Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids
Abstract
Simple Summary
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Culture
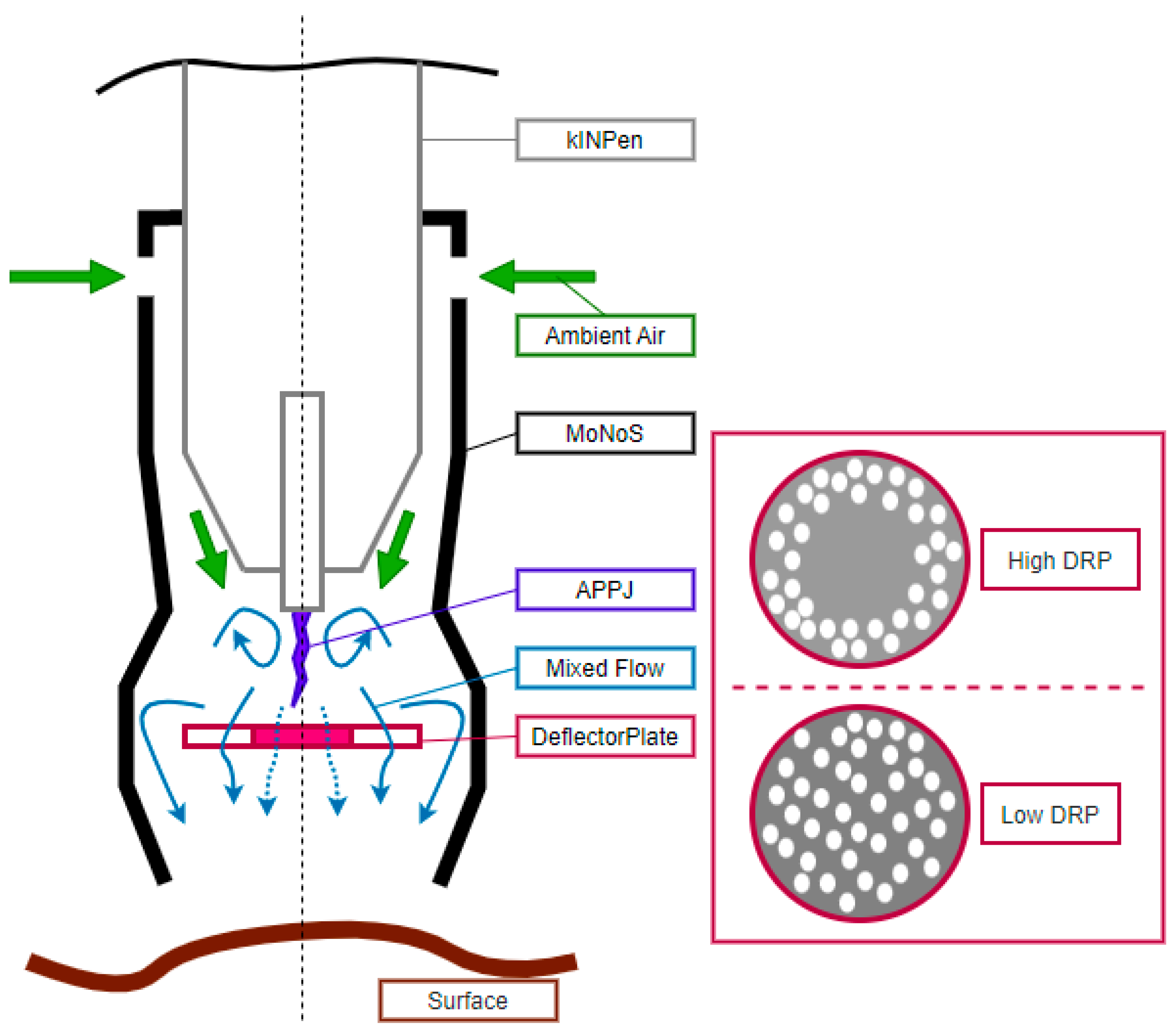
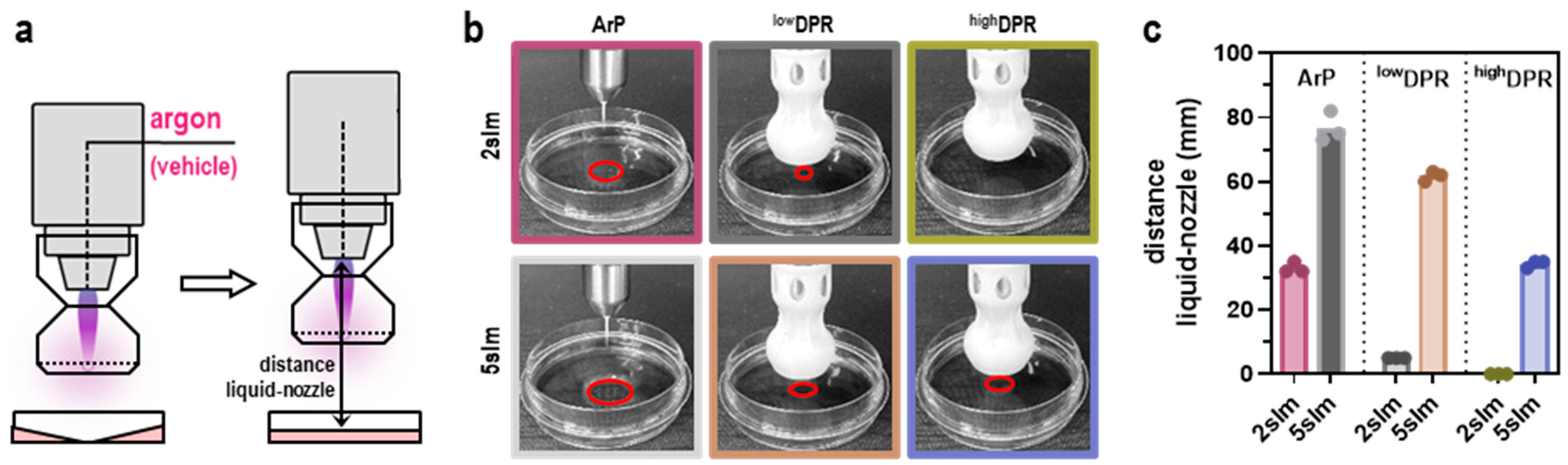
2.2. The Modular Nozzle System (MoNoS)
2.3. Gas Plasma Jet kINPen and Treatment
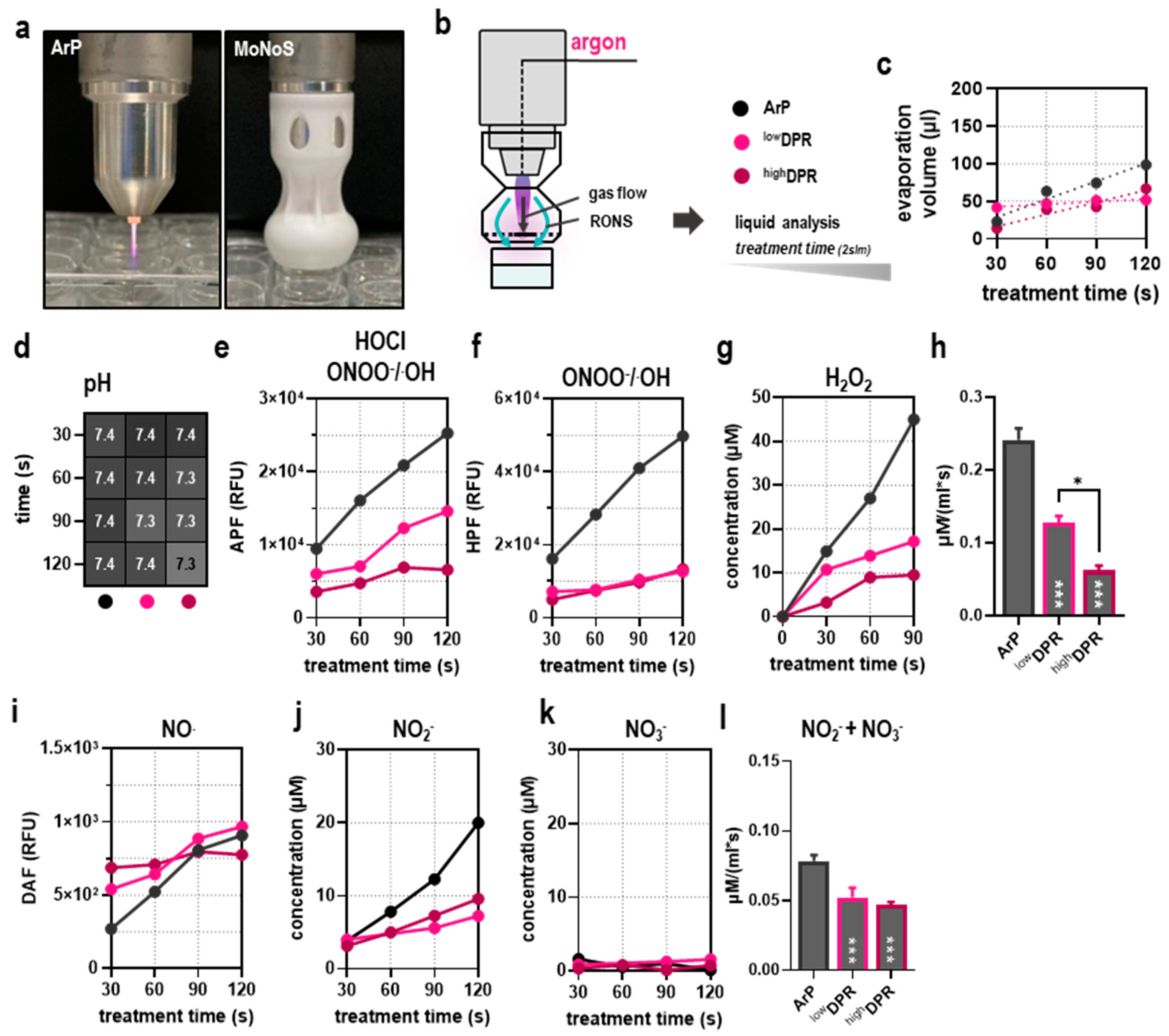
2.4. Profiling of Reactive Oxygen and Nitrogen Deposition
2.5. Metabolic Activity
2.6. Bioluminescence
2.7. Flow Cytometry
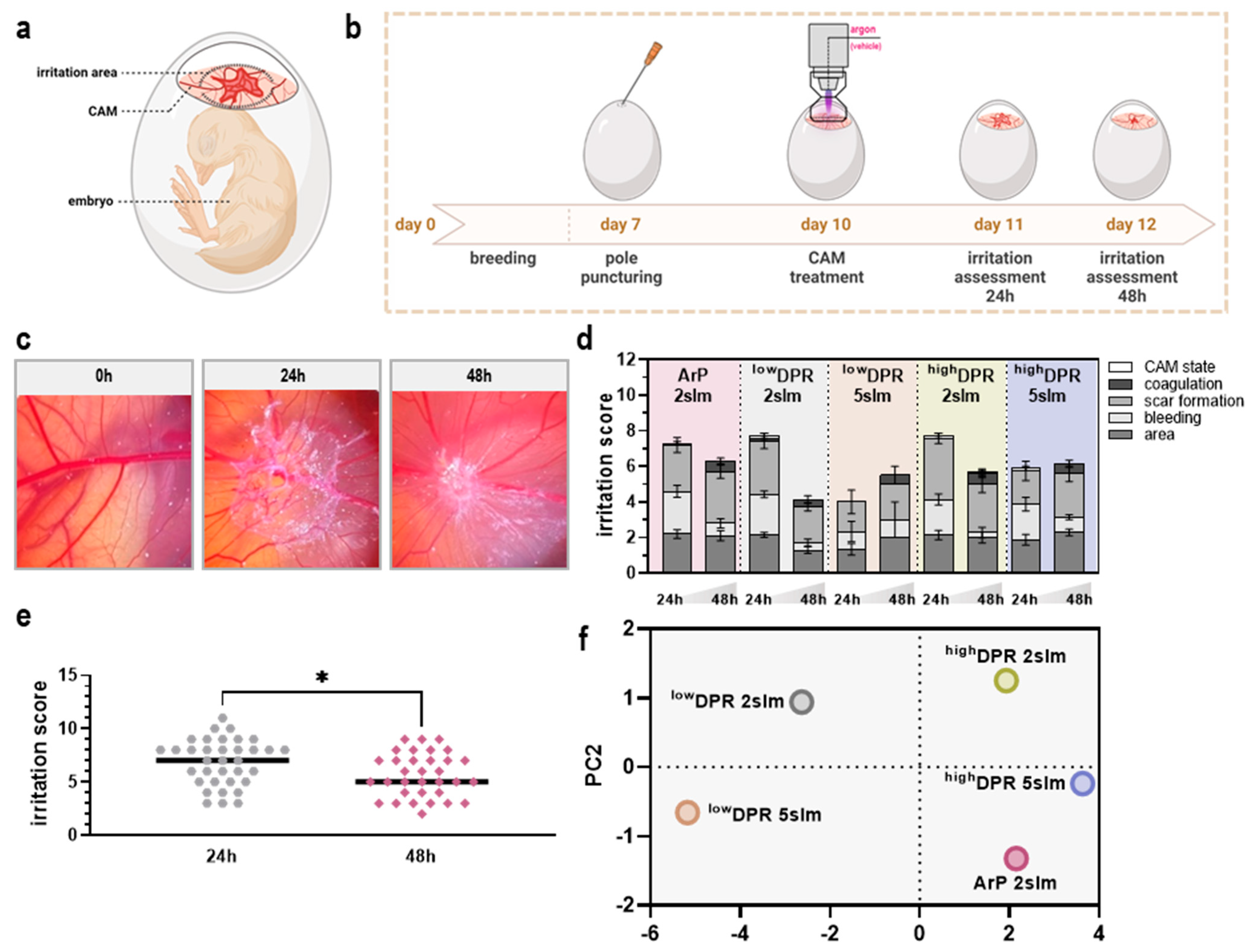
2.8. In Ovo Model
2.9. Chemokine and Cytokine Release Profiling
2.10. Software and Statistical Analysis
3. Results
3.1. MoNoS-Complemented kINPen Treatment at Modest Feed Gas Fluxes Yielded Reduced ROS Deposition in Liquids
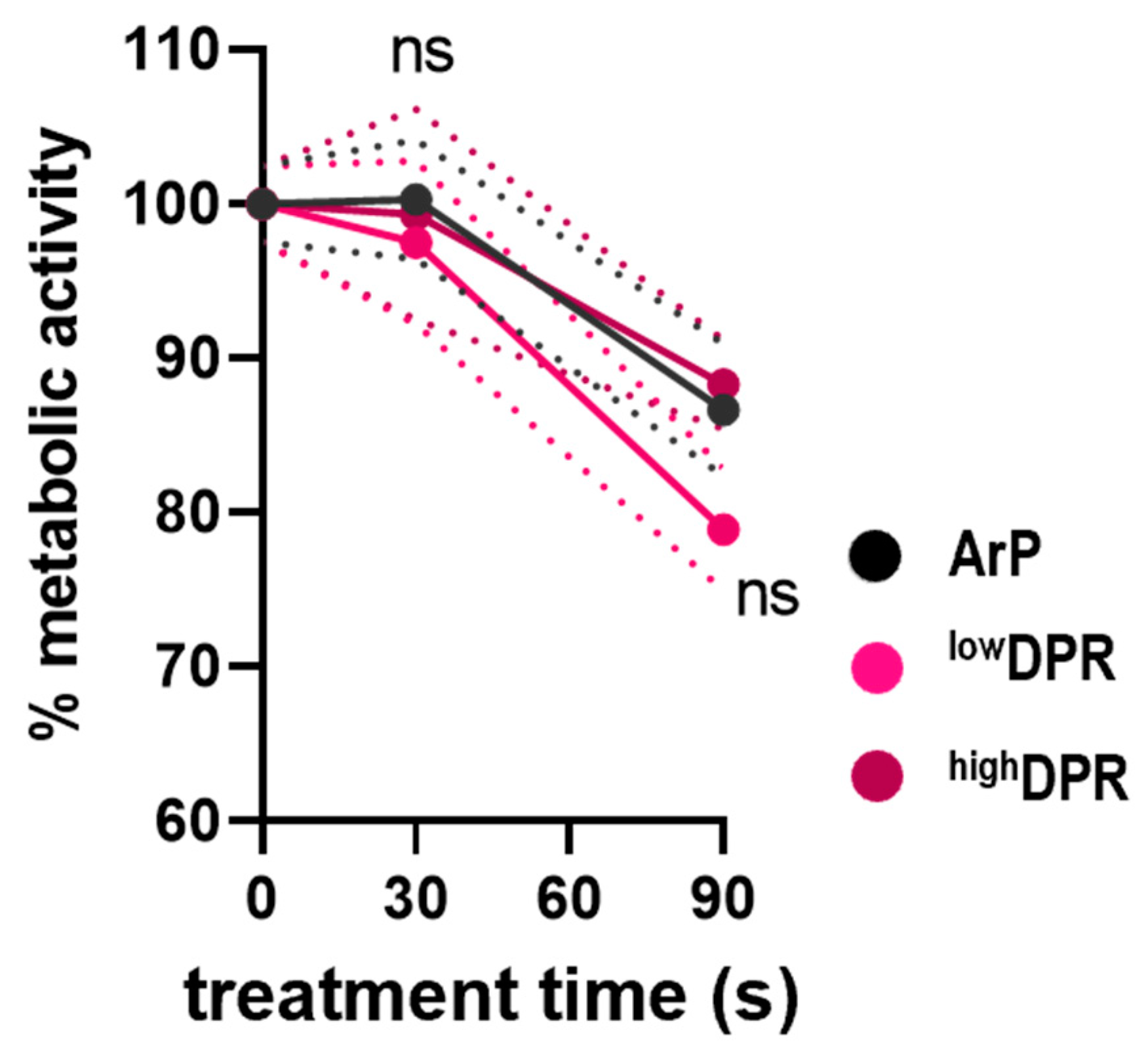
3.2. MoNoS-Complemented kINPen Treatment at Modest Gas Fluxes Does Not Augment Tumor Toxicity In Vitro and In Ovo
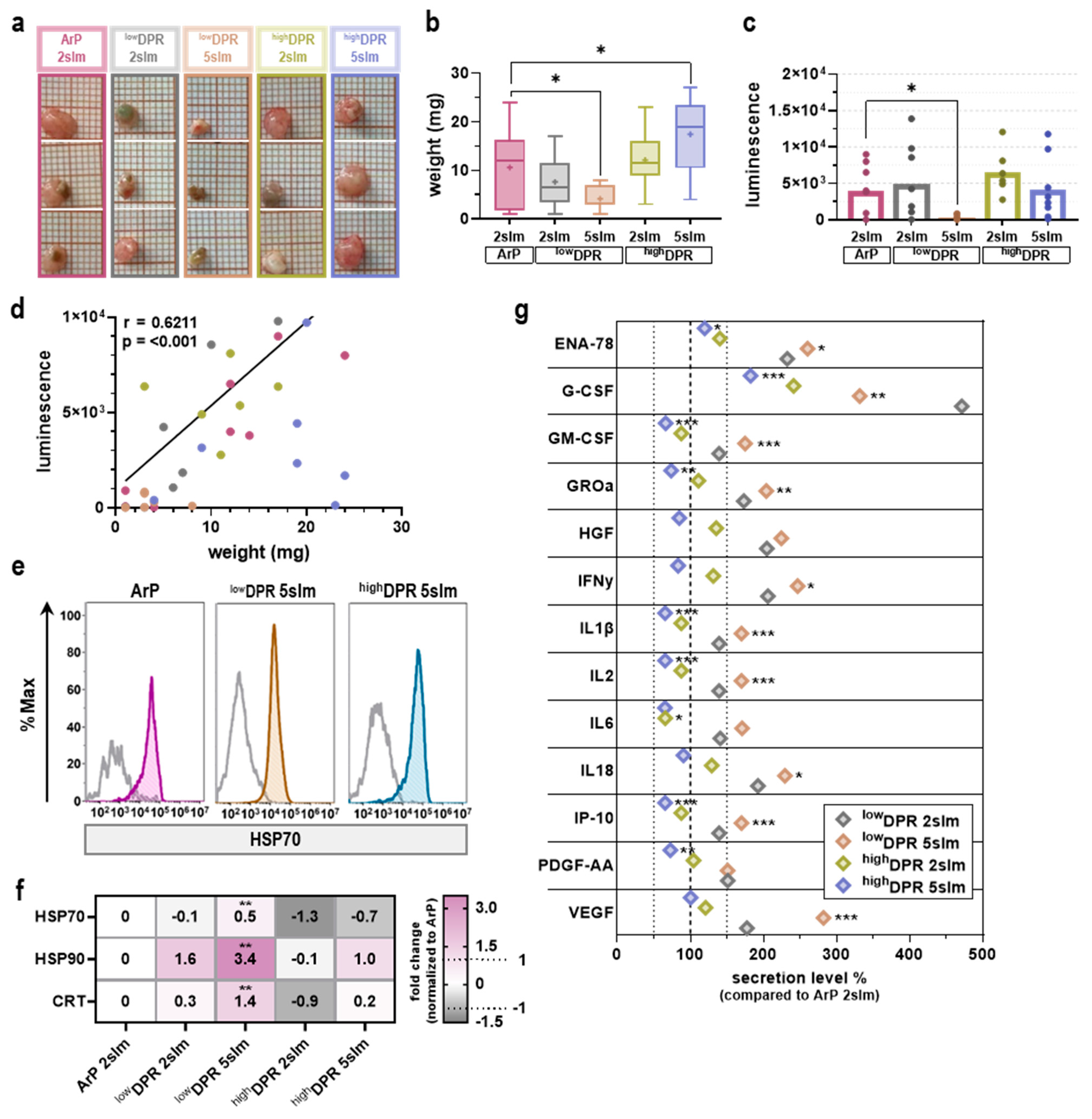
3.3. MoNoS-Complemented kINPen Treatment at 5 slm Augments Tumor Toxicity
3.4. MoNoS-Complemented kINPen Treatment Was Well-Tolerated in the HET-CAM Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| PC1 | PC2 | |
