Micro-Ultrasound: Current Role in Prostate Cancer Diagnosis and Future Possibilities
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
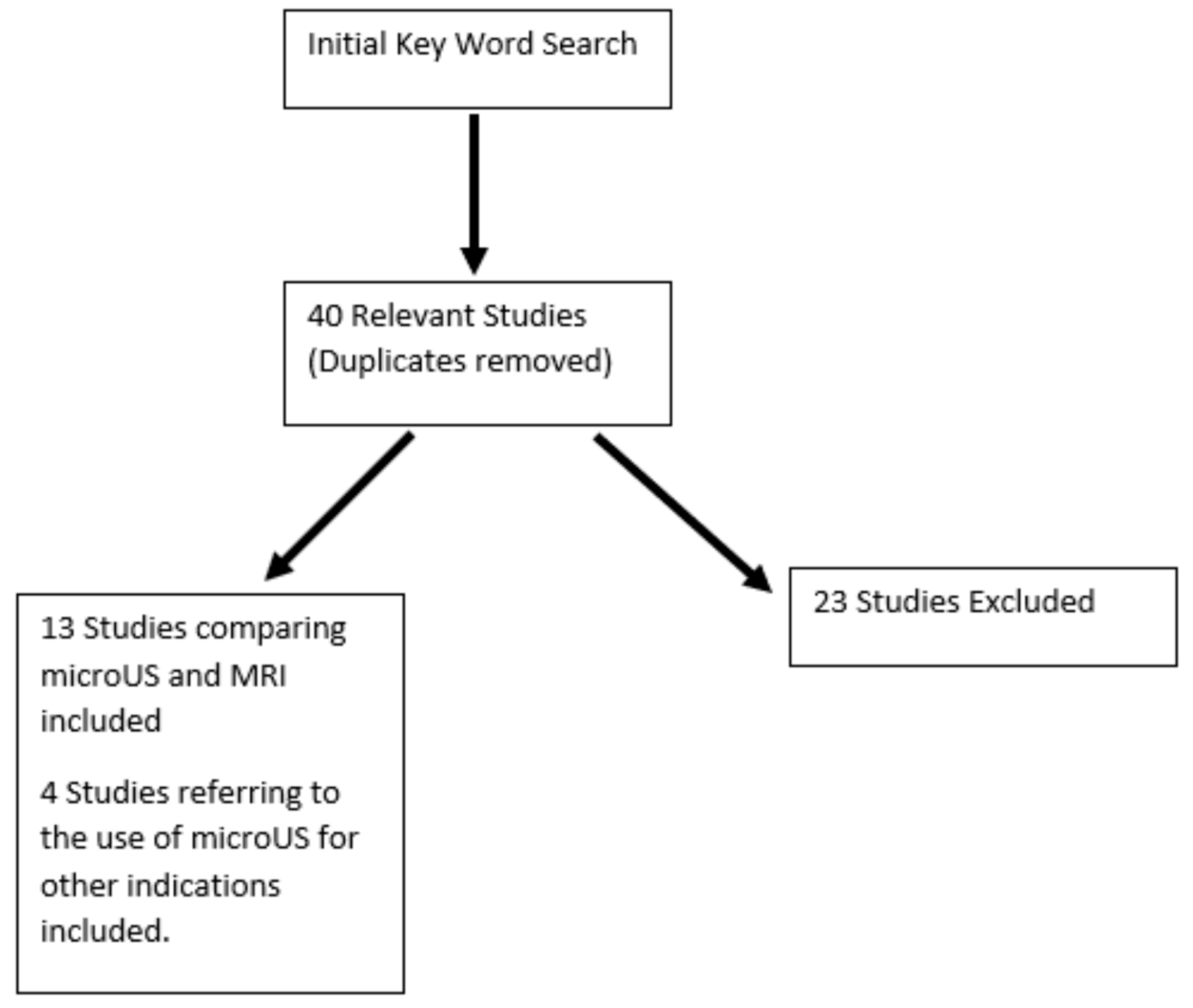
2.3. Search Results and Quality Assessment
3. Results and Discussion
3.1. QUADAS-2 Results
