The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
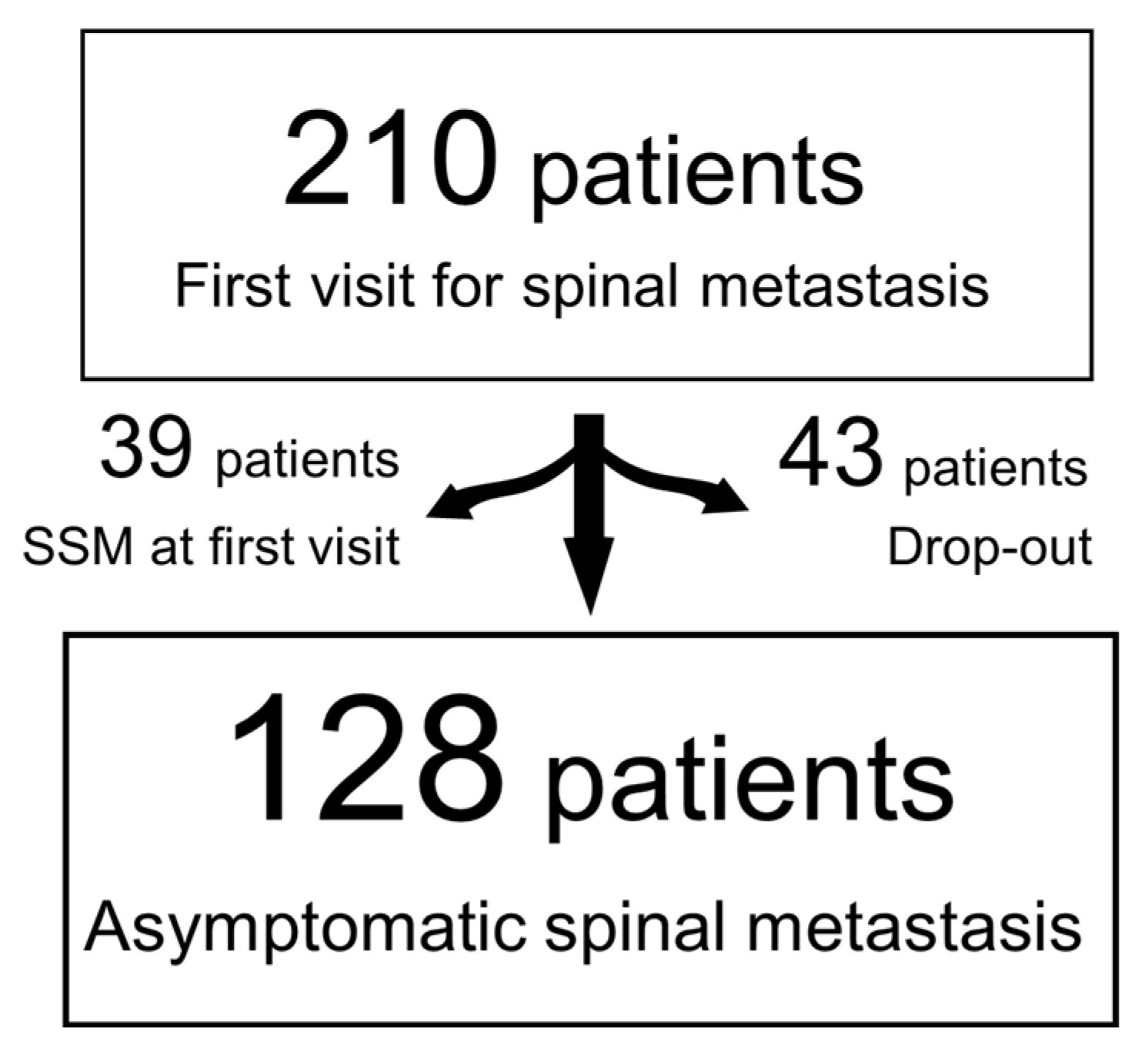
2.2. Patients
2.3. Statistical Analysis
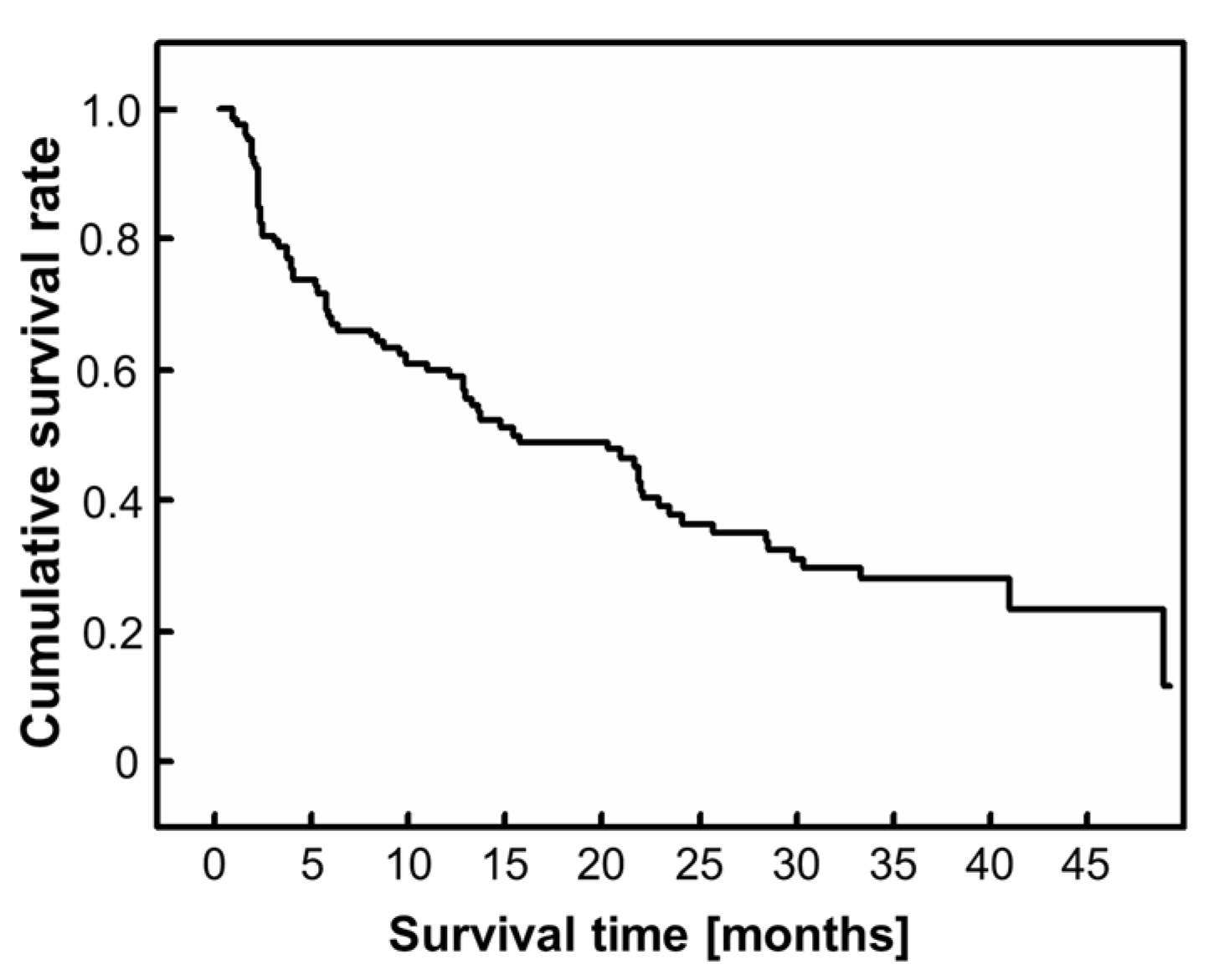
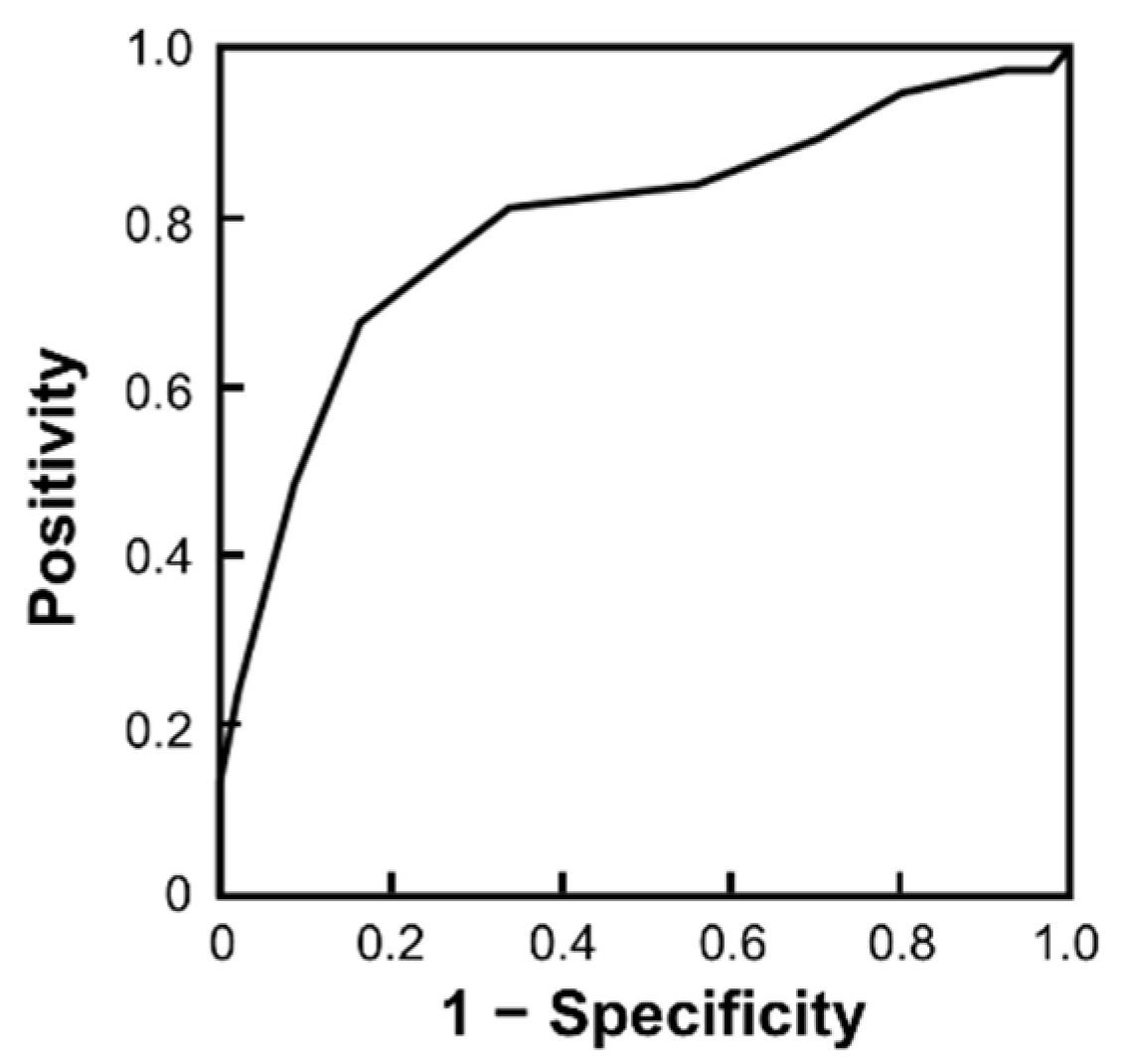
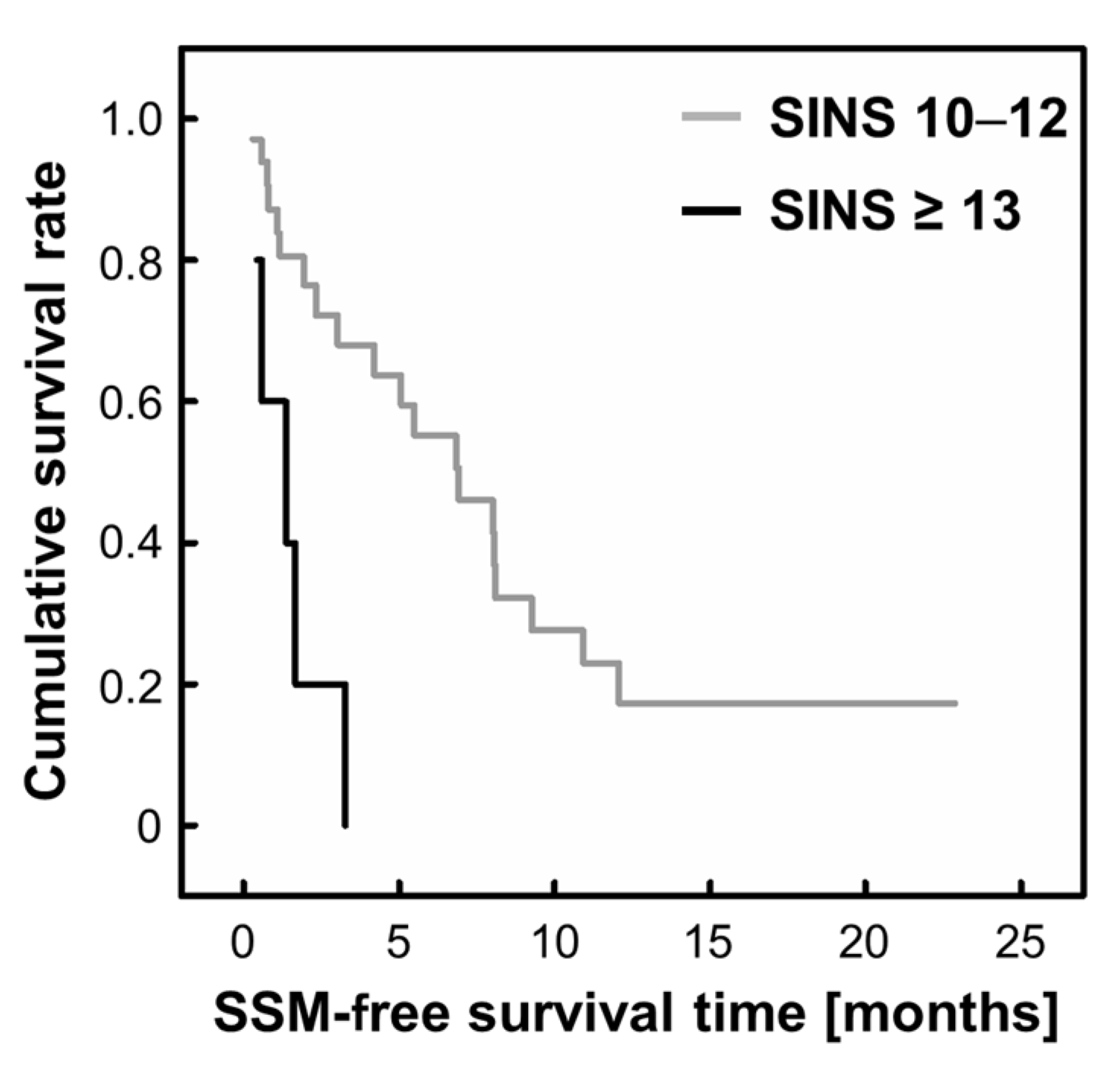
3. Results
3.1. Demographic and Clinical Data
3.2. Risk Factors for SSM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Witham, T.F.; Khavkin, Y.A.; Gallia, G.L.; Wolinsky, J.P.; Gokaslan, Z.L. Surgery insight: Current management of epidural spinal cord compression from metastatic spine disease. Nat. Clin. Pract. Neurol. 2006, 2, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.A.; Fornasier, V.L.; MacNab, I. Spinal metastases: The obvious, the occult, and the impostors. Spine 1990, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Klimo, P., Jr.; Schmidt, M.H. Surgical management of spinal metastases. Oncologist 2004, 9, 188–196. [Google Scholar] [CrossRef]
- Kakutani, K.; Sakai, Y.; Maeno, K.; Takada, T.; Yurube, T.; Kurakawa, T.; Miyazaki, S.; Terashima, Y.; Ito, M.; Hara, H.; et al. Prospective cohort study of performance status and activities of daily living after surgery for spinal metastasis. Clin. Spine Surg. 2017, 30, E1026–E1032. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kakutani, K.; Sakai, Y.; Ejima, Y.; Maeno, K.; Takada, T.; Yurube, T.; Terashima, Y.; Ito, M.; Kakiuchi, Y.; et al. Quality of life and cost-utility of surgical treatment for patients with spinal metastases: Prospective cohort study. Int. Orthop. 