|---|---|---|
| weight | 0.905 | 0.035 |
| luminescence | 0.620 | 0.739 |
| irritation score | 0.788 | 0.189 |
| IL1β | −0.989 | −0.105 |
| IP-10 | −0.989 | −0.105 |
| IL2 | −0.989 | −0.105 |
| GM-CSF | −0.989 | −0.120 |
| G-CSF | −0.750 | 0.569 |
| IL6 | −0.965 | −0.226 |
| HGF | −0.973 | 0.228 |
| IFNγ | −0.987 | 0.142 |
| ENA-78 | −0.959 | 0.202 |
| IL18 | −0.985 | 0.145 |
| GROα | −0.996 | 0.075 |
| PDFG-aa | −0.961 | 0.164 |
| VEGF | −0.949 | −0.073 |
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, S.R.; Yang, K.S.; Ahn, Y.; Kim, Y.J.; Stadtman, E.R.; Rhee, S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 2004, 101, 16419–16424. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, R.; Jaken, S. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 2000, 28, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Alpha-Tocopherol, B.C.C.P.S.G. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef]
- Shen, B.; He, P.J.; Shao, C.L. Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLoS ONE 2013, 8, e84610. [Google Scholar] [CrossRef]
- Brenneisen, P.; Reichert, A.S. Nanotherapy and Reactive Oxygen Species (ROS) in Cancer: A Novel Perspective. Antioxidants 2018, 7, 31. [Google Scholar] [CrossRef]
- Hak, A.; Ravasaheb Shinde, V.; Rengan, A.K. A review of advanced nanoformulations in phototherapy for cancer therapeutics. Photodiagnosis Photodyn. Ther. 2021, 33, 102205. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R. Plasma, cancer, immunity. J. Phys. D Appl. Phys. 2022, 55, 473003. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Bogaerts, A.; Pouvesle, J.M.; Robert, E.; Szili, E.J. Plasma–liquid interactions. J. Appl. Phys. 2021, 130, 200401. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; Metelmann, P. Treating cancer with cold physical plasma: On the way to evidence-based medicine. Contrib. Plasma Phys. 2018, 58, 415–419. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ROS and RNS delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Miebach, L.; Berner, J.; Bekeschus, S. In ovo model in cancer research and tumor immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Partecke, L.I.; Kindel, E.; Doring, F.; Lademann, J.; Heidecke, C.D.; Kramer, A.; Hubner, N.O. The modified HET-CAM as a model for the assessment of the inflammatory response to tissue tolerable plasma. Toxicol. Vitr. 2011, 25, 530–537. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Freund, E.; Spadola, C.; Schmidt, A.; Privat-Maldonado, A.; Bogaerts, A.; von Woedtke, T.; Weltmann, K.D.; Heidecke, C.D.; Partecke, L.I.; Kading, A.; et al. Risk Evaluation of EMT and Inflammation in Metastatic Pancreatic Cancer Cells Following Plasma Treatment. Front. Phys. 2020, 8, 569618. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Renschler, M.F. The emerging role of reactive oxygen species in cancer therapy. Eur. J. Cancer 2004, 40, 1934–1940. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wang, Z.; Obenchain, R.; Wen, D.; Li, H.; Wirz, R.E.; Gu, Z. Portable air-fed cold atmospheric plasma device for postsurgical cancer treatment. Sci. Adv. 2021, 7, eabg5686. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Kuchenbecker, M.; Bibinov, N.; Kaemlimg, A.; Wandke, D.; Awakowicz, P.; Viol, W. Characterization of DBD plasma source for biomedical applications. J. Phys. D Appl. Phys. 2009, 42, 045212. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Brandenburg, R.; von Woedtke, T.; Ehlbeck, J.; Foest, R.; Stieber, M.; Kindel, E. Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs). J. Phys. D Appl. Phys. 2008, 41, 194008. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Zimmermann, J.; Shimizu, T.; Stolz, W. Cold atmospheric plasma: A successful treatment of lesions in Hailey-Hailey disease. Arch. Dermatol. 2011, 147, 388–390. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. Rev. Sect. Phys. Lett. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Iseni, S.; Zhang, S.; van Gessel, A.F.H.; Hofmann, S.; van Ham, B.T.J.; Reuter, S.; Weltmann, K.D.; Bruggeman, P.J. Nitric oxide density distributions in the effluent of an RF argon APPJ: Effect of gas flow rate and substrate. New J. Phys. 2014, 16, 123011. [Google Scholar] [CrossRef]
- Reuter, S.; Winter, J.; Schmidt-Bleker, A.; Schroeder, D.; Lange, H.; Knake, N.; Schulz-von der Gathen, V.; Weltmann, K.D. Atomic oxygen in a cold argon plasma jet: TALIF spectroscopy in ambient air with modelling and measurements of ambient species diffusion. Plasma Sources Sci. Technol. 2012, 21, 024005. [Google Scholar] [CrossRef]
