertaining to this population. In preclinical models, a response to HER2, TKIs were seen in tumours with inactivated BRCA2 mutations. Furthermore, the addition of PARPi showed an enhanced effect and showed an advantage to combination therapy in this instance [46], suggestive of an advantage of dual therapy.
2.4. Hypoxia
Hypoxia-Inducible Factors (HIFs) and their regulatory molecules regulate multiple pathways through the transcription of genes that allow for cellular adaption to changes in oxygen tension, especially hypoxic environments [47]. Examples when HIF-1α is overexpressed include established downstream targets, such as carbonic anhydrase IX (CAIX) and glucose transporter-1 (Glut-1) [48]. HIFs are involved in the pathogenesis and progression of many cancer types, including triple-negative breast and BRCA1-associated breast cancer, as they provide a growth advantage to rapidly growing tumours where angiogenesis may not be adequate. HIF-1α overexpression correlates with poor prognosis in triple-negative breast cancer (TNBC), including familial-associated breast cancer [49,50,51,52,53,54,55].
There are some data to suggest that BRCA1 modulates the hypoxic response by regulating HIF-1α stability and, thereby, vascular endothelial growth factor levels (see below) [55]. Furthermore, possibly due to limiting angiogenesis, hypoxia-induced HIF-1α overexpression has been reported to be more than 2-fold more frequent in BRCA1-mutation-associated breast cancer than in sporadic breast cancers [56]. Conversely, BRCA2-mutation-related breast cancers express HIF-1α less frequently [55]. It has been proposed that the HIF pathway is integral to the progression of BRCA1-associated breast cancer and may be a target for emerging targeted therapies directed to this pathway and downstream targets [57].
2.5. Vascular Endothelial Growth Factor (VEGF)
As above, VEGF is an important HIF-upregulated gene, which plays a pivotal role in angiogenesis. VEGF has been shown to be important in breast carcinogenesis, with increased expression of VEGF in HER2, luminal B and basal-type breast cancers [58], and being an adverse prognostic factor for some breast cancer subsets [58]. In a study of 60 breast cancers [59], stratified by familial history and known BRCA status, there were significantly higher levels of VEGF and Angiopoietin-1 and Angiopoietin-2, two other angiogenic factors involved in vessel remodelling in patients with BRCA1/2 mutations. This suggests that VEGF with Tie2 signalling could stimulate sprouting angiogenesis in BRCA-related cancer, accounting for the high microvessel density often observed in these cancers (especially the triple-negative/basal-type breast cancer common in BRCA1 carriers) and suggest these breast tumours might be attractive targets for anti-angiogenic therapeutics.
2.6. Cell Cycle Regulation
Through differential gene expression analysis, Severson et al. [60] showed that the most common genes that were upregulated in BRCA1-like tumours were centred on the FOXM1 network. FOXM1 is a member of the forkhead superfamily transcription factors and a key regulator of cell cycle progression and DNA damage repair. FOXM1 is over-expressed in most human cancers, including epithelial cancers, such as prostate adenocarcinomas, gastrointestinal cancers, non-small-cell lung cancers and high-grade ovarian cancers, and is predictive of poorer survival in breast cancer. While only a small proportion of these BRCA1-like tumour harboured BRCA1 germline mutation, the finding that FOXM1 and downstream factors CDK4/6 are highly expressed in BRCA1-like tumours may have predictive implications in the setting of available CDK4/6-based therapies in BRCA1/2mut cancers.
2.7. Androgen Receptor
The androgen receptor (AR) is a steroid signalling family member and functions as a transcription factor. With ongoing development of AR-targeted therapies, including bicalutamide and ezalutamide (both nonsteroidal antiandrogen), showing clinical benefit [61,62], AR is a potentially useful biomarker for these therapies. It is more commonly associated with hormone-receptor-positive breast cancer, where AR expression is seen in >70% of tumours, and some small studies have shown AR expression in 30% (n = 13 of 43) of BRCA1mut and 78% (n = 14 of 18) of BRCA2mut tumours [63]. However, there is a complexity to the significance of AR expression in breast cancer, with studies showing that the prognostic and predictive power is dependent on the molecular subtype of the tumour, and other factors such as EGFR [62]. In TNBC subsets, AR positivity is associated with improved features, such as lower tumour grade, risk of nodal involvement and lower proliferative index. While AR is associated with improved DFS in TNBC, interestingly, AR expression may imply less sensitivity to chemotherapy [64]. Nonetheless, as ~20% of ER/PR-negative BRCA1mut breast cancers express AR, the modulation of AR may offer new treatment options for these high-risk cancers. Further study into the mechanism of AR function in familial breast cancers is needed, with some studies into prostate cancers showing improved efficacy of PARPi in conjunction with antiandrogen therapies, albeit in HR-proficient cell lines [65]. There are, as yet, no clinical trials on antiandrogen therapy in familial breast cancer.
2.8. Epidermal Growth Factor Receptor
While the high frequency of the epidermal growth factor receptor (EGFR) is well recognised in triple-negative breast cancers [66], and has been shown to be an independent prognostic factor disease and overall survival [67,68], paradoxically, in one report, low EGFR expression was observed in BRCAmut tumours compared with other TNBCs [69]. Notably, there are data to suggest the blockade of EGFR may alter DNA damage repair mechanisms, with decreased induction of Rad51 in DNA repair, and shuttling of BRCA1 protein to the cytoplasm in the cell in vitro [70]. Promisingly, in pre-clinical TNBC models, a combination of EGFR inhibition with PARPi-targeted therapies also showed synthetic lethality through abrogation of the HR-dependent mechanism [71]. Nevertheless, we were unable to identify, at the time of writing, studies that have evaluated EGFR as a prognostic marker in familial breast tumours, or the benefits of EGFR-targeted tyrosine kinase inhibitors, specifically in clinical familial breast cancer studies.
2.9. MicroRNA (miR)
MiR binds mRNA and, through this post-transcriptional mechanism, regulates DNA expression. They are aberrantly expressed in tumours [72] and, being stable, are able to be extracted in tissue, but also isolated from fluids, such as blood [73], saliva [74] and urine [75].
Some miRNAs have been used to stratify risk in patients with breast/ovarian cancer and are associated with BRCA1/2 mutations [73,76]. Different miRNA expression profiles have been identified in healthy women, women with sporadic breast cancer and women with BRCA-mut breast cancer [75,77], and, to some degree, between BRCA1-, BRCA2- and BRCAX-related tumours. Although only a small number of specimens was studied, Murria-Estal et al. [78] reported 15 differentially expressed miRNAs (miR 4756-5p, miR 1273c, miR 4519, miR 323-3p, miR 4731-5p, miR 4498, miR 4417, miR 4783-3p, miR 129-5p, miR 4680-3p, miR 583, miR 206, miR 423-3p, miR 1181, miR 3169) that differentiate between BRCA1, BRCA2, BRCAX and sporadic breast tumours, albeit with relatively low accuracy (75%). Yan et al. showed an miR signature correlating with reduced/negative staining for downstream protein FOXP1, Cyclin D1 and NRP1 able to predict germline BRCA1 mutation status with a sensitivity of 92%, specificity of 44%, positive predictive value of 38% and a negative predictive value of 94% [79]. Other classifiers based on variable numbers of miRNAs have been suggested to distinguish BRCA1/2-mut (n = 6) and hereditary breast cancers (n = 15) from non-mutated breast tumours, respectively, with relatively high accuracy [80,81]. However, validation studies of these are lacking.
Notably, over 100 miRNAs have been shown to be interactive for the BRCA proteins. While prognostic significance has not been seen specifically in familial breast cancer cohorts, studies in TNBC have identified several miRNAs that may show potential significance in BRCA1/2mut cancers. These include elevated levels of miR-21, miR-27a/b, miR-210 and miR-454, associated with shorter OS and high miR-548c-5p and high miR-29b-1-5p, associated with improved overall survival in breast cancer patients with basal or TNBCs [82,83,84,85,86]. Furthermore, low expression of miR-155 [87] was associated with poor overall survival in TNBC patients, and low expression of miR-374a/b [88] and high miR-214 and miR-454 expression correlated with shorter disease-free survival [83,89]. These miRNAs appear to function across a wide range of cellular mechanisms that include cell proliferation, apoptosis and immunoregulatory mechanisms. Direct associations are seen between the has-miR-548 family, which has binding sites in BRCA2, and epigenetic control of miR-155 by BRCA1.
With regard to predictive makers, several miRNAs, including miR-146a, miR-146b-5p and miR-182, have been reported to reduce BRCA1 protein expression, with miR-182 expression also showing sensitivity to PARPi in cancer cell lines [90,91]. Other studies have reported that miR-493-5p overexpression is able to restore genomic stability in BRCA2-mut cells, leading to acquired resistance to PARP inhibitors [92]. In vivo studies also suggest miR-664b-5p may be important in increasing chemosensitivity in BRCA1mut TNBC [93]. MiR-664b-5p, by targeting CCNE2 (a G1-cyclin binding CDK2) protein expression, acts as a tumour suppressor and increases sensitivity to PARP inhibitors. Indeed, MiR-664b-5p inhibited tumour growth compared with the control in tumour xenograft models, and CCNE2 expression was also inversely correlated with miR-664b-5p expression in 90 TNBC patient samples [93].