2017, 41, 1265–1271. [Google Scholar] [CrossRef]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Sze, W.M.; Shelley, M.; Held, I.; Mason, M. Palliation of metastatic bone pain: Single fraction versus multifraction radiotherapy—A systematic review of the randomised trials. Cochrane Database Syst. Rev. 2004, 2002, CD004721. [Google Scholar] [CrossRef]
- Kimura, T. Multidisciplinary approach for bone metastasis: A review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef]
- Sutcliffe, P.; Connock, M.; Shyangdan, D.; Court, R.; Kandala, N.B.; Clarke, A. A systematic review of evidence on malignant spinal metastases: Natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol. Assess. 2013, 17, 1–274. [Google Scholar] [CrossRef]
- Fisher, C.G.; DiPaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2891. [Google Scholar] [CrossRef] [PubMed]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Ejima, Y.; Matsuo, Y.; Sasaki, R. The current status and future of radiotherapy for spinal bone metastases. J. Orthop. Sci. 2015, 20, 585–592. [Google Scholar] [CrossRef]
- Shi, D.D.; Hertan, L.M.; Lam, T.C.; Skamene, S.; Chi, J.H.; Groff, M.; Cho, C.H.; Ferrone, M.L.; Harris, M.; Chen, Y.H.; et al. Assessing the utility of the spinal instability neoplastic score (SINS) to predict fracture after conventional radiation therapy (RT) for spinal metastases. Pract. Radiat. Oncol. 