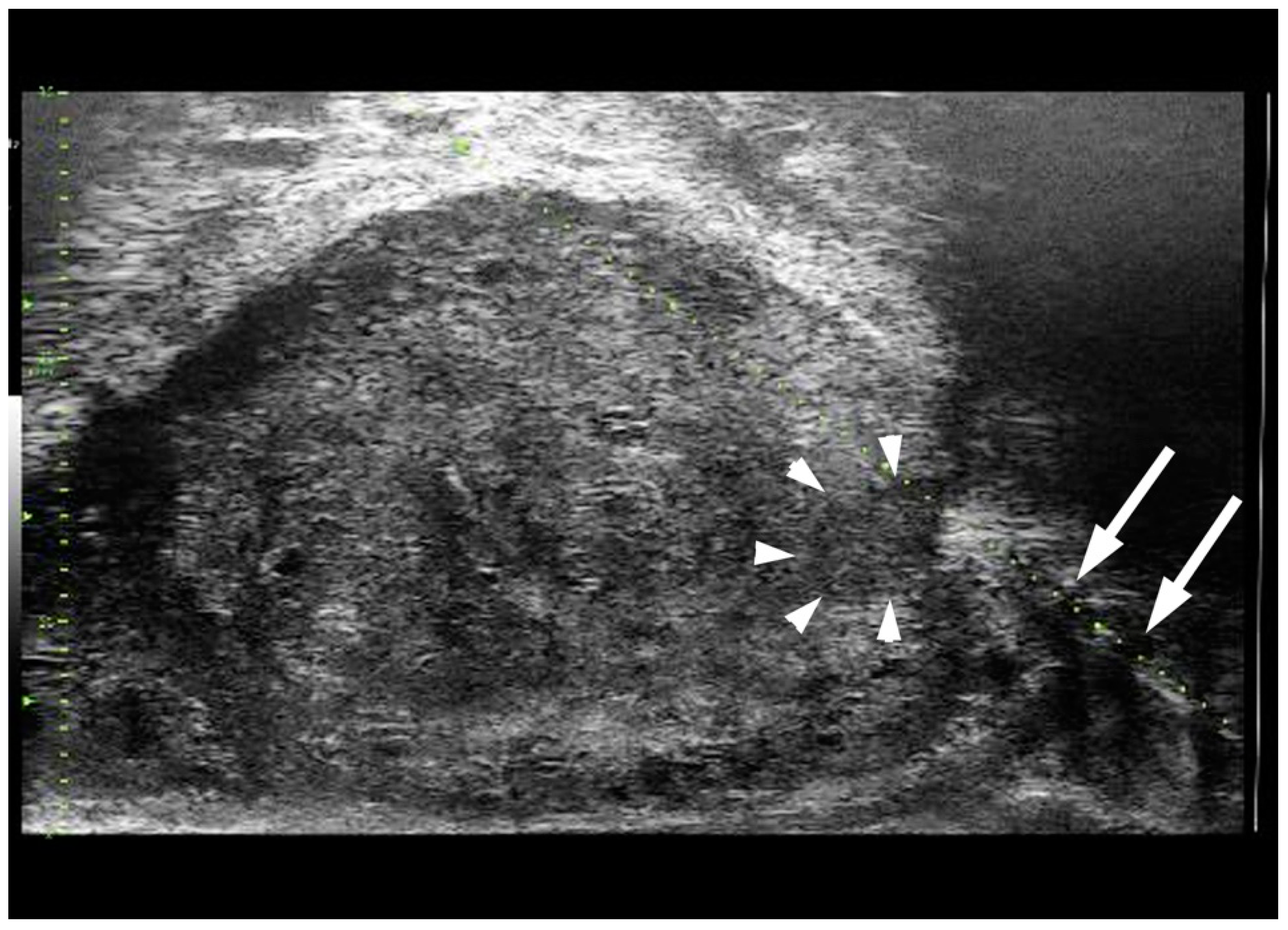
3.2. The Diagnostic Accuracy of MicroUS
3.3. mpMRI/microUS Fusion
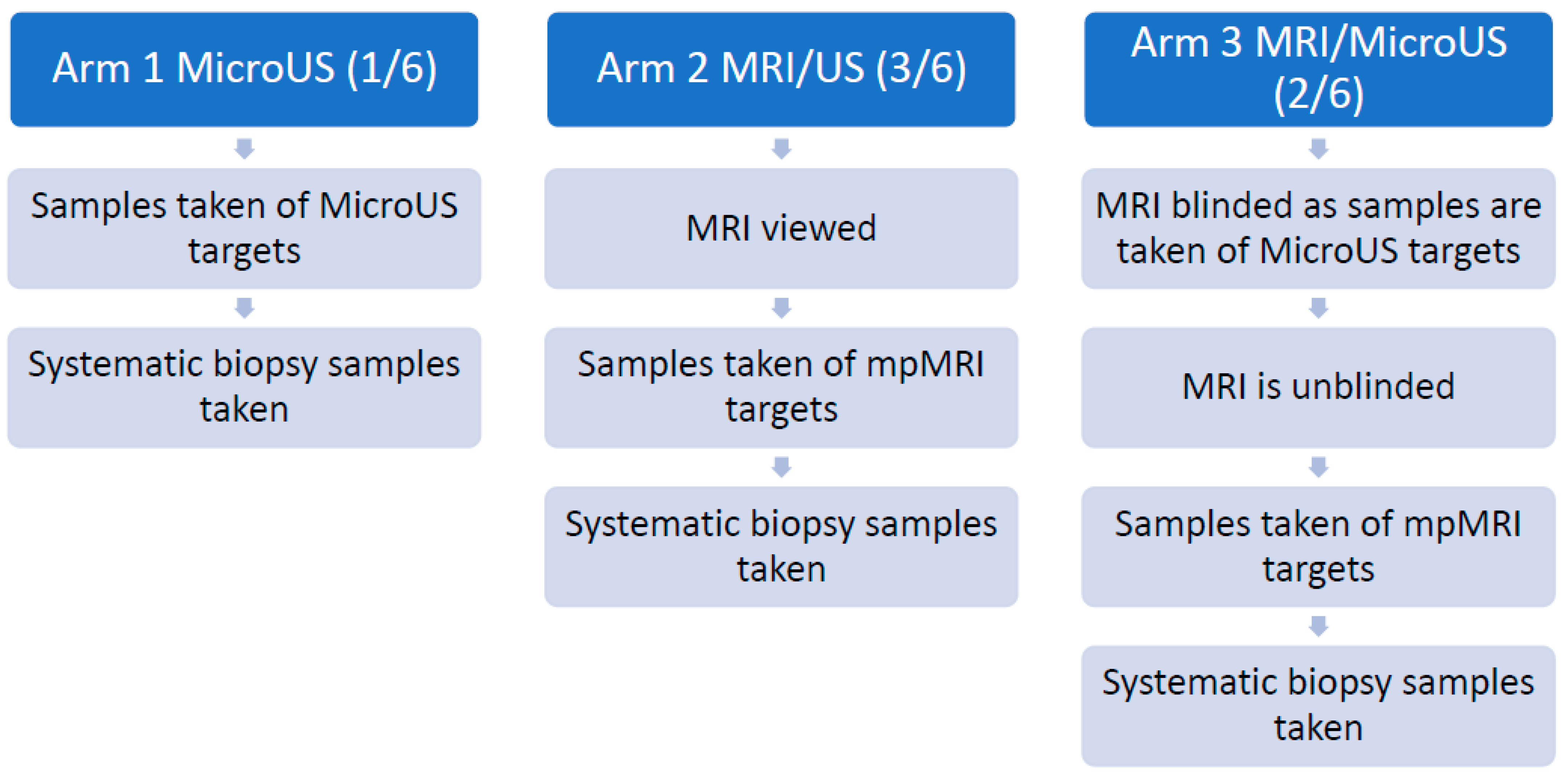
3.4. The OPTIMUM Trial
3.5. Other Indications of MicroUS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund International. Worldwide Cancer Data. 2022. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 2 November 2022).
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer Early Detection V.1.2022. © National Comprehensive Cancer Network, Inc., 2022. Available online: https://www.NCCN.org (accessed on 2 November 2022).