Recent studies showed that results of the treatment with PARP1 inhibitors in breast and ovarian cancers may be dependent on high expression, particularly for miRNAs, and low expression of BRCA1 [94]. In mouse models, increased expression of miRNA-9 inhibited tumour growth during treatment with a PARPi [94]. Using prediction algorithms, Moskwa et al. found miRNA-182 targeted BRCA1 in breast cancer [91], and overexpression of miRNA-182Its in MDA-MB231 cells was significantly more sensitive to PARPi [91].
In a setting of marked clinical and tumour heterogeneity, the use of miRs as both biomarkers and in therapy is highly appealing. These targets may be especially useful serum interval markers of treatment response and as early markers of progression to advanced disease. While there are no miR-based therapeutics available currently, there may be potential clinical application in various subsets of familial breast cancer. As a result of further investigation in this area, there is also potential for identification of relevant downstream gene and protein biomarkers in familial breast cancers, and increased use of utility of these biomarkers in directing therapies, with several clinical discovery studies underway and one validation clinical trial looking at the predictive value of treatment response to HER2 therapies also present (NCT02656589) [95]. As of yet, there are no miRNA-based clinical studies specifically focused on familial breast cancer.
2.10. Single-Nucleotide Polymorphisms (SNPs)/Single-Nucleotide Variants (SNVs)
Several studies have examined the potential risk modification of SNP/SNVs in familial breast cancers and, in particular, BRCA1, BRAC2, CHEK2, PALB2 and ATM mutation carriers. They have shown both a correlative and inverse association between SNP/SNV panels and risk of breast cancer when compared to the general population, suggestive of the importance of genetic context in potential risk stratification. There are, however, no SNV-based predictors for tumour outcome in familial cancer patients [96].
2.11. Commercial Expression Profile Assays
Arguably among the best studied and strongest predictive and prognostic tools in breast cancer, several small studies have reported on the application of molecular assays, such as Oncotype DX®, MammaPrint®, ProsignaTM and EndoPredict®, in hereditary breast cancers [97].
The use of a Breast Recurrence Score (RS) in the Oncotype DX® assay has been shown to be prognostically significant and is a useful predictive too in the treatment of early-stage hormone-receptor-positive breast cancer. Not surprisingly, compared with the general population, both BRCA1- and BRCA2-associated tumours showed higher mean RS scores (oncotype DX® 23.5-29 vs. 16) in 32 and 33 patients (25 BRCA2 and 8 BRCA1) [98,99] or a higher proportion of intermediate (18–30) or high (>30) RS scores (BRCA1—87.6%, BRCA2—82.8% vs. general population—46.6%, p < 0.001). The RS scores showed an association with higher tumour grade, and more frequent PR-negative status, but did not appear to be influenced by nodal status [100,101].
From a prognostic standpoint, there was no evidence of a statistically different OS between BRCA mutation carriers and the general population when stratified by RS score. Notably, while there were differences in treatment between the BRCA mutation carriers and the whole general population (with higher levels of chemotherapy in BRCAmut patients (54.5% vs. 32.6%, p = 0.0090) and lower rates of hormone therapy (81.8% vs. 91.7%, p = 0.489) and radiation therapy (24.2% vs. 48.5%, p = 0.0065)) [100,101], when matched by RS scores, the treatment protocols appear similar, with no significant differences.
As BRCAmut tumours are more frequently treated with chemotherapy and mastectomy, the utility of these tests may be somewhat arbitrary in treatment decision making. However, there is still an uncertainty as to whether these tests may provide similar prognostic and predictive value similar to that seen in the general population [99], or identify potential hormone-positive BRCAmut tumours, most likely of low RS that may not need chemotherapy, a group which may comprise an estimated 8–44% of hormone-positive BRCAmut tumours.
Currently, there are no studies on the utility of MammaPrint®, ProsignaTM and EndoPredict® in familial breast cancer. As the homologous recombination machinery is not evaluated by these assays, newer assays for homologous recombination deficiency may be of predictive use. Several models have been studied to determine the BRCA level of tumours and, in particular, those that may benefit most from platinum-based chemotherapy. Vollebergh et al. used an aCGH classifier to identify BRCA1-like tumours [102]. The majority of these tumours showed either BRCA1 mutation or methylation (63%) and improved benefit from platinum-based chemotherapy compared to conventional chemotherapy (Hazard Ratio—0.12, p = 0.006).
Several newer platforms have evaluated molecular signatures specific to HR deficiency, and the ‘BRCA likness’ of cancers. At this point, there are up to 49 different mutational signatures described, with many showing an association with specific mutations and pathways [103,104]. The HR-deficiency-specific pattern, “Signature 3” is seen with BRCA1/2 loss in several cancer streams, including breast, ovarian, prostate and gastric [103]. This signature may be identified by whole-genome sequencing and contemporary bioinformatics tools, such as SigMA [105], able to detect Signature 3 profiles from sequence data procured form large targeted panels used in clinical practice and bypassing whole-genome analysis.
Several commercial assays are now available for HR deficiency, of which two are approved by the FDA for ovarian cancers. Using formalin-fixed paraffin-embedded (FFPE) tissue, the Foundation Medicine’s FoundationFocus CDxBRCA LOH [106] and Myriad’s myChoice® CDx Plus assay [107,108] have been approved as companion diagnostics for identifying patients for rucaparib and olaparib treatment, respectively. The assays identify focused HR gene mutations and variable genomic changes, primarily through LOH for Foundation Medicine and, additionally, large-scale transitions and telomeric allelic imbalance for the Myriad assay, undertaken by targeted panel testing.
Other commercial panels include the SOPHiA GeneticsTM DDM Homolgous Recombination Deficiency Panel using targeted next-generation sequencing combined with low-pass whole-genome sequencing to identify mutations in 28 HR-pathway-associated genes and large-scale copy number changes, indicative of HR deficiency. The assay shows high concordance with Myriad myChoice® [109] and can be performed on FFPE or fresh-frozen material from breast cancers. The AmoyDx® HRD Focus Panel can be performed on FFPE tissue and evaluates for pathogenic mutations in BRCA genes, as well as genomic changes through SNPs, distributed evenly within the genome. HRDetect, developed by Zainal et al., is a weighted model combing six distinguishing mutational signatures using an assay that was able to accurately identify a BRCA1/2-deficient tumour with 98.7% accuracy [110]. This identified all tumours with germline BRCA1/2 loss (22 cases out of 560), 22 further cases of somatic BRCA1/2 loss and 47 tumours with BRCA1/2 deficiency where no mutation was detected, but it was limited by the requirement of whole-genome sequencing for data input.
2.12. PIK3CA
Of the limited descriptive studies to date in familial breast cancer, when compared with sporadic tumours, there is a markedly reduced frequency of somatic PIK3CA mutations in BRCA1/2mut tumours (5–16%) [111,112]. The clinical significance of this is unknown but may be of interest, as PIK3CA inhibition has been shown to result in HR deficiency. In particular, BRCA-proficient TNBCs have shown increased sensitivity to PARP inhibition following PIK3CA blockade. Furthermore, in ovarian cancer cells lines, dual blockade of PI3K and PARP in vitro resulted in downregulation of PI3K/AKT/mTOR signalling and impaired DNA damage response with HRR deficiency, with associated reductions in BRCA, in a setting of intact PIK3CAwt and BRCAwt [113,114,115].
2.13. Immunotherapy Biomarkers
Almost all known moderate and high-penetrance familial breast cancer tumour suppressor genes are associated with the maintenance of genomic stability and its integrity [116,117]. While dysregulation of these cancer pathways is integral to tumour development, as a by-product of gene mutation, chromosomal rearrangement and genomic instability, there appears to be increased tumour immunogenicity through multiple mechanisms, albeit with immune regulatory mechanisms also at play [116,117].
2.14. Tumour-Infiltrating Lymphocytes
The role of tumour-infiltrating lymphocytes (TILs) in breast cancer has been studied over the last decade. Notably, high numbers of TILs are frequently present in triple-negative breast tumours and also in HER2-positive cancers. Similar to the association seen in microsatellite unstable cancers, such as colorectal or endometrial carcinomas, loss of homologous recombination repair pathways, frequently seen in familial breast cancers, is thought to increase tumour neoantigens, with cancer models, particularly BRCA1 deficiency, showing increased somatic mutation loads [118]. Nevertheless, HRR deficiency is not always associated with high tumour mutational burden [119].
Overall, high stromal TILs appear to be predictive for higher rates of pathological complete response (pCR) in the setting of neoadjuvant chemotherapy and prolonged overall survival in certain subsets of breast tumours. Several large studies have shown increased rates of TIL-high tumours in BRCA1/2 carriers when compared with all WT BRCA breast cancer patients [120]. Notably, however, there appears to be a phenotypic bias, with the difference not always observed between BRCAmut and BRCAwt tumours, at least in triple-negative tumours (p = 0.391, p = 0.36) [121].
