Temporal Evolution and Prognostic Role of Indeterminate Response Sub-Groups in Patients with Differentiated Thyroid Cancer after Initial Therapy with Radioiodine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Follow-Up after Initial Therapy
2.2. Statistical Analysis
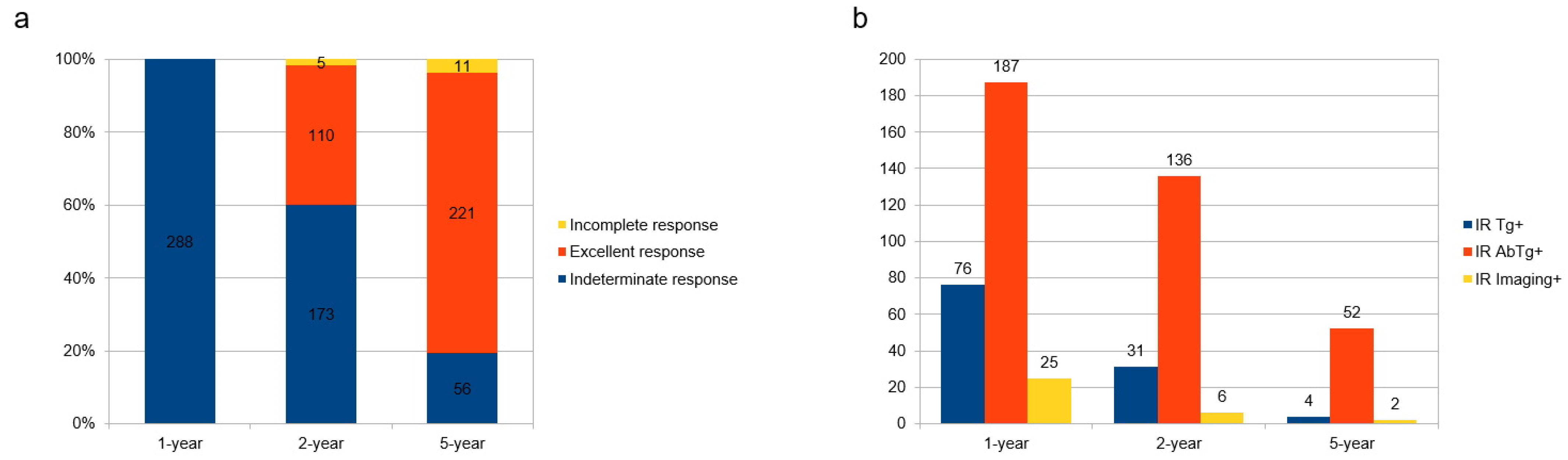
3. Results
3.1. Natural Course of IR Disease
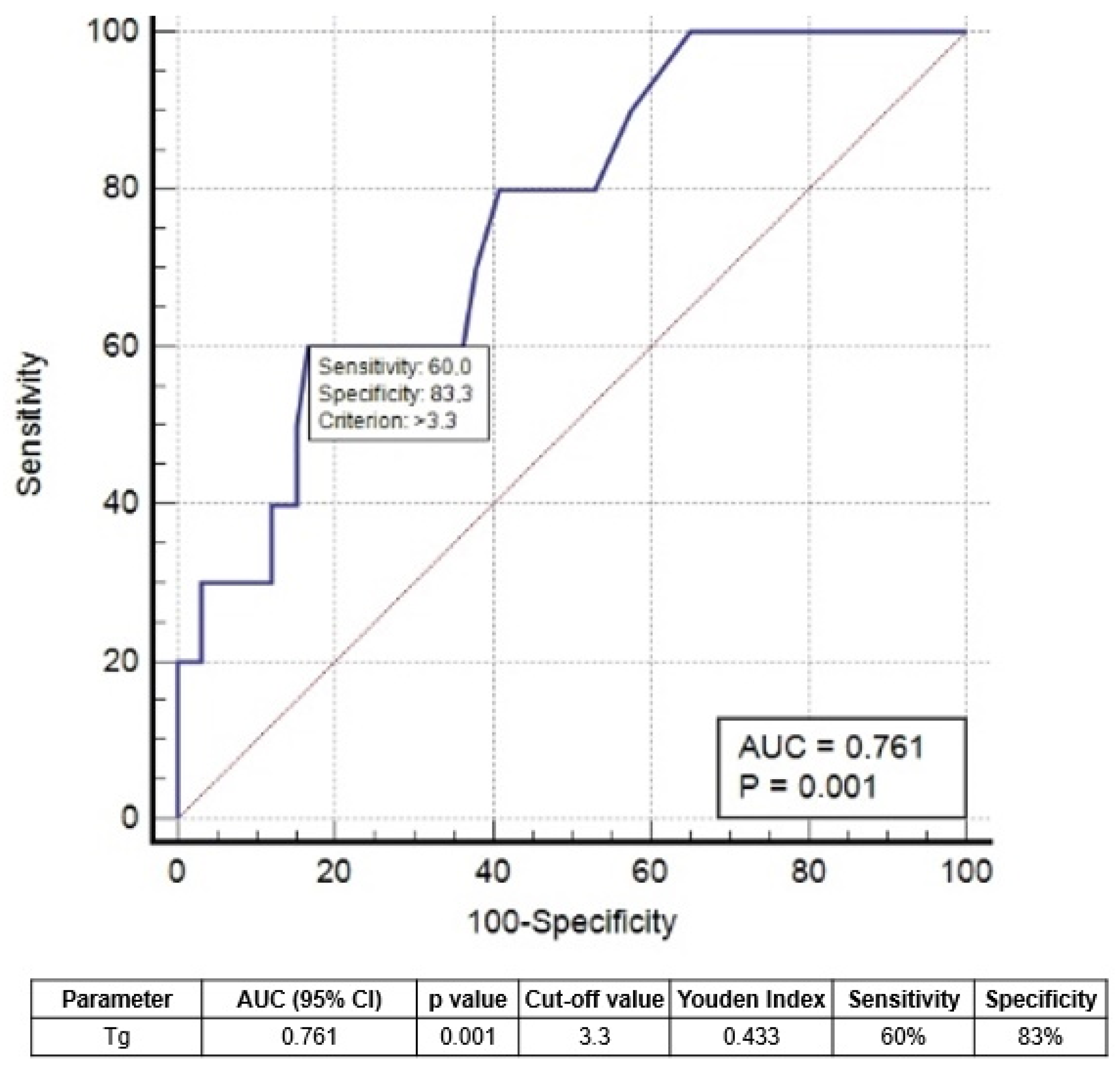
3.2. Features Associated with Excellent Response 2 and 5 Years after Therapy
3.3. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avram, A.M.; Giovanella, L.; Greenspan, B.; Lawson, S.A.; Luster, M.; Van Nostrand, D.; Peacock, J.G.; Ovčariček, P.P.; Silberstein, E.; Tulchinsky, M.; et al. SNMMI Procedure Standard/EANM Practice Guideline for Nuclear Medicine Evaluation and Therapy of Differentiated Thyroid Cancer: Abbreviated Version. J. Nucl. Med. 2022, 63, 15N–35N. [Google Scholar] [PubMed]
- Albano, D.; Bertagna, F.; Bonacina, M.; Durmo, R.; Cerudelli, E.; Gazzilli, M.; Panarotto, M.B.; Formenti, A.M.; Mazziotti, G.; Giustina, A.; et al. Possible delayed diagnosis and treatment of metastatic differentiated thyroid cancer by adopting the 2015 ATA guidelines. Eur. J. Endocrinol. 2018, 179, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: Using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 2010, 20, 1341–1349. [Google Scholar] [PubMed]
- Vaisman, F.; Momesso, D.; Bulzico, D.A.; Pessoa, C.H.; Dias, F.; Corbo, R.; Vaisman, M.; Tuttle, R.M. Spontaneous remission in thyroid cancer patients after biochemical incomplete response to initial therapy. Clin. Endocrinol. 2012, 77, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Bonacina, M.; Durmo, R.; Bertagna, F.; Giubbini, R. Efficacy of low radioiodine activity versus intermediate-high activity in the ablation of low-risk differentiated thyroid cancer. Endocrine 2020, 68, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Tuttle, R.M. Risk-adapted management of thyroid cancer. Endocr. Pract. 