- van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naïve Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [PubMed]
- Rouvière, O.; Puech, P.; Renard-Penna, R.; Claudon, M.; Roy, C.; Mège-Lechevallier, F.; Decaussin-Petrucci, M.; Dubreuil-Chambardel, M.; Magaud, L.; Remontet, L.; et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): A prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019, 20, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Ahdoot, M.; Wilbur, A.R.; Reese, S.E.; Lebastchi, A.H.; Mehralivand, S.; Gomella, P.T.; Bloom, J.; Gurram, S.; Siddiqui, M.; Pinsky, P.; et al. MRI-Targeted, Systematic, and Combined Biopsy for Prostate Cancer Diagnosis. N. Engl. J. Med. 2020, 382, 917–928. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam, 2022. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 2 November 2022).
- Ploussard, G.; Roubaud, G.; Barret, E.; Beauval, J.B.; Brureau, L.; Créhange, G.; Dariane, C.; Fiard, G.; Fromont, G.; Gauthé, M.; et al. French AFU Cancer Committee Guidelines—Update 2022-2024: Prostate cancer—Diagnosis and management of localised disease. Progrès Urol. 2022, 32, 1275–1372. [Google Scholar] [CrossRef]
- Klotz, L.; Lughezzani, G.; Maffei, D.; Sanchez, A.; Pereira, J.G.; Staerman, F.; Cash, H.; Luger, F.; Lopez, L.; Sanchez-Salas, R.; et al. Comparison of micro-ultrasound and multiparametric magnetic resonance imaging for prostate cancer: A multicenter, prospective analysis. Can. Urol. Assoc. J. 2021, 15, E11–E16. [Google Scholar] [CrossRef]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; Hurle, R.; Buffi, N.M.; Guazzoni, G.; Casale, P. Comparison of the Diagnostic Accuracy of Micro-ultrasound and Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies for the Diagnosis of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef]
- Cornud, F.; Lefevre, A.; Flam, T.; Dumonceau, O.; Galiano, M.; Soyer, P.; Camparo, P.; Barral, M. MRI-directed high-frequency (29MhZ) TRUS-guided biopsies: Initial results of a single-center study. Eur. Radiol. 2020, 30, 4838–4846. [Google Scholar] [CrossRef]
- Dias, A.B.; O’Brien, C.; Correas, J.M.; Ghai, S. Multiparametric ultrasound and micro-ultrasound in prostate cancer: A comprehensive review. Br. J. Radiol. 2022, 95, 20210633. [Google Scholar] [CrossRef]
- Ghai, S.; Perlis, N.; Atallah, C.; Jokhu, S.; Corr, K.; Lajkosz, K.; Incze, P.F.; Zlotta, A.R.; Jain, U.; Fleming, H.; et al. Comparison of Micro-US and Multiparametric MRI for Prostate Cancer Detection in Biopsy-Naive Men. Radiology 2022, 305. [Google Scholar] [CrossRef]
- Ghai, S.; Eure, G.; Fradet, V.; Hyndman, M.E.; McGrath, T.; Wodlinger, B.; Pavlovich, C.P. Assessing Cancer Risk on Novel 29 MHz Micro-Ultrasound Images of the Prostate: Creation of the Micro-Ultrasound Protocol for Prostate Risk Identification. J. Urol. 2016, 196, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Dariane, C.; Ploussard, G.; Barret, E.; Beauval, J.-B.; Brureau, L.; Créhange, G.; Fromont, G.; Gauthé, M.; Mathieu, R.; Renard-Penna, R.; et al. Micro-ultrasound-guided biopsies versus systematic biopsies in the detection of prostate cancer: A systematic review and meta-analysis. World J. Urol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, R.; Wu, Y.; Jing, J.; Chen, S.; Zhang, G.; Xu, B.; Liu, C.; Chen, M. Micro-Ultrasound Imaging for Accuracy of Diagnosis in Clinically Significant Prostate Cancer: A Meta-Analysis. Front. Oncol. 