There are a few limited studies reporting on the prognostic and predictive relevance of TILs as a biomarker in only familial breast cancers. A study by Sonderstrup of 411 BRCA1/2mut early breast cancers showed that stromal TILs increasing in 10% intervals were significantly associated with OS (Hazard Ratio 0.92, 95% CI 0.84–1.00, p = 0.05) in BRCA1 and BRCA2mut breast cancers [122]. The association in the BRCA1 subset was a 10% reduction and a 13% reduction in risk of DFS events with each 10% increment in stromal TILs, even after adjustment for ER status. However, no significant association with survival was observed in the BRCA2 subgroup by itself. Using almost the same cohort, in 414 BRCA1/2mut cancers, Jorgensen et al. [123] examined other immune markers and observed a 26% reduction in risk of disease-free survival for a 10% increase in CD8-positive cells, with a similar trend seen for CD4 and FOXP3 expression, the latter most prominently in BRCA1mut-associated tumours. Mortality rates showed a 28%, 46% and 12% reduction for each 10% increase in CD4 and CD8 expression and for each 1% increase in FOXP3 expression, respectively. Interestingly, many of these associations are also seen in studies of TNBCs [124,125,126]. Nevertheless, some studies have observed a direct correlation between increased numbers of FOXP3 T-regulatory cells and improved mortality, which is somewhat unexpected given the immune regulatory role of these cells, and is contrary to the association with poor survival in breast cancer studies unselected for familial breast cancers [127].
Examination of specific T-cell subsets, including T cells (CD3, CD4, CD8), B cells (CD20) and checkpoints (PD-1, PD-L1, see below), to date, has shown no difference between BRCA1mut and BRCA2mut tumours, and between BRCAmut and BRCAwt tumours, albeit with the latter studies almost universally performed in triple-negative breast cancers [120,128]. Interestingly, this is somewhat different to what in seen in prostate cancer, where BRCA2mut tumours showed increased ratios of intratumoral to extratumoral immune cells, and lower CD8:FOXP3 ratios when compared with BRCA1mut and ATMmut cancers, suggestive of at least the possibility of a more suppressed microenvironment in some tumour types when stratified by BRCA mutation status [129].
A recent study by Grandal et al. is the only to examine TILs in the setting of pre- and post-neoadjuvant therapy in a large patient cohort with BRCA germline status available [121]. Similar treatment responses were seen in triple-negative and HER2 tumours when stratified for BRCAmut versus BRCAwt. There was a markedly superior pCR rate in the luminal BRCAmut tumours when compared to BRCAwt lesions (33% vs. 5%, p = 0.006). Most notably, in this group, there was also a high percentage of post-neoadjuvant stromal TILS in the BRCAmut luminal cancers when compared with wild type (p = 0.0091). Such a difference was not observed in the TNBC and HER2 subgroups. Notably, there was no difference between any of the three phenotypes (luminal, TNBC and HER2) between the BRCAmut and BRCAwt tumours in the pre-neoadjuvant biopsy.
2.15. Programmed Cell Death Ligand-1 (PDL-1)
Within cancer immunology, there are now well-described immunomodulatory and suppressive mechanisms that allow potentially highly immunogenic tumours to survive in anergic micro-environments. Key regulators within this area are PD-1 (Programmed Cell Death Protein 1) (CD274 or B7-H1) and PDL-1. The transmembrane glycoprotein PD-1 is strongly expressed by activated T cells and in tumour-specific T cells, B cells and NK. [130].
When the receptor activates the ligand, regulatory and anergic mechanisms are initiated, most importantly resulting in inhibition of normal activation and proliferation of tumour-specific T cells, thereby abrogating the PI3K/AKT/mTOR and Ras pathways and consequently the cell-killing effect of tumour-specific T cells [131,132]. Downstream inhibition of pro-inflammatory factors and reduced antigen presenting of dendritic cells also occur [133].
In breast cancer, PDL-1 and PD-1 have been best studied in triple-negative breast cancers. Expression appears to be higher both within tumour and immune cells of triple-negative tumours when compared with hormone-positive lesions [134]. In some studies, an association between tumour PDL-1 expression and prognostic factors, such as higher grade, larger tumour size and younger age of onset, have been reported; however, reproducibility and guidelines for robust biomarkers have been hampered by the use of different PD-L1 antibodies raised against different epitopes, different immunohistochemical staining platforms and detection methods and a mix-and-match approach to score immune cells versus tumour staining (Figure 2), and with probable tropism bias, with liver and nodal lesions consistently showing higher levels of PDL-1 tumour expression compared to other sites [135,136].
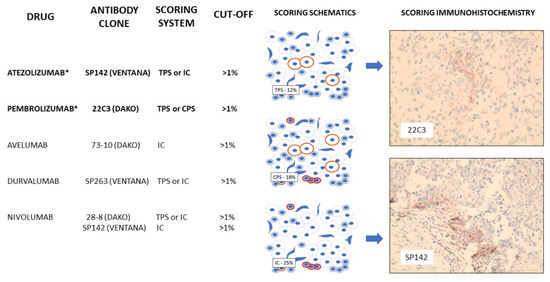
Figure 2.
PD-L1 inhibitors currently studied in breast cancer, including those approved by the FDA for use in TNBC (*). Scoring schematics demonstrate counting methodology for tumour proportion score (TPS—percentage of tumour staining positively), immune score (IC—percentage of immune cells staining positively) and combine proportion score (CPS—percentage of tumour and immune cells staining positively) with patient immunohistochemical staining of tumour cells (22C3) and TILs (SP142) present.
To date, PD-L1 inhibition has shown clinical benefit in triple-negative breast cancer in some studies. IMpassion130 [137], the first randomized controlled study in first-line therapy in unresected metastatic TNBC, showed a significant prolonged progression-free survival in patients receiving combination nab-paclitaxel and atezolizumab (anti PD-L1) compared with nab-paclitaxel alone, the strongest effect in patients in tumours with >1% staining of immune cells (ICs)(PD-L1 positive). However, IMpassion131 [138], which had a slightly different trial design, did not confirm the clinical effectiveness, leading to withdrawal of atezolizumab by the FDA [139]. The reason for this is unclear but is the subject of much speculation [140]. Another checkpoint inhibitor study, KEYNOTE-355 [141], compared pembrolizumab (anti-PD1) with nab-paclitaxel, paclitaxel or carboplatin and gemcitabine in first-line therapy for metastatic TNBC patients and reported improved progression-free survival and OS in PD-L1-positive tumours (combined positive score (CPS) of >10) and led to the approval of pembrolizumab. Although approval for atezolizumab was withdrawn, a more detailed analysis of Impassion130 showed immune cell PD-L1 positivity was associated with clinical benefit, irrespective of TIL density. Furthermore, either germline or somatic BRCA mutation status was not associated with PD-L1 expression levels in immune cells [142], and the benefit of atezolizumab was noted in patients with PD-L1-positive immune cells, regardless of BRCA mutation status [142]; however, given the smaller numbers, it was suggested that further study is required in larger numbers to understand why this confirmatory trial was negative.
Innate immunity and Cyclic GMP–AMP synthase (cGAS)-stimulator of interferon genes (STING).
Increasingly recognised as a potent stimulator of anti-tumour immunity, activation of cyclic GMP–AMP synthase (cGAS)-stimulator of interferon genes (STING) results in a cascade sequence within the innate immune system, driving interferon production and heightening T-cell responses [143]. This pathway may be particularly significant in familial breast cancer, as compared to homologous recombination proficient tumours. In vitro studies of PARP inhibitors show a significant anti-tumour response dependent on BRCA1/2 deficiency, activation of the cGAS/STING pathway and recruitment of CD8+ T cells [144]. While the role players in the pathway are relatively well described, in several tumour streams, efficacy has been shown to be negatively impacted by predominantly downstream players, such as dendritic cell inactivity and PD-L1 activation, both of which were able to be overcome in vitro. While in vivo biomarkers have not yet been validated in this pathway, potential candidates include TANK-binding kinase 1 (TBK1), interferon regulatory factor 3 (IRF3) and IFN-related genes [145], the levels of the latter in the sera of a general cohort of 451 breast cancer patients being associated with poorer prognosis [146].
2.16. CXCL10/CXCR3
Several studies have shown a role for CXCL10, a key component of the Th1-associated immune response, as well as its receptor in the progression of several cancers, which are known to be significantly overexpressed in basal breast tumours [147,148]. Secreted by a host of immune (activated T lymphocytes, monocytes), stromal (fibroblasts, endothelial cells) and epithelial cells, the chemokine attracts other immune cells, including NK cells, with autocrine promotion of tumour growth also seen. In a study of familial breast cancers, tumour expression of both CXCL10 and the Th1-associated transcription factor T-BET was associated with higher tumour grade, higher proliferation index, tumour p53 expression, increased peritumour CD4+ and CD8+ lymphocytes. In relation to FOXP3-positive Tregs, there was no association, suggestive of independence from this immune-regulatory pathway. Furthermore, in the BRCA1 subset, CXCL10-positive expression showed a worse prognosis, suggesting that the axis may serve as a potential target in these tumours [148].