2018, 8, e285–e294. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, W.J.; Chang, J.S.; Chang, S.K.; Koom, W.S. Evaluation of predictive factors of vertebral compression fracture after conventional palliative radiotherapy for spinal metastasis from colorectal cancer. J. Neurosurg. Spine 2018, 28, 333–340. [Google Scholar]
- Chang, S.Y.; Ha, J.H.; Seo, S.G.; Chang, B.S.; Lee, C.K.; Kim, H. Prognosis of single spinal metastatic tumors: Predictive value of the Spinal Instability Neoplastic Score system for spinal adverse events. Asian Spine J. 2018, 12, 919–926. [Google Scholar]
- Aiba, H.; Kimura, T.; Yamagami, T.; Watanabe, N.; Sakurai, H.; Kimura, H.; Shimozaki, S.; Yamada, S.; Otsuka, T. Prediction of skeletal-related events in patients with non-small cell lung cancer. Support. Care Cancer 2016, 24, 3361–3367. [Google Scholar] [CrossRef]
- Versteeg, A.L.; van der Velden, J.M.; Verkooijen, H.M.; van Vulpen, M.; Oner, F.C.; Fisher, C.G.; Verlaan, J.J. The effect of introducing the Spinal Instability Neoplastic Score in routine clinical practice for patients with spinal metastases. Oncologist 2016, 21, 95–101. [Google Scholar] [CrossRef]