2019, 9, 1368. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Eure, G.; Fanney, D.; Lin, J.; Wodlinger, B.; Ghai, S. Comparison of conventional transrectal ultrasound, magnetic resonance imaging, and micro-ultrasound for visualizing prostate cancer in an active surveillance population: A feasibility study. Can. Urol. Assoc. J. 2019, 13, E70–E77. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Maffei, D.; Saita, A.; Paciotti, M.; Diana, P.; Buffi, N.M.; Colombo, P.; Elefante, G.M.; Hurle, R.; Lazzeri, M.; et al. Diagnostic Accuracy of Microultrasound in Patients with a Suspicion of Prostate Cancer at Magnetic Resonance Imaging: A Single-institutional Prospective Study. Eur. Urol. Focus 2021, 7, 1019–1026. [Google Scholar] [CrossRef]
- Socarrás, M.E.R.; Rivas, J.G.; Rivera, V.C.; Elbers, J.R.; González, L.L.; Mercado, I.M.; del Alamo, J.F.; del Dago, P.J.; Sancha, F.G. Prostate Mapping for Cancer Diagnosis: The Madrid Protocol. Transperineal Prostate Biopsies Using Multiparametric Magnetic Resonance Imaging Fusion and Micro-Ultrasound Guided Biopsies. J. Urol. 2020, 204, 726–732. [Google Scholar] [CrossRef]
- Avolio, P.P.; Lughezzani, G.; Paciotti, M.; Maffei, D.; Uleri, A.; Frego, N.; Hurle, R.; Lazzeri, M.; Saita, A.; Guazzoni, G.; et al. The use of 29 MHz transrectal micro-ultrasound to stratify the prostate cancer risk in patients with PI-RADS III lesions at multiparametric MRI: A single institutional analysis. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 832.e1–832.e7. [Google Scholar]
- Wiemer, L.; Hollenbach, M.; Heckmann, R.; Kittner, B.; Plage, H.; Reimann, M.; Asbach, P.; Friedersdorff, F.; Schlomm, T.; Hofbauer, S.; et al. Evolution of Targeted Prostate Biopsy by Adding Micro-Ultrasound to the Magnetic Resonance Imaging Pathway. Eur. Urol. Focus 2021, 7, 1292–1299. [Google Scholar] [CrossRef]
- Hofbauer, S.L.; Luger, F.; Harland, N.; Plage, H.; Reimann, M.; Hollenbach, M.; Gusenleitner, A.; Stenzl, A.; Schlomm, T.; Wiemer, L.; et al. A non-inferiority comparative analysis of micro-ultrasonography and MRI-targeted biopsy in men at risk of prostate cancer. BJU Int. 2022, 129, 648–654. [Google Scholar] [CrossRef]
- Sountoulides, P.; Pyrgidis, N.; Polyzos, S.A.; Mykoniatis, I.; Asouhidou, E.; Papatsoris, A.; Dellis, A.; Anastasiadis, A.; Lusuardi, L.; Hatzichristou, D. Micro-Ultrasound-Guided vs. Multiparametric Magnetic Resonance Imaging-Targeted Biopsy in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. J. Urol. 2021, 205, 1254–1262. [Google Scholar] [CrossRef]
- You, C.; Li, X.; Du, Y.; Peng, L.; Wang, H.; Zhang, X.; Wang, A. The Microultrasound-Guided Prostate Biopsy in Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. J. Endourol. 2022, 36, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ukimura, O.; Marien, A.; Palmer, S.; Villers, A.; Aron, M.; Abreu, A.L.D.C.; Leslie, S.; Shoji, S.; Matsugasumi, T.; Gross, M.; et al. Trans-rectal ultrasound visibility of prostate lesions identified by magnetic resonance imaging increases accuracy of image-fusion targeted biopsies. World J. Urol. 2015, 33, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Claros, O.R.; Tourinho-Barbosa, R.R.; Fregeville, A.; Gallardo, A.C.; Muttin, F.; Carneiro, A.; Stabile, A.; Moschini, M.; Macek, P.; Cathala, N.; et al. Comparison of Initial Experience with Transrectal Magnetic Resonance Imaging Cognitive Guided Micro-Ultrasound Biopsies versus Established Transperineal Robotic Ultrasound Magnetic Resonance Imaging Fusion Biopsies for Prostate Cancer. J. Urol. 2020, 203, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Scialpi, M.; D’Andrea, A.; Martorana, E.; Malaspina, C.M.; Aisa, M.C.; Napoletano, M.; Orlandi, E.; Rondoni, V.; Scialpi, P.; Pacchiarini, D.; et al. Biparametric MRI of the prostate. Turk J. Urol. 2017, 43, 401–409. [Google Scholar] [CrossRef]