2008, 4, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, R.M.; Leboeuf, R. Follow up approaches in thyroid cancer: A risk adapted paradigm. Endocrinol. Metab. Clin. N. Am. 2008, 7, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Furtado, M.D.S.; Mourão, G.F.; Calsolari, M.R. Patients with Papillary Thyroid Carcinoma at Intermediate Risk of Recurrence According to American Thyroid Association Criteria Can Be Reclassified as Low Risk When the Postoperative Thyroglobulin Is Low. Thyroid 2015, 25, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Landenberger, G.M.C.; de Souza Salerno, M.L.; Golbert, L.; de Souza Meyer, E.L. Thyroglobulin Antibodies as a Prognostic Factor in Papillary Thyroid Carcinoma Patients with Indeterminate Response After Initial Therapy. Horm. Metab. Res. 2021, 53, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, N.; Aljamei, H.; Aljomaiah, A.; Moria, Y.; Alzahrani, A.S. Natural Course of the American Thyroid Association Response to Therapy Statuses (Dynamic Risk Stratification) in Differentiated Thyroid Cancer. Eur. Thyroid J. 2021, 10, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Ahn, J.H.; Song, E.; Han, J.M.; Kim, W.G.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jeon, M.J. Individualized Follow-Up Strategy for Patients with an Indeterminate Response to Initial Therapy for Papillary Thyroid Carcinoma. Thyroid 2019, 29, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, P.; Tumino, D.; Russo, M.; Marescalco, S.; Fulco, R.A.; Frasca, F. Surveillance of patients with differentiated thyroid cancer and indeterminate response: A longitudinal study on basal thyroglobulin trend. J. Endocrinol. Investig. 2019, 42, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Yoon, J.K.; Lee, S.J.; Soh, E.Y.; Lee, J.; An, Y.S. Risk Factors for Indeterminate Response After Radioactive Iodine Therapy in Patients With Differentiated Thyroid Cancer. Clin. Nucl. Med. 2019, 44, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mourão, G.F.; Calsolari, M.R. Definition of the Response to Initial Therapy with Radioiodine in Patients with Differentiated Thyroid Carcinoma: Basal or Stimulated Thyroglobulin? Horm. Metab. Res. 2019, 51, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Rosario, P.W.; Mourão, G.F. Natural history, predictive factors of apparent disease (structural or biochemical) and spontaneous excellent response in patients with papillary thyroid carcinoma and indeterminate response to initial therapy with radioiodine. Endocrine 2022, 76, 671–676. [Google Scholar] [CrossRef] [PubMed]


| Mean ± SD (Range) | Patients, n (%) | |
|---|---|---|
| Age | 49 ± 15 (18–84) | |
| Gender | ||
| Female | 235 (82%) | |
| Male | 53 (18%) | |
| Histotype | ||
| Papillary | 118 (41%) | |
| Follicular variant of Papillary | 92 (32%) | |
| Follicular | 53 (18%) | |
| Aggressive variant | 25 (9%) | |
| Tumor size, mm | 16 ± 13 (1–130) | |
| Multicentricity | 168 (58%) | |
| Central lymphadenectomy | 100 (35%) | |
| Lateral lymphadecentomy | 59 (20%) | |
| TNM | ||
| Tx | 2 (1%) | |
| T1b | 214 (74%) | |
| T2 | 50 (17%) | |
| T3 | 22 (8%) | |
| N0 | 226 (78%) | |
| N1a | 42 (15%) | |
| N1b | 20 (7%) | |
| ATA initial risk | ||
| low | 217 (75%) | |
| intermediate | 71 (25%) | |
| Tg at the time of ablation, ng/mL | 3.4 ± 5.5 (0.2–114) | |
| TgAb positivity at ablation | 198 (69%) | |
| First RAI activities administrated, GBq | 2.85 ± 1.1 (0.9–4) |
| 2-Year Treatment Response | |||||
|---|---|---|---|---|---|
| Variable | Univariate Analysis | Multivariate Analysis | |||
| ER (n = 110) | Not ER (n = 178) | p-value | HR (95%CI) | p-value | |
| Gender F:M | 86:24 | 149:29 | 0.241 | ||
| Age at diagnosis | 48.2 | 48.7 | 0.807 | ||
| Tumor size | 15.4 | 17 | 0.333 | ||
| Multicentricity | 60 | 108 | 0.768 | ||
| ATA initial risk low | 85 | 132 | 0.198 | ||
| T stage: T1–2 vs. T3 | 102:8 | 162:16 | 0.501 | ||
| N metastases | 20 | 42 | 0.470 | ||
| Tg at ablation | 6.7 | 5.6 | 0.324 | ||
| First RAI administrated, GBq | 2.5 | 2.7 | 0.191 | ||
| 1-year sTg | 1.8 | 2.7 | <0.001 | 5.02 (2.454–8.909) | <0.001 |
| 5-Year Treatment Response | |||||
| Variable | Univariate Analysis | Multivariate Analysis | |||