3. Conclusions
While a lot of research has focused on cancer germline predisposition, there is a paucity of data relating to prognostic and predictive biomarkers in familial breast cancers. Study into this is inherently difficult in some instances, as BRCA1/2 germline mutation status is often not known before treatment begins. The identification of BRCAX patients is, in some instances, even more difficult, with few studies segregating these tumours from clear sporadic cancers. Recommended Guidelines, such as those produced by NICE, focus on proper identification of familial case, genetic screening and threshold for germline testing, surveillance of breast and other cancers and risk reduction. There are no treatment recommendations specific for familial breast cancers outside of generic breast cancer treatment guidelines. Similarly, the NCCN only provides guidelines for the assessment of high-risk/potential familial breast cancers that may require germline testing and testing panels.
While cancers arising in BRCA1/2 mutation carriers certainly show marked sensitivity to platinum-based chemotherapeutic regimens and immunotherapies, there is still some heterogeneity in regards to treatment response. A small number of these tumours appear more phenotypically and genotypically similar to sporadic cancers, and they may require alternate therapies. Further evaluation is, therefore, required to identify possible cancers that may require alternate therapies. Furthermore, identification of treatment-resistant pathways is integral in either tailoring primary therapies or developing secondary and third-line treatment options.
Large studies into non-BRCA1/2 familial cancers are also needed. With ever-expanding familial databases and collaboration, further biomarkers in PALB2 and CHEK2 familial breast cancers should be explored, with some small case series showing some benefit of platinum-based chemotherapy in these cancers [149]. Description of BRCAX tumours has also been difficult with explorative studies general of modest size, with marked variability of inclusion criteria, and are often historically composed of genetic testing limited to BRCA1/2 only. These studies are universally retrospective and also show a lack of replication of findings, and frequently lack adequate controls. Certainly, there are some differences that are alluded to from sporadic cases of breast cancer. Whether there are specific predictive and prognostic biomarkers in this group, and whether they require alternate therapies, is, as yet, unknown. Prospective clinical trials evaluating novel combination therapies and identifying specific subgroups to most benefit from these treatments are warranted. Promising further work on immunotherapies in combination therapies with PIK3CA or PARP inhibitors [150] may also demonstrate additional benefits and potential new biomarkers. While almost all current studies asses these biomarkers at the time of cancer diagnosis, the optimal timing of the utility of these biomarkers is also somewhat unknown, with further studies required to assess promising targets in pre-clinical and in situ disease.
Author Contributions
Conceptualization, S.D. and S.B.F.; investigation S.D. and A.C.; writing—original draft preparation, S.D. and A.C.; writing—review and editing, S.D. and S.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Claus, E.B.; Risch, N.; Thompson, W.D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am. J. Hum. Genet. 1991, 48, 232–242. [Google Scholar] [PubMed]
- Newman, B.; Austin, M.A.; Lee, M.; King, M.C. Inheritance of human breast cancer: Evidence for autosomal dominant transmission in high-risk families. Proc. Natl. Acad. Sci. USA 1988, 85, 3044–3048. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Reis-Filho, J.S. Basal-like breast cancer and the BRCA1 phenotype. Oncogene 2006, 25, 5846–5853. [Google Scholar] [CrossRef]
- Deb, S.; Jene, N.; Fox, S.B. Genotypic and phenotypic analysis of familial male breast cancer shows under representation of the HER2 and basal subtypes in BRCA-associated carcinomas. BMC Cancer 2012, 12, 510. [Google Scholar] [CrossRef]
- Chen, S.; Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Li, W.; Bai, H.; Zhang, Z. PARP inhibitors in ovarian cancer: Sensitivity prediction and resistance mechanisms. J. Cell. Mol. Med. 2019, 23, 2303–2313. [Google Scholar] [CrossRef]
- Miglietta, F.; Cinquini, M.; Dieci, M.V.; Cortesi, L.; Criscitiello, C.; Montemurro, F.; Del Mastro, L.; Zambelli, A.; Biganzoli, L.; Levaggi, A.; et al. PARP-inhibitors for BRCA1/2-related advanced HER2-negative breast cancer: A meta-analysis and GRADE recommendations by the Italian Association of Medical Oncology. Breast 2022, 66, 293–304. [Google Scholar] [CrossRef]
- Geenen, J.J.J.; Linn, S.C.; Beijnen, J.H.; Schellens, J.H.M. PARP Inhibitors in the Treatment of Triple-Negative Breast Cancer. Clin. Pharmacokinet. 2018, 57, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.; de Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef] [PubMed]
- de Gooijer, M.C.; Buil, L.C.M.; Çitirikkaya, C.H.; Hermans, J.; Beijnen, J.H.; van Tellingen, O. ABCB1 Attenuates the Brain Penetration of the PARP Inhibitor AZD2461. Mol. Pharm. 2018, 15, 5236–5243. [Google Scholar] [CrossRef] [PubMed]
- Pettitt, S.J.; Krastev, D.B.; Brandsma, I.; Dréan, A.; Song, F.; Aleksandrov, R.; Harrell, M.I.; Menon, M.; Brough, R.; Campbell, J.; et al. Genome-wide and high-density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat. Commun. 2018, 9, 1849. [Google Scholar] [CrossRef]
- Gogola, E.; Duarte, A.A.; de Ruiter, J.R.; Wiegant, W.W.; Schmid, J.A.; de Bruijn, R.; James, D.I.; Guerrero Llobet, S.; Vis, D.J.; Annunziato, S.; et al. Selective Loss of PARG Restores PARylation and Counteracts PARP Inhibitor-Mediated Synthetic Lethality. Cancer Cell 2018, 33, 1078–1093. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef]
- Ray Chaudhuri, A.; Callen, E.; Ding, X.; Gogola, E.; Duarte, A.A.; Lee, J.E.; Wong, N.; Lafarga, V.; Calvo, J.A.; Panzarino, N.J.; et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 2016, 535, 382–387. [Google Scholar] [CrossRef]
- Rondinelli, B.; Gogola, E.; Yücel, H.; Duarte, A.A.; van de Ven, M.; van der Sluijs, R.; Konstantinopoulos, P.A.; Jonkers, J.; Ceccaldi, R.; Rottenberg, S.; et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 2017, 19, 1371–1378. [Google Scholar] [CrossRef]
- Yazinski, S.A.; Comaills, V.; Buisson, R.; Genois, M.M.; Nguyen, H.D.; Ho, C.K.; Todorova Kwan, T.; Morris, R.; Lauffer, S.; Nussenzweig, A.; et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev. 2017, 31, 318–332. [Google Scholar] [CrossRef]
- Godin, S.K.; Sullivan, M.R.; Bernstein, K.A. Novel insights into RAD51 activity and regulation during homologous recombination and DNA replication. Biochem. Cell Biol. 2016, 94, 407–418. [Google Scholar] [CrossRef]