- Howick, J.; Chalmers, I.; Glasziou, P.; Greenhalgh, T.; Heneghan, C.; Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H. The Oxford Levels of Evidence 2; Centre for Evidence-Based Medicine: Oxford, UK, 2011. [Google Scholar]
- Klotz, L.; Andriole, G.; Cash, H.; Cooperberg, M.; Crawford, E.D.; Emberton, M.; Gomez-Sancha, F.; Klein, E.; Lughezzani, G.; Marks, L.; et al. Optimization of prostate biopsy—Micro-Ultrasound versus MRI (OPTIMUM): A 3-arm randomized controlled trial evaluating the role of 29 MHz micro-ultrasound in guiding prostate biopsy in men with clinical suspicion of prostate cancer. Contemp. Clin. Trials 2022, 112, 106618. [Google Scholar] [CrossRef]
- Regis, F.; Casale, P.; Persico, F.; Colombo, P.; Cieri, M.; Guazzoni, G.; Buffi, N.M.; Lughezzani, G. Use of 29-MHz Micro-ultrasound for Local Staging of Prostate Cancer in Patients Scheduled for Radical Prostatectomy: A Feasibility Study. Eur. Urol. Open Sci. 2020, 19, 20–23. [Google Scholar] [CrossRef]
- de Rooij, M.; Hamoen, E.H.J.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef]
- Fasulo, V.; Buffi, N.M.; Regis, F.; Paciotti, M.; Persico, F.; Maffei, D.; Uleri, A.; Saita, A.; Casale, P.; Hurle, R.; et al. Use of high-resolution micro-ultrasound to predict extraprostatic extension of prostate cancer prior to surgery: A prospective single-institutional study. World J. Urol. 2022, 40, 435–442. [Google Scholar] [CrossRef]
- Albers, P.; Wang, B.; Broomfield, S.; Medina Martín, A.; Fung, C.; Kinnaird, A. Micro-ultrasound Versus Magnetic Resonance Imaging in Prostate Cancer Active Surveillance. Eur. Urol. Open Sci. 2022, 46, 33–35. [Google Scholar] [CrossRef]
- Saita, A.; Lughezzani, G.; Buffi, N.M.; Hurle, R.; Nava, L.; Colombo, P.; Diana, P.; Fasulo, V.; Paciotti, M.; Elefante, G.M.; et al. Assessing the Feasibility and Accuracy of High-resolution Microultrasound Imaging for Bladder Cancer Detection and Staging. Eur. Urol. 2020, 77, 727–732. [Google Scholar] [CrossRef]

| First Author and Reference | Year | # of Patients | Institutions | Reference Standard | Standard Biopsy Performed? | MRI Blinded or Unblinded? | MRI Parameters | MicroUS Operators | mpMRI Readers | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Eure [18] | 2018 | 9 | 1 | MRI/US fusion targeted biopsy | Yes | Blinded | 3.0 Tesla Toshiba TitanTM no endo-rectal coil (ERC). Three-plane T2 imaging, diffusion-weighted imaging (b-value 2000) and dynamic contrast-enhanced sequences were used according to PI-RADS v2. | 1 | 1 | mpMRI found 7 lesions graded GG of 7 or above while microUS found 8. |
| Lughezzani [10] | 2019 | 104 | 1 | MRI/US fusion targeted biopsy | Yes | Blinded | Not reported | 1 | Not reported | MicroUS Sensitivity 94% Specificity 28% |
| Socarras [20] | 2020 | 194 | 1 | MRI/US fusion targeted biopsy (relative) | Yes | Blinded | Not reported | 6 | Not reported | MRI Sensitivity 84.3% Specificity 18.8% MicroUS Sensitivity 99.7% Specificity 23.1% |