| ER (n = 221) | Not ER (n = 67) | p-value | HR (95%CI) | p-value | |
| Gender F:M | 175:46 | 60:7 | 0.106 | ||
| Age at diagnosis | 49 | 47 | 0.320 | ||
| Tumor size | 15.8 | 18.1 | 0.236 | ||
| Multicentricity | 132 | 36 | 0.200 | ||
| ATA initial risk low | 172 | 42 | 0.509 | ||
| T stage: T1–2 vs. T3 | 204:17 | 60:7 | 0.765 | ||
| N metastases | 38 | 24 | 0.001 | 2.345 (1.134–3.190) | 0.012 |
| Tg at ablation, ng/mL | 6.8 | 7.9 | 0.599 | ||
| First RAI administrated, GBq | 2.8 | 3.1 | 0.065 | ||
| 1-year sTg, ng/mL | 1.9 | 3.3 | 0.002 | 4.102 (2.601–7.879) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, D.; Bellini, P.; Dondi, F.; Calabrò, A.; Casella, C.; Taboni, S.; Lombardi, D.; Treglia, G.; Bertagna, F. Temporal Evolution and Prognostic Role of Indeterminate Response Sub-Groups in Patients with Differentiated Thyroid Cancer after Initial Therapy with Radioiodine. Cancers 2023, 15, 1270. https://doi.org/10.3390/cancers15041270
Albano D, Bellini P, Dondi F, Calabrò A, Casella C, Taboni S, Lombardi D, Treglia G, Bertagna F. Temporal Evolution and Prognostic Role of Indeterminate Response Sub-Groups in Patients with Differentiated Thyroid Cancer after Initial Therapy with Radioiodine. Cancers. 2023; 15(4):1270. https://doi.org/10.3390/cancers15041270
Chicago/Turabian StyleAlbano, Domenico, Pietro Bellini, Francesco Dondi, Anna Calabrò, Claudio Casella, Stefano Taboni, Davide Lombardi, Giorgio Treglia, and Francesco Bertagna. 2023. "Temporal Evolution and Prognostic Role of Indeterminate Response Sub-Groups in Patients with Differentiated Thyroid Cancer after Initial Therapy with Radioiodine" Cancers 15, no. 4: 1270. https://doi.org/10.3390/cancers15041270
APA StyleAlbano, D., Bellini, P., Dondi, F., Calabrò, A., Casella, C., Taboni, S., Lombardi, D., Treglia, G., & Bertagna, F. (2023). Temporal Evolution and Prognostic Role of Indeterminate Response Sub-Groups in Patients with Differentiated Thyroid Cancer after Initial Therapy with Radioiodine. Cancers, 15(4), 1270. https://doi.org/10.3390/cancers15041270