- Yun, M.H.; Hiom, K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 2009, 459, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Wakimoto, H.; Peters, C.; Martuza, R.L.; Rabkin, S.D. Rad51 Degradation: Role in Oncolytic Virus-Poly(ADP-Ribose) Polymerase Inhibitor Combination Therapy in Glioblastoma. J. Natl. Cancer Inst. 2017, 109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Noordermeer, S.M.; Adam, S.; Setiaputra, D.; Barazas, M.; Pettitt, S.J.; Ling, A.K.; Olivieri, M.; Álvarez-Quilón, A.; Moatti, N.; Zimmermann, M.; et al. The shieldin complex mediates 53BP1-dependent DNA repair. Nature 2018, 560, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Dev, H.; Chiang, T.W.; Lescale, C.; de Krijger, I.; Martin, A.G.; Pilger, D.; Coates, J.; Sczaniecka-Clift, M.; Wei, W.; Ostermaier, M.; et al. Shieldin complex promotes DNA end-joining and counters homologous recombination in BRCA1-null cells. Nat. Cell Biol. 2018, 20, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Jacot, W.; Thezenas, S.; Senal, R.; Viglianti, C.; Laberenne, A.C.; Lopez-Crapez, E.; Bibeau, F.; Bleuse, J.P.; Romieu, G.; Lamy, P.J. BRCA1 promoter hypermethylation, 53BP1 protein expression and PARP-1 activity as biomarkers of DNA repair deficit in breast cancer. BMC Cancer 2013, 13, 523. [Google Scholar] [CrossRef]
- Neboori, H.J.; Haffty, B.G.; Wu, H.; Yang, Q.; Aly, A.; Goyal, S.; Schiff, D.; Moran, M.S.; Golhar, R.; Chen, C.; et al. Low p53 binding protein 1 (53BP1) expression is associated with increased local recurrence in breast cancer patients treated with breast-conserving surgery and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e677–e683. [Google Scholar] [CrossRef]
- Blum, J.L.; Laird, A.D.; Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Martin, M.; Roché, H.H.; Lee, K.H.; Goodwin, A.; et al. Determinants of Response to Talazoparib in Patients with HER2-Negative, Germline BRCA1/2-Mutated Breast Cancer. Clin. Cancer Res. 2022, 28, 1383–1390. [Google Scholar] [CrossRef]
- Castroviejo-Bermejo, M.; Cruz, C.; Llop-Guevara, A.; Gutiérrez-Enríquez, S.; Ducy, M.; Ibrahim, Y.H.; Gris-Oliver, A.; Pellegrino, B.; Bruna, A.; Guzmán, M.; et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol. Med. 2018, 10, e9172. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Elattar, A.; Cerbinskaite, A.; Wilkinson, S.J.; Drew, Y.; Kyle, S.; Los, G.; Hostomsky, Z.; Edmondson, R.J.; Curtin, N.J. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin. Cancer Res. 2010, 16, 2344–2351. [Google Scholar] [CrossRef]
- Naipal, K.A.; Verkaik, N.S.; Ameziane, N.; van Deurzen, C.H.; Ter Brugge, P.; Meijers, M.; Sieuwerts, A.M.; Martens, J.W.; O’Connor, M.J.; Vrieling, H.; et al. Functional ex vivo assay to select homologous recombination-deficient breast tumors for PARP inhibitor treatment. Clin. Cancer Res. 2014, 20, 4816–4826. [Google Scholar] [CrossRef]
- Cruz, C.; Castroviejo-Bermejo, M.; Gutiérrez-Enríquez, S.; Llop-Guevara, A.; Ibrahim, Y.H.; Gris-Oliver, A.; Bonache, S.; Morancho, B.; Bruna, A.; Rueda, O.M.; et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Xu, H.; Waddell, N.; Shield-Artin, K.; Haviv, I.; McKay, M.J.; Fox, S.B. Enhanced RAD21 cohesin expression confers poor prognosis in BRCA2 and BRCAX, but not BRCA1 familial breast cancers. Breast Cancer Res. 2012, 14, R69. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.A.; Ouchi, T. BRCA1 phosphorylation regulates caspase-3 activation in UV-induced apoptosis. Cancer Res. 2005, 65, 10657–10662. [Google Scholar] [CrossRef] [PubMed]
- López de Silanes, I.; Gorospe, M.; Taniguchi, H.; Abdelmohsen, K.; Srikantan, S.; Alaminos, M.; Berdasco, M.; Urdinguio, R.G.; Fraga, M.F.; Jacinto, F.V.; et al. The RNA-binding protein HuR regulates DNA methylation through stabilization of DNMT3b mRNA. Nucleic Acids Res. 2009, 37, 2658–2671. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Chu, H.; Guan, Y.; Bi, J.; Wang, B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int. J. Mol. Sci. 2013, 14, 10015–10041. [Google Scholar] [CrossRef]
- Heinonen, M.; Fagerholm, R.; Aaltonen, K.; Kilpivaara, O.; Aittomäki, K.; Blomqvist, C.; Heikkilä, P.; Haglund, C.; Nevanlinna, H.; Ristimäki, A. Prognostic role of HuR in hereditary breast cancer. Clin. Cancer Res. 2007, 13, 6959–6963. [Google Scholar] [CrossRef]
- Takada, M.; Nagai, S.; Haruta, M.; Sugino, R.P.; Tozuka, K.; Takei, H.; Ohkubo, F.; Inoue, K.; Kurosumi, M.; Miyazaki, M.; et al. BRCA1 alterations with additional defects in DNA damage response genes may confer chemoresistance to BRCA-like breast cancers treated with neoadjuvant chemotherapy. Genes Chromosomes Cancer 2017, 56, 405–420. [Google Scholar] [CrossRef]
- Paterson, M.C.; Dietrich, K.D.; Danyluk, J.; Paterson, A.H.; Lees, A.W.; Jamil, N.; Hanson, J.; Jenkins, H.; Krause, B.E.; McBlain, W.A.; et al. Correlation between c-erbB-2 amplification and risk of recurrent disease in node-negative breast cancer. Cancer Res. 1991, 51, 556–567. [Google Scholar]
- Ross, J.S.; Fletcher, J.A. The HER-2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. Stem Cells 1998, 16, 413–428. [Google Scholar] [CrossRef]
- Incorvati, J.A.; Shah, S.; Mu, Y.; Lu, J. Targeted therapy for HER2 positive breast cancer. J. Hematol. Oncol. 2013, 6, 38. [Google Scholar] [CrossRef]
- Tomasello, G.; Gambini, D.; Petrelli, F.; Azzollini, J.; Arcanà, C.; Ghidini, M.; Peissel, B.; Manoukian, S.; Garrone, O. Characterization of the HER2 status in BRCA-mutated breast cancer: A single institutional series and systematic review with pooled analysis. ESMO Open 2022, 7, 100531. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.G.; Couch, F.J.; Visscher, D.W.; Lindor, N.M. Non-BRCA familial breast cancer: Review of reported pathology and molecular findings. Pathology 2017, 49, 363–370. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Brezden-Masley, C.; Cazzaniga, M.; Dalvi, T.; Walker, G.; Bennett, J.; Ohsumi, S. Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: Global results from the real-world, observational BREAKOUT study. Breast Cancer Res. 2020, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Curtit, E.; Benhamo, V.; Gruel, N.; Popova, T.; Manie, E.; Cottu, P.; Mariani, O.; Stoppa-Lyonnet, D.; Pivot, X.; Stern, M.H.; et al. First description of a sporadic breast cancer in a woman with BRCA1 germline mutation. Oncotarget 2015, 6, 35616–35624. [Google Scholar] [CrossRef] [PubMed]
- Viansone, A.; Pellegrino, B.; Omarini, C.; Pistelli, M.; Boggiani, D.; Sikokis, A.; Uliana, V.; Zanoni, D.; Tommasi, C.; Bortesi, B.; et al. Prognostic significance of germline BRCA mutations in patients with HER2-POSITIVE breast cancer. Breast 2022, 65, 145–150. [Google Scholar] [CrossRef]
- Conlon, N.T.; Kooijman, J.J.; van Gerwen, S.J.C.; Mulder, W.R.; Zaman, G.J.R.; Diala, I.; Eli, L.D.; Lalani, A.S.; Crown, J.; Collins, D.M. Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br. J. Cancer 2021, 124, 1249–1259. [Google Scholar] [CrossRef]
- Liu, W.; Shen, S.M.; Zhao, X.Y.; Chen, G.Q. Targeted genes and interacting proteins of hypoxia inducible factor-1. Int. J. Biochem. Mol. Biol. 2012, 3, 165–178. [Google Scholar]
- Wykoff, C.C.; Beasley, N.J.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.D.; Turley, H.; Talks, K.L.; Maxwell, P.H.; et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083. [Google Scholar]