- Pennington, Z.; Ahmed, A.K.; Westbroek, E.M.; Cottrill, E.; Lubelski, D.; Goodwin, M.L.; Sciubba, D.M. SINS score and stability: Evaluating the need for stabilization within the uncertain category. World Neurosurg. 2019, 128, e1034–e1047. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.C.; Uno, H.; Krishnan, M.; Lutz, S.; Groff, M.; Cheney, M.; Balboni, T. Adverse outcomes after palliative radiation therapy for uncomplicated spine metastases: Role of spinal instability and single-fraction radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, D.; Lu, Y.; Curtis, D.; Wen, X.; Yao, Y.; Zhao, H. Systematic literature review and network meta-analysis comparing bone-targeted agents for the prevention of skeletal-related events in cancer patients with bone metastasis. Oncologist 2015, 20, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Kakutani, K.; Sakai, Y.; Yurube, T.; Miyazaki, S.; Takada, T.; Hoshino, Y.; Kuroda, R. Prospective cohort study of surgical outcome for spinal metastases in patients aged 70 years or older. Bone Jt. J. 2020, 102, 1709–1716. [Google Scholar] [CrossRef]

| Variables | Variables | ||
|---|---|---|---|
| Age (no. (%)) | Barthel index (mean [range]) (pts) | 79.7 [5–100] | |
| ≥65 | 85 (66.4) | SINS (total score) (median [interquartile range]) (pts) | 8 [7–10] |
| ˂65 | 43 (33.6) | Location | 2 [2–3] |
| Sex (no. (%)) | Pain | 1 [1–3] | |
| Male | 75 (58.6) | Bone lesion | 2 [1–2] |
| Female | 53 (41.4) | Alignment | 0 [0–0] |
| Malignancy of primary site (no. (%)) | Collapses | 1 [1–2] | |