- Park, J.C.; Krishnakumar, H.N.; Saladi, S.V. Current and Future Biomarkers for Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma. Curr. Oncol. 2022, 29, 4185–4198. [Google Scholar] [CrossRef]
- Lang, F.; Schrors, B.; Lower, M.; Tureci, O.; Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat. Rev. Drug Discov. 2022, 21, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Barroso-Sousa, R.; Garrido-Castro, A.C.; McAllister, S.S.; Guerriero, J.L.; Mittendorf, E.; Curigliano, G.; Tolaney, S.M. Understanding resistance to immune checkpoint inhibitors in advanced breast cancer. Expert Rev. Anticancer Ther. 2022, 22, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Panaretakis, T.; Joza, N.; Tufi, R.; Tesniere, A.; van Endert, P.; Zitvogel, L.; Kroemer, G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007, 14, 1848–1850. [Google Scholar] [CrossRef]
- Adkins, I.; Fucikova, J.; Garg, A.D.; Agostinis, P.; Spisek, R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 2014, 3, e968434. [Google Scholar] [CrossRef]
- Rebe, C.; Ghiringhelli, F. Interleukin-1beta and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Hernandez, R.; Poder, J.; LaPorte, K.M.; Malek, T.R. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat. Rev. Immunol. 2022, 22, 614–628. [Google Scholar] [CrossRef]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Marklund, S. Spectrophotometric study of spontaneous disproportionation of superoxide anion radical and sensitive direct assay for superoxide dismutase. J. Biol. Chem. 1976, 251, 7504–7507. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, R.; Daeschlein, G.; von Woedtke, T.; Smeets, R.; Gosau, M.; Metelmann, H.R. Long-term Risk Assessment for Medical Application of Cold Atmospheric Pressure Plasma. Diagnostics 2020, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Evert, K.; Kocher, T.; Schindler, A.; Müller, M.; Müller, K.; Pink, C.; Holtfreter, B.; Schmidt, A.; Dombrowski, F.; Schubert, A.; et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci. Rep. 2021, 11, 20672. [Google Scholar] [CrossRef]