- Zhao, M.; Zhang, Y.; Zhang, H.; Wang, S.; Zhang, M.; Chen, X.; Wang, H.; Zeng, G.; Chen, X.; Liu, G.; et al. Hypoxia-induced cell stemness leads to drug resistance and poor prognosis in lung adenocarcinoma. Lung Cancer 2015, 87, 98–106. [Google Scholar] [CrossRef]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Bos, R.; van der Groep, P.; Greijer, A.E.; Shvarts, A.; Meijer, S.; Pinedo, H.M.; Semenza, G.L.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 2003, 97, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; van Diest, P.J.; van der Wall, E. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Trastour, C.; Benizri, E.; Ettore, F.; Ramaioli, A.; Chamorey, E.; Pouysségur, J.; Berra, E. HIF-1alpha and CA IX staining in invasive breast carcinomas: Prognosis and treatment outcome. Int. J. Cancer J. Int. Cancer 2007, 120, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Vleugel, M.M.; Greijer, A.E.; Shvarts, A.; van der Groep, P.; van Berkel, M.; Aarbodem, Y.; van Tinteren, H.; Harris, A.L.; van Diest, P.J.; van der Wall, E. Differential prognostic impact of hypoxia induced and diffuse HIF-1alpha expression in invasive breast cancer. J. Clin. Pathol. 2005, 58, 172–177. [Google Scholar] [CrossRef]
- Yan, M.; Rayoo, M.; Takano, E.A.; Fox, S.B. BRCA1 tumours correlate with a HIF-1alpha phenotype and have a poor prognosis through modulation of hydroxylase enzyme profile expression. Br. J. Cancer 2009, 101, 1168–1174. [Google Scholar] [CrossRef]
- van der Groep, P.; Bouter, A.; Menko, F.H.; van der Wall, E.; van Diest, P.J. High frequency of HIF-1alpha overexpression in BRCA1 related breast cancer. Breast Cancer Res. Treat. 2008, 111, 475–480. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, S.; Shrivastava, N. Therapeutic Targeting Hypoxia-Inducible Factor (HIF-1) in Cancer: Cutting Gordian Knot of Cancer Cell Metabolism. Front. Genet. 2022, 13, 849040. [Google Scholar] [CrossRef]
- Liu, Y.; Tamimi, R.M.; Collins, L.C.; Schnitt, S.J.; Gilmore, H.L.; Connolly, J.L.; Colditz, G.A. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: Results from the Nurses’ Health Study. Breast Cancer Res. Treat. 2011, 129, 175–184. [Google Scholar] [CrossRef]
- Danza, K.; Pilato, B.; Lacalamita, R.; Addati, T.; Giotta, F.; Bruno, A.; Paradiso, A.; Tommasi, S. Angiogenetic axis angiopoietins/Tie2 and VEGF in familial breast cancer. Eur. J. Hum. Genet. 2013, 21, 824–830. [Google Scholar] [CrossRef]
- Severson, T.M.; Peeters, J.; Majewski, I.; Michaut, M.; Bosma, A.; Schouten, P.C.; Chin, S.F.; Pereira, B.; Goldgraben, M.A.; Bismeijer, T.; et al. BRCA1-like signature in triple negative breast cancer: Molecular and clinical characterization reveals subgroups with therapeutic potential. Mol. Oncol. 2015, 9, 1528–1538. [Google Scholar] [CrossRef]
- Kolyvas, E.A.; Caldas, C.; Kelly, K.; Ahmad, S.S. Androgen receptor function and targeted therapeutics across breast cancer subtypes. Breast Cancer Res. 2022, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Fujii, T.; Lim, B.; Karuturi, M.S.; Tripathy, D.; Ueno, N.T. Androgen Receptor Function and Androgen Receptor-Targeted Therapies in Breast Cancer: A Review. JAMA Oncol. 2017, 3, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Pristauz, G.; Petru, E.; Stacher, E.; Geigl, J.B.; Schwarzbraun, T.; Tsybrovskyy, O.; Winter, R.; Moinfar, F. Androgen receptor expression in breast cancer patients tested for BRCA1 and BRCA2 mutations. Histopathology 2010, 57, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, J.; Gast, K.; Quiroga, D.; Lustberg, M.; Williams, N. Triple-negative breast cancer: Promising prognostic biomarkers currently in development. Expert Rev. Anticancer. Ther. 2021, 21, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Karanika, S.; Yang, G.; Wang, J.; Park, S.; Broom, B.M.; Manyam, G.C.; Wu, W.; Luo, Y.; Basourakos, S.; et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci. Signal. 2017, 10, eaam7479. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Jang, M.H.; Kim, E.J.; Kim, H.J.; Lee, H.J.; Kim, Y.J.; Kim, J.H.; Kang, E.; Kim, S.W.; Kim, I.A.; et al. High EGFR gene copy number predicts poor outcome in triple-negative breast cancer. Mod. Pathol. 2014, 27, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Conchas, G.A.; Rodriguez-Romo, L.; Hernandez-Barajas, D.; Gonzalez-Guerrero, J.F.; Rodriguez-Fernandez, I.A.; Verdines-Perez, A.; Templeton, A.J.; Ocana, A.; Seruga, B.; Tannock, I.F.; et al. Epidermal growth factor receptor overexpression and outcomes in early breast cancer: A systematic review and a meta-analysis. Cancer Treat. Rev. 2018, 62, 1–8. [Google Scholar] [CrossRef]
- Abdelrahman, A.E.; Rashed, H.E.; Abdelgawad, M.; Abdelhamid, M.I. Prognostic impact of EGFR and cytokeratin 5/6 immunohistochemical expression in triple-negative breast cancer. Ann. Diagn. Pathol. 2017, 28, 43–53. [Google Scholar] [CrossRef]
- Danzinger, S.; Tan, Y.Y.; Rudas, M.; Kastner, M.T.; Weingartshofer, S.; Muhr, D.; Singer, C.F. Differential Claudin 3 and EGFR Expression Predicts BRCA1 Mutation in Triple-Negative Breast Cancer. Cancer Investig. 2018, 36, 378–388. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Yang, E.S.; Arteaga, C.L.; Xia, F. Erlotinib attenuates homologous recombinational repair of chromosomal breaks in human breast cancer cells. Cancer Res. 2008, 68, 9141–9146. [Google Scholar] [CrossRef]
- Nowsheen, S.; Cooper, T.; Stanley, J.A.; Yang, E.S. Synthetic lethal interactions between EGFR and PARP inhibition in human triple negative breast cancer cells. PLoS ONE 2012, 7, e46614. [Google Scholar] [CrossRef]
- Morales, S.; Monzo, M.; Navarro, A. Epigenetic regulation mechanisms of microRNA expression. Biomol. Concepts 2017, 8, 203–212. [Google Scholar] [CrossRef]
- Nassar, F.J.; Chamandi, G.; Tfaily, M.A.; Zgheib, N.K.; Nasr, R. Peripheral Blood-Based Biopsy for Breast Cancer Risk Prediction and Early Detection. Front. Med. 2020, 7, 28. [Google Scholar] [CrossRef]
- Setti, G.; Pezzi, M.E.; Viani, M.V.; Pertinhez, T.A.; Cassi, D.; Magnoni, C.; Bellini, P.; Musolino, A.; Vescovi, P.; Meleti, M. Salivary MicroRNA for Diagnosis of Cancer and Systemic Diseases: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 907. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Casorelli, A.; Bardhi, E.; Besharat, A.R.; Savone, D.; Ruscito, I.; Farooqi, A.A.; Papadia, A.; Mueller, M.D.; Ferretti, E.; et al. Beyond circulating microRNA biomarkers: Urinary microRNAs in ovarian and breast cancer. Tumour Biol. 2017, 39, 1010428317695525. [Google Scholar] [CrossRef] [PubMed]
- Shen, J. Evaluation of environmental and personal susceptibility characteristics that modify genetic risks. Methods Mol. Biol. 2009, 471, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Yu, B.P.; Chung, H.Y.; Jung, H.A.; Choi, J.S. Epigenetic modifications of gene expression by lifestyle and environment. Arch. Pharmacal Res. 2017, 40, 1219–1237. [Google Scholar] [CrossRef]
- Murria Estal, R.; Palanca Suela, S.; de Juan Jiménez, I.; Egoavil Rojas, C.; García-Casado, Z.; Juan Fita, M.J.; Sánchez Heras, A.B.; Segura Huerta, A.; Chirivella González, I.; Sánchez-Izquierdo, D.; et al. MicroRNA signatures in hereditary breast cancer. Breast Cancer Res. Treat. 2013, 142, 19–30. [Google Scholar] [CrossRef]
- Yan, M.; Shield-Artin, K.; Byrne, D.; Deb, S.; Waddell, N.; Haviv, I.; Fox, S.B. Comparative microRNA profiling of sporadic and BRCA1 associated basal-like breast cancers. BMC Cancer 2015, 15, 506. [Google Scholar] [CrossRef]