| Lung, osteosarcoma, stomach, bladder, esophagus, pancreas | 40 (31.3) | Spinal element | 1 [0.25–3] |
| Liver, gallbladder, unidentified | 18 (14.1) | Radiotherapy (no. (%)) | |
| Others | 26 (20.3) | Yes | 90 (70.3) |
| Kidney, uterus | 19 (14.8) | No | 38 (29.7) |
| Rectum | 4 (3.1) | Chemotherapy (no. [%]) | |
| Thyroid, breast, prostate, carcinoid tumor | 21 (16.4) | Yes | 31 (24.2) |
| ECOGPS grade (no. (%)) | No | 97 (75.8) | |
| PS 0 | 14 (10.9) | Molecularly targeted drugs or hormonal therapy (no. (%)) | |
| PS 1 | 45 (35.2) | Yes | 43 (33.6) |
| PS 2 | 35 (27.3) | No | 85 (66.4) |
| PS 3 | 23 (18.0) | Bone-modifying agents (no. (%)) | |
| PS 4 | 11 (8.6) | Yes | 73 (57.0) |
| Median PS | 2 | No | 55 (43.0) |
| Primary Tumor | No. (%) of Patients |
|---|---|
| Lung | 33 (25.8) |
| Kidney | 15 (11.7) |
| Breast | 10 (7.8) |
| Liver | 10 (7.8) |
| Unknown | 9 (7.0) |
| Thyroid | 6 (4.7) |
| Prostate | 5 (3.9) |
| Lymphoma | 5 (3.9) |
| Myeloma | 4 (3.1) |
| Bladder | 4 (3.1) |
| Colorectal | 4 (3.1) |
| Others | 23 (23.8) |
| Variables | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Age (≥65) | 1.062 | 0.399–2.830 | 0.904 |
| Sex (male) | 1.076 | 0.391–2.965 | 0.887 |
| Malignancy of primary tumor | 1.007 | 0.749–1.353 | 0.963 |
| ECOGPS | 1.419 | 0.645–3.126 | 0.384 |
| Barthel Index | 1.024 | 0.991–1.059 | 0.159 |
| SINS (total score) | 1.739 | 1.345–2.250 | <0.001 * |
| Radiotherapy | 2.413 | 0.745–7.811 | 0.142 |