| Lughezzani [19] | 2021 | 320 | 1 | MRI/US fusion targeted biopsy (relative) | Yes | Blinded | Either a 1.5 T scanner with an ERC or with a 3.0-T scanner. | 2 | Not reported | MicroUS Sensitivity 89.7% Specificity 26.0% |
| Klotz [9] | 2021 | 1040 | 11 | MRI/US fusion targeted biopsy (relative) | Yes | 9 sites unblinded 2 sites blinded | Site A: b-value ≥ 1400 no ERC Site B: 3T Toshiba Titan no ERC b-value = 2000 Site C: 1.5T and 3T Site D: 3T Siemens Skyra no ERC Site E: 3T with pelvic phased array coil no ERC Site F: 1.5T and 3T Site G: Siemens and Phillips 3T, no ERC Site H: 1.5T and 3T Site I: 3T no ERC Site J: 1.5T and 3T Site K: 1.5T and 3T | Multiple, specific number of operators not reported | Multiple, specific number of readers not reported | MicroUS provides non-inferior Specificity and PPV and superior Sensitivity and NPV to mpMRI |
| Avolio [21] | 2021 | 111 | 1 | MRI/US fusion targeted biopsy (relative) | Yes | Blinded | 1.5T scanner with an ERC or with a 3.0T scanner | 2 | Not reported | For MicroUS in PI- RADS 3 cases Sensitivity 100% Specificity 27.2% |
| Wiemer [22] | 2021 | 159 | 1 | MRI/US fusion targeted biopsy (relative) | Yes | Blinded | 3.0T scanner with a pelvic phased-array coil without an ERC. High-spatial- resolution T2-weighted turbo spin-echo sequences in transverse and coronal orientation and transverse diffusion-weighted images (measured b values 0, 500 and 1000 s/mm2, calculated b value 1400 s/mm2) and when indicated based on the PI-RADS category of dominant sequence, a dynamic contrast-enhanced sequence. | 3 | Not reported | In 17% (27/159) of patients negative on mpMRI targeted biopsy were positive on microUS targeted biopsy, 7 cases were clinically insignificant prostate cancer while 20 patients had csPCa. |
| Hofbauer [23] | 2022 | 203 | 3 | MRI/US fusion targeted biopsy | Yes | Blinded | 3.0T scanner with a pelvic phased-array surface coil without an ERC. T2-weighted-, diffusion-weighted-and dynamic contrast-enhanced sequences were acquired according to a PI-RADS-complaint protocol. | 7 | Not reported | MicroUS non-inferior to mpMRI for csPCa detection |
| Ghai [13] | 2022 | 94 | 1 | MRI/US fusion targeted biopsy (relative) | Yes | Blinded | 3.0T Siemens Magnetom Skyra Fit magnet without an ERC. Sequences: T2- weighted imaging in all three planes, diffusion-weighted imaging (b values of 50, 900, 1600 s/mm2, with generation of apparent diffusion coefficient maps) and dynamic contrast-enhanced imaging (injection rate, mL/s). | 1 | 1 | csPCa (GG ≥ 2) rate comparable between microUS and mpMRI (35% vs. 39%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso Dias, A.; Ghai, S. Micro-Ultrasound: Current Role in Prostate Cancer Diagnosis and Future Possibilities. Cancers 2023, 15, 1280. https://doi.org/10.3390/cancers15041280
Basso Dias A, Ghai S. Micro-Ultrasound: Current Role in Prostate Cancer Diagnosis and Future Possibilities. Cancers. 2023; 15(4):1280. https://doi.org/10.3390/cancers15041280
Chicago/Turabian StyleBasso Dias, Adriano, and Sangeet Ghai. 2023. "Micro-Ultrasound: Current Role in Prostate Cancer Diagnosis and Future Possibilities" Cancers 15, no. 4: 1280. https://doi.org/10.3390/cancers15041280
APA StyleBasso Dias, A., & Ghai, S. (2023). Micro-Ultrasound: Current Role in Prostate Cancer Diagnosis and Future Possibilities. Cancers, 15(4), 1280. https://doi.org/10.3390/cancers15041280