- Tanic, M.; Yanowski, K.; Gómez-López, G.; Rodriguez-Pinilla, M.S.; Marquez-Rodas, I.; Osorio, A.; Pisano, D.G.; Martinez-Delgado, B.; Benítez, J. MicroRNA expression signatures for the prediction of BRCA1/2 mutation-associated hereditary breast cancer in paraffin-embedded formalin-fixed breast tumors. Int. J. Cancer J. Int. Cancer 2015, 136, 593–602. [Google Scholar] [CrossRef]
- Pessôa-Pereira, D.; Evangelista, A.F.; Causin, R.L.; da Costa Vieira, R.A.; Abrahão-Machado, L.F.; Santana, I.V.V.; da Silva, V.D.; de Souza, K.C.B.; de Oliveira-Silva, R.J.; Fernandes, G.C.; et al. miRNA expression profiling of hereditary breast tumors from BRCA1- and BRCA2-germline mutation carriers in Brazil. BMC Cancer 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Block, I.; Burton, M.; Sørensen, K.P.; Andersen, L.; Larsen, M.J.; Bak, M.; Cold, S.; Thomassen, M.; Tan, Q.; Kruse, T.A. Association of miR-548c-5p, miR-7-5p, miR-210-3p, miR-128-3p with recurrence in systemically untreated breast cancer. Oncotarget 2018, 9, 9030–9042. [Google Scholar] [CrossRef]
- Cao, Z.G.; Li, J.J.; Yao, L.; Huang, Y.N.; Liu, Y.R.; Hu, X.; Song, C.G.; Shao, Z.M. High expression of microRNA-454 is associated with poor prognosis in triple-negative breast cancer. Oncotarget 2016, 7, 64900–64909. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Katsaros, D.; Lu, L.; Preti, M.; Durando, A.; Arisio, R.; Mu, L.; Yu, H. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast Cancer Res. Treat. 2009, 117, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Kondo, N.; Endo, Y.; Sugiura, H.; Yoshimoto, N.; Iwasa, M.; Takahashi, S.; Fujii, Y.; Yamashita, H. High expression of microRNA-210 is an independent factor indicating a poor prognosis in Japanese triple-negative breast cancer patients. Jpn. J. Clin. Oncol. 2012, 42, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhao, B.; Ren, X.; Wu, S.; Liu, M.; Wang, Z.; Liu, W. MiR-27a-3p Targeting GSK3β Promotes Triple-Negative Breast Cancer Proliferation and Migration Through Wnt/β-Catenin Pathway. Cancer Manag. Res. 2020, 12, 6241–6249. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Nuzzo, S.; Condorelli, G.; Salvatore, M.; Incoronato, M. Prognostic and Clinicopathological Significance of MiR-155 in Breast Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 5834. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, A.; Bao, P.P.; Lin, L.; Wang, Y.; Wu, H.; Shu, X.O.; Liu, A.; Cai, Q. MicroRNA-374b inhibits breast cancer progression through regulating CCND1 and TGFA genes. Carcinogenesis 2021, 42, 528–536. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, S.; Dong, L.; Li, Y.; Mao, Y.; Liang, Y.; Tao, Y.; Ma, J. Inhibition of miR-214 attenuates the migration and invasion of triple-negative breast cancer cells. Mol. Med. Rep. 2019, 19, 4035–4042. [Google Scholar] [CrossRef]
- Garcia, A.I.; Buisson, M.; Bertrand, P.; Rimokh, R.; Rouleau, E.; Lopez, B.S.; Lidereau, R.; Mikaélian, I.; Mazoyer, S. Down-regulation of BRCA1 expression by miR-146a and miR-146b-5p in triple negative sporadic breast cancers. EMBO Mol. Med. 2011, 3, 279–290. [Google Scholar] [CrossRef]
- Moskwa, P.; Buffa, F.M.; Pan, Y.; Panchakshari, R.; Gottipati, P.; Muschel, R.J.; Beech, J.; Kulshrestha, R.; Abdelmohsen, K.; Weinstock, D.M.; et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Mol. Cell 2011, 41, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Meghani, K.; Fuchs, W.; Detappe, A.; Drané, P.; Gogola, E.; Rottenberg, S.; Jonkers, J.; Matulonis, U.; Swisher, E.M.; Konstantinopoulos, P.A.; et al. Multifaceted Impact of MicroRNA 493-5p on Genome-Stabilizing Pathways Induces Platinum and PARP Inhibitor Resistance in BRCA2-Mutated Carcinomas. Cell Rep. 2018, 23, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Tang, L.; Xu, Y.; Xu, J.; Zhang, W.; Xie, H.; Wang, S.; Guan, X. PARP inhibitor increases chemosensitivity by upregulating miR-664b-5p in BRCA1-mutated triple-negative breast cancer. Sci. Rep. 2017, 7, 42319. [Google Scholar] [CrossRef] [PubMed]
- Strumidło, A.; Skiba, S.; Scott, R.J.; Lubiński, J. The potential role of miRNAs in therapy of breast and ovarian cancers associated with BRCA1 mutation. Hered. Cancer Clin. Pract. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Sempere, L.F.; Azmi, A.S.; Moore, A. microRNA-based diagnostic and therapeutic applications in cancer medicine. RNA 2021, 12, e1662. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.; Hughes, E.; Wagner, S.; Tshiaba, P.; Rosenthal, E.; Roa, B.B.; Kurian, A.W.; Domchek, S.M.; Garber, J.; Lancaster, J.; et al. Association of a Polygenic Risk Score With Breast Cancer Among Women Carriers of High- and Moderate-Risk Breast Cancer Genes. JAMA Netw. Open 2020, 3, e208501. [Google Scholar] [CrossRef]
- Zeng, C.; Zhang, J. A narrative review of five multigenetic assays in breast cancer. Transl. Cancer Res. 2022, 11, 897–907. [Google Scholar] [CrossRef]
- Blanter, J.; Zimmerman, B.; Tharakan, S.; Ru, M.; Cascetta, K.; Tiersten, A. BRCA Mutation Association with Recurrence Score and Discordance in a Large Oncotype Database. Oncology 2020, 98, 248–251. [Google Scholar] [CrossRef]
- Layman, R.M.; Lin, H.; Gutierrez Barrera, A.M.; Karuturi, M.S.; Yam, C.; Arun, B.K. Clinical outcomes and Oncotype DX Breast Recurrence Score® in early-stage BRCA-associated hormone receptor-positive breast cancer. Cancer Med. 2022, 11, 1474–1483. [Google Scholar] [CrossRef]
- Halpern, N.; Sonnenblick, A.; Uziely, B.; Divinsky, L.; Goldberg, Y.; Hamburger, T.; Peretz, T.; Kadouri, L. Oncotype Dx recurrence score among BRCA1/2 germline mutation carriers with hormone receptors positive breast cancer. Int. J. Cancer J. Int. Cancer 2017, 140, 2145–2149. [Google Scholar] [CrossRef]
- Lewin, R.; Sulkes, A.; Shochat, T.; Tsoref, D.; Rizel, S.; Liebermann, N.; Hendler, D.; Neiman, V.; Ben-Aharon, I.; Friedman, E.; et al. Oncotype-DX recurrence score distribution in breast cancer patients with BRCA1/2 mutations. Breast Cancer Res. Treat. 2016, 157, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Vollebergh, M.A.; Lips, E.H.; Nederlof, P.M.; Wessels, L.F.A.; Schmidt, M.K.; van Beers, E.H.; Cornelissen, S.; Holtkamp, M.; Froklage, F.E.; de Vries, E.G.E.; et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann. Oncol. 2011, 22, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, A.; Zou, X.; Amarante, T.D.; Martinez-Martinez, A.; Koh, G.C.C.; Dias, J.M.L.; Heskin, L.; Chmelova, L.; Rinaldi, G.; Wang, V.Y.W.; et al. Substitution mutational signatures in whole-genome-sequenced cancers in the UK population. Science 2022, 376, abl9283. [Google Scholar] [CrossRef] [PubMed]
- Gulhan, D.C.; Lee, J.J.; Melloni, G.E.M.; Cortes-Ciriano, I.; Park, P.J. Detecting the mutational signature of homologous recombination deficiency in clinical samples. Nat. Genet. 2019, 51, 912–919. [Google Scholar] [CrossRef]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef]
- Telli, M.L.; Timms, K.M.; Reid, J.; Hennessy, B.; Mills, G.B.; Jensen, K.C.; Szallasi, Z.; Barry, W.T.; Winer, E.P.; Tung, N.M.; et al. Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2016, 22, 3764–3773. [Google Scholar] [CrossRef]
- Myriad_Genetics. myChoice HRD Technical Specifications Effective Date: June 2017. Available online: https://myriad-web.s3.amazonaws.com/myChoice/downloads/myChoiceHRDTechSpecs.pdf (accessed on 6 February 2023).