- Bekeschus, S.; Freund, E.; Spadola, C.; Privat-Maldonado, A.; Hackbarth, C.; Bogaerts, A.; Schmidt, A.; Wende, K.; Weltmann, K.D.; von Woedtke, T.; et al. Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers 2019, 11, 1237. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berner, J.; Miebach, L.; Herold, L.; Höft, H.; Gerling, T.; Mattern, P.; Bekeschus, S. Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids. Cancers 2023, 15, 1254. https://doi.org/10.3390/cancers15041254
Berner J, Miebach L, Herold L, Höft H, Gerling T, Mattern P, Bekeschus S. Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids. Cancers. 2023; 15(4):1254. https://doi.org/10.3390/cancers15041254
Chicago/Turabian StyleBerner, Julia, Lea Miebach, Luise Herold, Hans Höft, Torsten Gerling, Philipp Mattern, and Sander Bekeschus. 2023. "Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids" Cancers 15, no. 4: 1254. https://doi.org/10.3390/cancers15041254
APA StyleBerner, J., Miebach, L., Herold, L., Höft, H., Gerling, T., Mattern, P., & Bekeschus, S. (2023). Gas Flow Shaping via Novel Modular Nozzle System (MoNoS) Augments kINPen-Mediated Toxicity and Immunogenicity in Tumor Organoids. Cancers, 15(4), 1254. https://doi.org/10.3390/cancers15041254