| Chemotherapy | 1.603 | 0.478–5.369 | 0.444 |
| Molecularly targeted drugs or hormonal therapy | 1.012 | 0.330–3.100 | 0.984 |
| Bone-modifying agents | 0.535 | 0.200–1.431 | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakutani, K.; Kanda, Y.; Yurube, T.; Takeoka, Y.; Miyazaki, K.; Ohnishi, H.; Matsuo, T.; Ryu, M.; Kuroshima, K.; Kumagai, N.; et al. The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients. Cancers 2023, 15, 1251. https://doi.org/10.3390/cancers15041251
Kakutani K, Kanda Y, Yurube T, Takeoka Y, Miyazaki K, Ohnishi H, Matsuo T, Ryu M, Kuroshima K, Kumagai N, et al. The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients. Cancers. 2023; 15(4):1251. https://doi.org/10.3390/cancers15041251
Chicago/Turabian StyleKakutani, Kenichiro, Yutaro Kanda, Takashi Yurube, Yoshiki Takeoka, Kunihiko Miyazaki, Hiroki Ohnishi, Tomoya Matsuo, Masao Ryu, Kohei Kuroshima, Naotoshi Kumagai, and et al. 2023. "The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients" Cancers 15, no. 4: 1251. https://doi.org/10.3390/cancers15041251
APA StyleKakutani, K., Kanda, Y., Yurube, T., Takeoka, Y., Miyazaki, K., Ohnishi, H., Matsuo, T., Ryu, M., Kuroshima, K., Kumagai, N., Hiranaka, Y., Hayashi, S., Hoshino, Y., Hara, H., Sakai, Y., & Kuroda, R. (2023). The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients. Cancers, 15(4), 1251. https://doi.org/10.3390/cancers15041251