- Buisson, A.; Saintigny, P.; Pujade-Lauraine, E.; Montoto-Grillot, C.; Vacirca, D.; Barberis, M.; Colombo, N.; Harle, A.; Gilson, P.; Roma, C.; et al. A deep learning solution for detection of homologous recombination deficiency in ovarian cancer using low pass whole-genome sequencing: Evaluation of the analytical performance. J. Clin. Oncol. 2022, 40, e17599. [Google Scholar] [CrossRef]
- Davies, H.; Glodzik, D.; Morganella, S.; Yates, L.R.; Staaf, J.; Zou, X.; Ramakrishna, M.; Martin, S.; Boyault, S.; Sieuwerts, A.M.; et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat. Med. 2017, 23, 517–525. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, G.; Li, X.; Ren, C.; Wang, Y.; Li, K.; Mok, H.; Cao, L.; Wen, L.; Jia, M.; et al. Comparison of BRCA versus non-BRCA germline mutations and associated somatic mutation profiles in patients with unselected breast cancer. Aging 2020, 12, 3140–3155. [Google Scholar] [CrossRef] [PubMed]
- Ruangapirom, L.; Sutivijit, N.; Teerapakpinyo, C.; Mutirangura, A.; Doungkamchan, C. Identification of Shared Neoantigens in BRCA1-Related Breast Cancer. Vaccines 2022, 10, 1597. [Google Scholar] [CrossRef]
- De, P.; Sun, Y.; Carlson, J.H.; Friedman, L.S.; Leyland-Jones, B.R.; Dey, N. Doubling down on the PI3K-AKT-mTOR pathway enhances the antitumor efficacy of PARP inhibitor in triple negative breast cancer model beyond BRCA-ness. Neoplasia 2014, 16, 43–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, C.; Zhang, Y.; Wang, M.; Jiang, N.; Xiang, L.; Li, T.; Roberts, T.M.; Zhao, J.J.; Cheng, H.; et al. Combined inhibition of PI3K and PARP is effective in the treatment of ovarian cancer cells with wild-type PIK3CA genes. Gynecol. Oncol. 2016, 142, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, M.; Jiang, N.; Zhang, Y.; Bian, X.; Wang, X.; Roberts, T.M.; Zhao, J.J.; Liu, P.; Cheng, H. Effective use of PI3K inhibitor BKM120 and PARP inhibitor Olaparib to treat PIK3CA mutant ovarian cancer. Oncotarget 2016, 7, 13153–13166. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.Y.; Guner, G.; Guven, D.C.; Arslan, C.; Dizdar, O. Exploiting DNA repair defects in breast cancer: From chemotherapy to immunotherapy. Expert Rev. Anticancer. Ther. 2019, 19, 589–601. [Google Scholar] [CrossRef]
- Chabanon, R.M.; Rouanne, M.; Lord, C.J.; Soria, J.C.; Pasero, P.; Postel-Vinay, S. Targeting the DNA damage response in immuno-oncology: Developments and opportunities. Nat. Rev. Cancer 2021, 21, 701–717. [Google Scholar] [CrossRef]
- Nolan, E.; Savas, P.; Policheni, A.N.; Darcy, P.K.; Vaillant, F.; Mintoff, C.P.; Dushyanthen, S.; Mansour, M.; Pang, J.B.; Fox, S.B.; et al. Combined immune checkpoint blockade as a therapeutic strategy for BRCA1-mutated breast cancer. Sci. Transl. Med. 2017, 9, eaal4922. [Google Scholar] [CrossRef]
- van Wilpe, S.; Tolmeijer, S.H.; Koornstra, R.H.T.; de Vries, I.J.M.; Gerritsen, W.R.; Ligtenberg, M.; Mehra, N. Homologous Recombination Repair Deficiency and Implications for Tumor Immunogenicity. Cancers 2021, 13, 2249. [Google Scholar] [CrossRef]
- Solinas, C.; Marcoux, D.; Garaud, S.; Vitória, J.R.; Van den Eynden, G.; de Wind, A.; De Silva, P.; Boisson, A.; Craciun, L.; Larsimont, D.; et al. BRCA gene mutations do not shape the extent and organization of tumor infiltrating lymphocytes in triple negative breast cancer. Cancer Lett. 2019, 450, 88–97. [Google Scholar] [CrossRef]
- Grandal, B.; Evrevin, C.; Laas, E.; Jardin, I.; Rozette, S.; Laot, L.; Dumas, E.; Coussy, F.; Pierga, J.Y.; Brain, E.; et al. Impact of BRCA Mutation Status on Tumor Infiltrating Lymphocytes (TILs), Response to Treatment, and Prognosis in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy. Cancers 2020, 12, 3681. [Google Scholar] [CrossRef]
- Sønderstrup, I.M.H.; Jensen, M.B.; Ejlertsen, B.; Eriksen, J.O.; Gerdes, A.M.; Kruse, T.A.; Larsen, M.J.; Thomassen, M.; Laenkholm, A.V. Evaluation of tumor-infiltrating lymphocytes and association with prognosis in BRCA-mutated breast cancer. Acta Oncol. 2019, 58, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.; Hviid, T.V.F.; Nielsen, L.B.; Sønderstrup, I.M.H.; Eriksen, J.O.; Ejlertsen, B.; Gerdes, A.M.; Kruse, T.A.; Thomassen, M.; Jensen, M.B.; et al. Tumour-infiltrating CD4-, CD8- and FOXP3-positive immune cells as predictive markers of mortality in BRCA1- and BRCA2-associated breast cancer. Br. J. Cancer 2021, 125, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.R.; Provenzano, E.; Dawson, S.J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Bottai, G.; Raschioni, C.; Losurdo, A.; Di Tommaso, L.; Tinterri, C.; Torrisi, R.; Reis-Filho, J.S.; Roncalli, M.; Sotiriou, C.; Santoro, A.; et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res. 2016, 18, 121. [Google Scholar] [CrossRef]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Angarita, F.A.; Yan, L.; Matsuyama, R.; Zsiros, E.; Ishikawa, T.; Endo, I.; Takabe, K. Abundance of Regulatory T Cell (Treg) as a Predictive Biomarker for Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 3038. [Google Scholar] [CrossRef]
- Kos, K.; de Visser, K.E. The Multifaceted Role of Regulatory T Cells in Breast Cancer. Annu. Rev. Cancer Biol. 2021, 5, 291–310. [Google Scholar] [CrossRef]
- Sobral-Leite, M.; Van de Vijver, K.; Michaut, M.; van der Linden, R.; Hooijer, G.K.J.; Horlings, H.M.; Severson, T.M.; Mulligan, A.M.; Weerasooriya, N.; Sanders, J.; et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018, 7, e1509820. [Google Scholar] [CrossRef]
- Jenzer, M.; Keß, P.; Nientiedt, C.; Endris, V.; Kippenberger, M.; Leichsenring, J.; Stögbauer, F.; Haimes, J.; Mishkin, S.; Kudlow, B.; et al. The BRCA2 mutation status shapes the immune phenotype of prostate cancer. Cancer Immunol. Immunother. 2019, 68, 1621–1633. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef]
- Sheppard, K.A.; Fitz, L.J.; Lee, J.M.; Benander, C.; George, J.A.; Wooters, J.; Qiu, Y.; Jussif, J.M.; Carter, L.L.; Wood, C.R.; et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004, 574, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yuan, P.; Wu, H.; Chen, J.; Fu, J.; Li, P.; Lu, J.; Wei, W. Dendritic cells with an increased PD-L1 by TGF-β induce T cell anergy for the cytotoxicity of hepatocellular carcinoma cells. Int. Immunopharmacol. 2014, 20, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, H.; Zhao, S.; Wang, Y.; Pu, H.; Wang, Y.; Zhang, Q. Expression of PD-L1 and prognosis in breast cancer: A meta-analysis. Oncotarget 2017, 8, 31347–31354. [Google Scholar] [CrossRef] [PubMed]
- Rozenblit, M.; Huang, R.; Danziger, N.; Hegde, P.; Alexander, B.; Ramkissoon, S.; Blenman, K.; Ross, J.S.; Rimm, D.L.; Pusztai, L. Comparison of PD-L1 protein expression between primary tumors and metastatic lesions in triple negative breast cancers. J. Immunother. Cancer 2020, 8, e001558. [Google Scholar] [CrossRef]
- Pang, J.B.; Castles, B.; Byrne, D.J.; Button, P.; Hendry, S.; Lakhani, S.R.; Sivasubramaniam, V.; Cooper, W.A.; Armes, J.; Millar, E.K.A.; et al. SP142 PD-L1 Scoring Shows High Interobserver and Intraobserver Agreement in Triple-negative Breast Carcinoma But Overall Low Percentage Agreement with Other PD-L1 Clones SP263 and 22C3. Am. J. Surg. Pathol. 2021, 45, 1108–1117. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Roche., N.r. Roche Provides Update on Tecentriq US Indication for PD-L1-Positive, Metastatic Triple-Negative Breast Cancer. 27 August 2021. Available online: https://www.globenewswire.com/news-release/2021/08/27/2287799/0/en/Roche-provides-update-on-Tecentriq-US-indication-for-PD-L1-positive-metastatic-triple-negative-breast-cancer.html (accessed on 6 February 2023).
- Franzoi, M.A.; de Azambuja, E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials - how to explain different results? ESMO Open 2020, 5, e001112. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Emens, L.A.; Molinero, L.; Loi, S.; Rugo, H.S.; Schneeweiss, A.; Diéras, V.; Iwata, H.; Barrios, C.H.; Nechaeva, M.; Nguyen-Duc, A.; et al. Atezolizumab and nab-Paclitaxel in Advanced Triple-Negative Breast Cancer: Biomarker Evaluation of the IMpassion130 Study. J. Natl. Cancer Inst. 2021, 113, 1005–1016. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Pantelidou, C.; Sonzogni, O.; De Oliveria Taveira, M.; Mehta, A.K.; Kothari, A.; Wang, D.; Visal, T.; Li, M.K.; Pinto, J.; Castrillon, J.A.; et al. PARP Inhibitor Efficacy Depends on CD8(+) T-cell Recruitment via Intratumoral STING Pathway Activation in BRCA-Deficient Models of Triple-Negative Breast Cancer. Cancer Discov. 2019, 9, 722–737. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Zhang, J.; Meng, Q.; Ma, L. Correlation of TBK1, AR, and other serum cancer-related biomarkers in breast cancer patients: An observational study. Medicine 2022, 101, e29996. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef]
- Mulligan, A.M.; Raitman, I.; Feeley, L.; Pinnaduwage, D.; Nguyen, L.T.; O’Malley, F.P.; Ohashi, P.S.; Andrulis, I.L. Tumoral lymphocytic infiltration and expression of the chemokine CXCL10 in breast cancers from the Ontario Familial Breast Cancer Registry. Clin. Cancer Res. 2013, 19, 336–346. [Google Scholar] [CrossRef]
- Isaac, D.; Karapetyan, L.; Tamkus, D. Association of Germline PALB2 Mutation and Response to Platinum-Based Chemotherapy in Metastatic Breast Cancer: A Case Series. JCO Precis. Oncol. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020, 10, 570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).